Abstract
Medicinal herbs may cause clinically relevant drug interactions with antiretroviral agents. Ginkgo biloba extract is a popular herbal product among HIV-infected patients because of its positive effects on cognitive function. Raltegravir, an HIV integrase inhibitor, is increasingly being used as part of combined antiretroviral therapy. Clinical data on the potential inhibitory or inductive effect of ginkgo biloba on the pharmacokinetics of raltegravir were lacking, and concomitant use was not recommended. We studied the effect of ginkgo biloba extract on the pharmacokinetics of raltegravir in an open-label, randomized, two-period, crossover phase I trial in 18 healthy volunteers. Subjects were randomly assigned to a regimen of 120 mg of ginkgo biloba twice daily for 15 days plus a single dose of raltegravir (400 mg) on day 15, a washout period, and 400 mg of raltegravir on day 36 or the test and reference treatments in reverse order. Pharmacokinetic sampling of raltegravir was performed up to 12 h after intake on an empty stomach. All subjects (9 male) completed the trial, and no serious adverse events were reported. Geometric mean ratios (90% confidence intervals) of the area under the plasma concentration-time curve from dosing to infinity (AUC0-∞) and the maximum plasma concentration (Cmax) of raltegravir with ginkgo biloba versus raltegravir alone were 1.21 (0.93 to 1.58) and 1.44 (1.03 to 2.02). Ginkgo biloba did not reduce raltegravir exposure. The potential increase in the Cmax of raltegravir is probably of minor importance, given the large intersubject variability of raltegravir pharmacokinetics and its reported safety profile.
INTRODUCTION
Approximately 60% of HIV-infected patients use complementary and alternative medicines to treat HIV-related symptoms and the side effects of conventional antiretroviral therapy. Herbal medicines can cause clinically significant interactions with antiretroviral agents, with potential drug failure as a result (17, 26).
Among the most popular herbal products used worldwide is ginkgo biloba extract, which is made from the leaves of the ginkgo biloba tree. It is used for the treatment of peripheral vascular disease and is frequently taken for its claimed beneficial effects on concentration, memory, depressive disorders, and dementia (1). Because cognitive impairment is one of the most feared complications among HIV-infected patients, the popularity of ginkgo biloba within this patient group is easily explained (23). Although ginkgo biloba extract has potential beneficial effects, self-medication with ginkgo biloba may lead to undesirable drug interactions with regular medication. For example, a study in healthy subjects showed that plasma concentrations of midazolam (a CYP3A probe) were significantly reduced after ginkgo biloba intake (22). If this were also true with antiretroviral agents, it could place individual patients at risk for virological failure.
In past years, a few articles have been published about the potential negative effects of ginkgo biloba on the pharmacokinetics of antiretroviral agents. One case report described virological failure in an HIV-infected patient taking an efavirenz-based regimen due to concomitant use of ginkgo biloba. Although the underlying mechanism remained unclear, terpene lactones in ginkgo biloba may lower plasma efavirenz levels by the induction of CYP2B6, CYP3A4, and/or P-glycoprotein (P-gp) (28). Unlike the inductive effects of ginkgo biloba on the pharmacokinetics of midazolam, ginkgo biloba extract did not change the exposure of lopinavir (a protease inhibitor and CYP3A substrate) when used with low-dose ritonavir. The use of low-dose ritonavir, a potent CYP3A inhibitor, may have offset the effect of ginkgo biloba on the pharmacokinetics of lopinavir (22).
In vivo data on ginkgo biloba with other antiretroviral drug classes, such as HIV integrase inhibitors, like raltegravir, are lacking. According to current guidelines, raltegravir in combination with tenofovir/emtricitabine is recommended as one of the preferred regimens for antiretroviral-naïve patients (8, 21). Raltegravir targets the HIV-1 integrase enzyme and prevents the integration of viral DNA into the genome of the host cell. Raltegravir has shown sustained antiretroviral activity, is generally well tolerated, and has little propensity to interact with other drugs. The primary route of metabolism is glucuronidation via UDP-glucuronyltransferase 1A1 (UGT1A1) in the liver, with minor contributions from UGT1A3 and UGT1A9 (6, 12, 13). In vitro studies suggest that raltegravir is a weak P-gp substrate (20). There is in vitro and animal evidence that ginkgo biloba modulates UGT enzymes. Ginkgo biloba extract and ginkgolides induce the expression of UGT1A1 in human primary hepatocytes, although inhibitory effects on UGT enzymes of ginkgo biloba extract, or one of its components, have been described, as well (16, 18, 19, 29). Other investigations showed that long-term use of ginkgo biloba inhibits P-gp-mediated drug transport, but contrary results have also been reported (1, 9, 10, 30, 31).
Given the inconclusive data on the potential inhibitory or inductive effect of ginkgo biloba extract on glucuronidation or P-gp-mediated transport, ginkgo biloba may theoretically influence the exposure to raltegravir. Therefore, concomitant use is currently not recommended. The objectives of this study were to assess the effect of steady-state ginkgo biloba on the pharmacokinetics of a single dose of raltegravir in healthy volunteers and to evaluate the safety and tolerability of the combination.
MATERIALS AND METHODS
Study design.
This open-label, randomized, two-period, crossover, single-center, phase I trial was conducted from December 2010 to March 2011 at the Radboud University Nijmegen Medical Centre (Nijmegen, The Netherlands). The study was designed to examine the effect of multiple doses of ginkgo biloba on the pharmacokinetics of a single dose of raltegravir by intrasubject comparison. The secondary objective was to evaluate the safety of the combined use of raltegravir and ginkgo biloba.
Eighteen healthy volunteers (9 females and 9 males) were stratified according to gender in groups A and B. In group A, participants received 120 mg of ginkgo biloba twice daily for 14 days, followed by a single dose on day 15, together with a single dose of 400 mg of raltegravir. After a washout period of 3 weeks, the participants received a single dose of 400 mg of raltegravir on day 36. In group B, participants received the test and reference treatments in reverse order. They received a single dose of 400 mg of raltegravir on day 15, followed by a washout period of 1 week. On day 22, they started ginkgo biloba treatment (120 mg twice daily for 14 days). On day 36, participants took a single dose of ginkgo biloba together with a single dose of 400 mg of raltegravir. The trial was approved by the Investigational Review Board of the Radboud University Nijmegen Medical Centre, Nijmegen, The Netherlands. The trial was conducted in accordance with good clinical practice and the Declaration of Helsinki. The study was registered at http://www.ClinicalTrials.gov (NCT01246804).
Study population.
Healthy male and female participants between the ages of 18 and 55 years and with a body mass index of 18 to 30 kg/m2 (extremes included) were eligible for enrollment. The included participants had to be in good, age-appropriate health, as established by physical examination, medical history, electrocardiography, and biochemical, hematologic, and urinalysis testing within 4 weeks prior to day 1. The subjects had to be able and willing to sign an informed consent form prior to screening evaluations. The main exclusion criteria were a history of sensitivity or idiosyncrasy to medicinal products or excipients, a positive HIV or hepatitis B or C test result, or therapy with any drug (for 2 weeks preceding dosing), except for acetaminophen. Other exclusion criteria were participation in a drug trial or blood donation within 60 days prior to day 1 of the study. Pregnant and breastfeeding females were also excluded.
Study drug and dosing.
Ginkgo biloba extract is a complex mixture of chemical constituents, and the actual contents may vary depending on the part of the plant being processed, the season, or the manufacturing process (1). The industry standard for powdered extracts, as used in this study, is 24% flavonoids (quercetin, kaempferol, and isorhamnetin, among others) and 6% terpene lactones (ginkgolides and bilobalide) by weight. In this trial, the commercial product Tavonin (a tablet with 40 mg of ginkgo biloba extract) was used because of its standardized composition of 9.6 mg of flavonoids and 2.4 mg of terpene lactone. Tavonin (Willmar Schwabe GmbH, Karlsruhe, Germany) is licensed in The Netherlands for the treatment of occlusive peripheral arterial disease. The ginkgo biloba dose used in this trial (120 mg twice daily) is the recommended dosage for the prevention of cognitive decline and memory support (3, 24). A treatment period of 14 days of ginkgo biloba was chosen to reach steady state and to assess potential effects on metabolizing enzymes or drug transporters. Raltegravir (Isentress; Merck Sharp & Dohme Limited, Hoddesdon, United Kingdom) was administered as a single dose of 400 mg on an empty stomach, followed by a standardized breakfast 2 h after intake. The breakfast consisted of a glass of milk and two slices of wheat bread with cheese and cervelat.
Pharmacokinetic sampling and safety assessments.
Blood samples for assessment of the pharmacokinetic parameters of raltegravir were collected during a 12-hour period at 0 (predose), 0.5, 1, 1.5, 2, 3, 4, 5, 6, 8, 10, and 12 h after intake of a single dose of 400 mg of raltegravir on days 15 and 36. Blood samples were collected in heparinized tubes and centrifuged for 10 min at 2,930 × g at 20°C. Plasma was transferred to polypropylene tubes and stored at −40°C until further bioanalysis. Blood samples for serum biochemistry and hematology were taken on days 15 and 36, as well as before and during ginkgo biloba treatment (days 1 and 8 of treatment). Subjects were asked about the presence of adverse events at each visit day. Screening for drugs of abuse in urine was performed on days 15 and 36; blood glucose testing and urinalysis were carried out on day 36. Pregnancy was checked by performing a human chorionic gonadotropin (hCG) blood test on all female subjects on days 15 and 36.
Compliance.
All intake of medication at the clinical trial unit was supervised and recorded by the study personnel. Intake of ginkgo biloba tablets at home was monitored by the use of microelectronic monitoring system (MEMS) caps (Aardex Ltd., Zug, Switzerland), which record the opening of the medication bottle. In addition, ginkgo biloba tablets were counted on each visit day during ginkgo biloba treatment to assess adherence. Subjects were asked to write down the exact times of intake in a booklet.
Bioanalysis of raltegravir in plasma.
The concentrations of raltegravir in plasma were analyzed by use of a validated reversed-phase high-pressure liquid chromatography (HPLC) method with fluorescence detection. Sample preparation consisted of a liquid-liquid extraction by adding 500 μl of acetate buffer (pH 4.0; 0.2 M), 5 ml of hexane/dichloromethane (1:1 [vol/vol]), and 50 μl of internal standard (lormetazepam in methanol-water [1:1 {vol/vol}]) to 500 μl of plasma. The samples were mixed on a vortex mixer for 5 min, followed by centrifugation at 11,500 × g for 5 min. After freezing at −40°C for 5 min, the organic supernatant was decanted and evaporated at 37°C under a stream of nitrogen gas. The residue was reconstituted in 200 μl of eluent (acetonitrile-phosphate buffer, pH 4.8; 20 mM; 35:65 [vol/vol]). Forty microliters of the reconstituted solution was injected onto a SymmetryShield RP 18 column (3.5 μm; 100 by 4.6 mm). The flow rate was set at 1.5 ml/min. Raltegravir was detected by the use of a fluorescence detector (λexcitation, 240 nm; λemission, 412 nm). The lower limit of quantification was 0.014 mg/liter. The linear calibration ranges in plasma were from 0.014 to 10 mg/liter. The validation results displayed accuracies of the quality control samples of 100%, 102%, and 107% at plasma concentrations of 0.060, 0.400, and 4.00 mg/liter. At the same concentrations, the precision values (within-day coefficients of variation [CV]) were 3.7%, 1.8%, and 0%, respectively. The raltegravir assay was performed at the laboratory of the Pharmacy of Radboud University Nijmegen Medical Centre (Nijmegen, The Netherlands) and was externally validated through the International Interlaboratory Quality Control Program for Measurement of Antiretroviral Drugs in Plasma (5).
Pharmacokinetic analysis.
Pharmacokinetic parameters for raltegravir were calculated by noncompartmental methods using the WinNonlin software package (version 5.2; Pharsight, Mountain View, CA) and the linear log trapezoidal rule. Based on the individual plasma concentration-time data, the following pharmacokinetic parameters of raltegravir were determined: the area under the plasma concentration-time curve from dosing to infinity (AUC0-∞) (in mg · h/liter), the area under the plasma concentration-time curve from 0 to 12 h after intake (AUC0-12) (in mg · h/liter), the maximum plasma concentration of the drug (Cmax) (in mg/liter), the time to reach Cmax (Tmax) (in h), the apparent volume of distribution (V/F) (in liters), the apparent oral clearance (CL/F) (in liters/h), and the apparent elimination half-life (T1/2) (in h).
Sample size and statistical analysis.
For the identification of a clinically relevant drug interaction, the bioequivalence approach was used, as described previously (25). The main pharmacokinetic parameter to be evaluated in this respect is the exposure to raltegravir, expressed as the AUC. Sample size calculation was performed using the method for two-period designs of Diletti et al. (7) The required sample size was calculated (power of 80%) assuming no difference in the AUC of raltegravir with or without ginkgo biloba and an estimated intrasubject coefficient of variation of the log-transformed AUC values for raltegravir of 20%. The required number of participants was 16. Taking dropouts into account, a total of 18 subjects were included. Geometric mean ratios (GMRs) with 90% confidence intervals (CI) were calculated for AUC0-∞, AUC0-12 h, Cmax, and T1/2 after log transformation of within-subject ratios of raltegravir combined with ginkgo biloba versus raltegravir alone. GMRs with 90% CI falling entirely within the range of 0.80 to 1.25 were considered to indicate no significant interaction. Statistical and descriptive analyses were carried out using SPSS for Windows, version 16.0.1 (SPSS, Chicago, IL), and Microsoft Office Excel 2007.
RESULTS
Baseline characteristics.
A total of 18 subjects (9 male and 9 female, all Caucasian) were enrolled in the study and received treatment. The mean (range) age, body weight, and body mass index were 38 (22 to 55) years, 72 (52 to 93) kg, and 23 (19 to 28) kg/m2, respectively. The subjects were in good general health, according to medical histories, physical examinations, vital signs, and laboratory data. All included subjects completed the trial and were available for statistical evaluation.
Compliance.
The compliance with the ginkgo biloba treatment of all 18 subjects was good, as indicated by their statements about the intake of the drug doses as noted in the booklets, the number of ginkgo biloba tablets counted on each visit day, and the MEMS caps (data not shown). Only two subjects admitted to having missed one ginkgo biloba dose.
Pharmacokinetics.
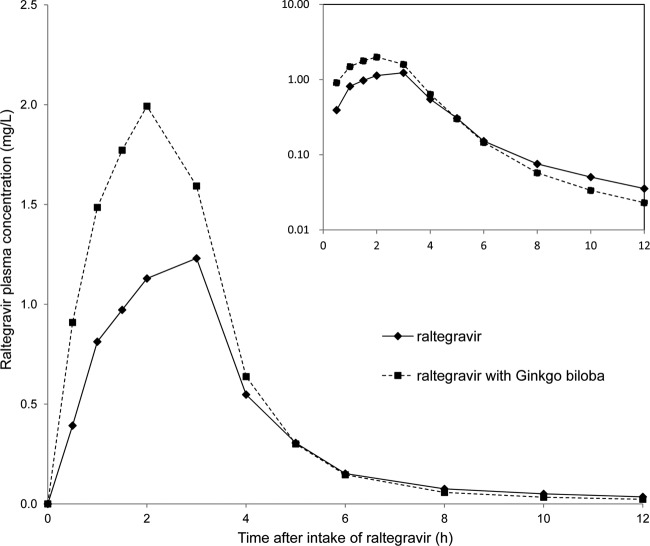
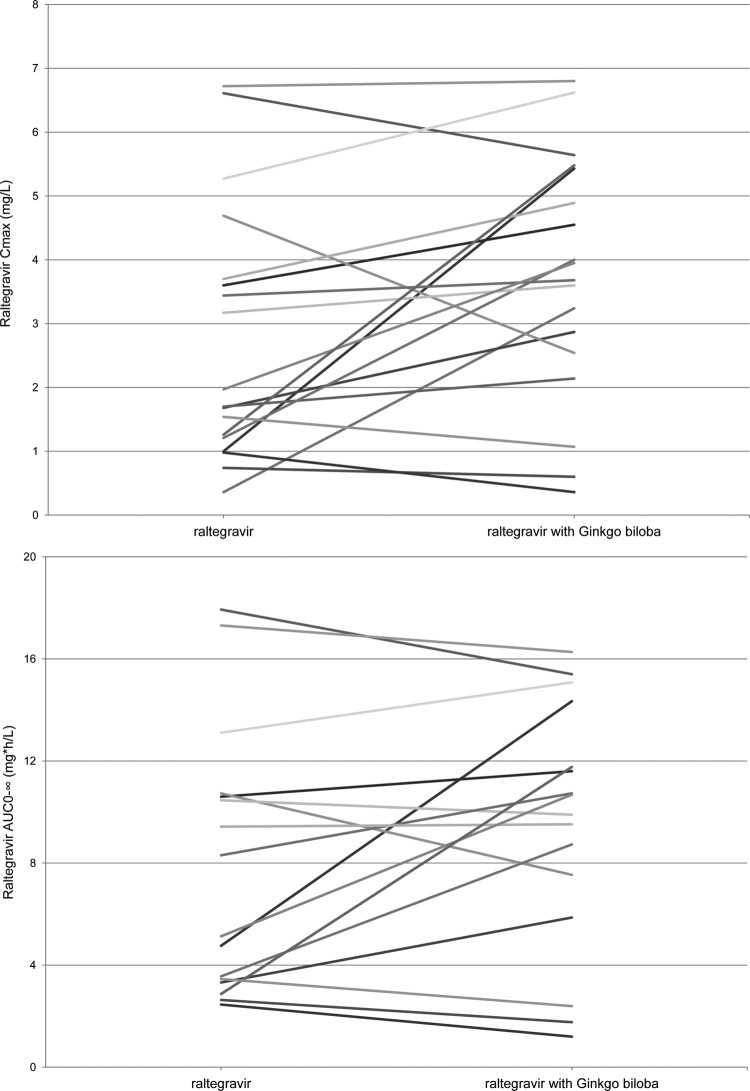
The pharmacokinetic parameters and the plasma concentration versus time curves of raltegravir in the presence and absence of steady-state ginkgo biloba are shown in Table 1 and Fig. 1. Ginkgo biloba increased the maximum plasma concentration (Cmax) and the area under the plasma concentration-time curve from dosing to infinity (AUC0-∞) of raltegravir. The apparent elimination half-life of raltegravir did not appear to be influenced by ginkgo biloba. The median time to reach Cmax of raltegravir was 2.0 h irrespective of ginkgo biloba treatment. For raltegravir coadministered with ginkgo biloba relative to raltegravir alone, the GMRs (90% confidence intervals) were 1.21 (0.93 to 1.58) for AUC0-∞, 1.44 (1.03 to 2.02) for Cmax, and 0.93 (0.73 to 1.17) for T1/2 (Table 1). Two subjects were excluded from the calculation of the GMRs of AUC0-∞ and T1/2 because the T1/2 could not be determined in these subjects. Figure 2 shows the individual-subject changes in the Cmax and AUC0-∞ of raltegravir alone and coadministered with ginkgo biloba. Although a small majority of the subjects showed an increase in the Cmax upon coadministration, considerable variation in the amount and direction of the effect was seen, as well as variation between individuals. This also applies to the observed individual changes in the AUC0-∞. The coefficients of variation of the AUC0-∞ values of raltegravir alone and raltegravir with ginkgo biloba were 66% and 51%, respectively.
Table 1.
Comparison of single-dose pharmacokinetic parameters of raltegravir with or without coadministerion of multiple doses of ginkgo biloba in healthy volunteers
| Pharmacokinetic parameter | Raltegravir alone |
Raltegravir + ginkgo biloba |
Raltegravir + ginkgo biloba/raltegravir alone |
|||
|---|---|---|---|---|---|---|
| Geometric mean | 95% CI | Geometric mean | 95% CI | GMR | 90% CI | |
| AUC0-∞ (mg · h/liter) | 6.35b | 4.39–9.18 | 7.44 | 5.10–10.9 | 1.21b | 0.93–1.58 |
| AUC0-12 (mg · h/liter) | 5.93 | 4.21–8.34 | 7.33 | 5.01–10.7 | 1.24 | 0.97–1.58 |
| Cmax (mg/liter) | 2.08 | 1.39–3.12 | 3.01 | 2.00–4.52 | 1.44 | 1.03–2.02 |
| Tmaxa (h) | 2.00 | 1.13–3.00 | 2.00 | 1.50–2.00 | ||
| CL/F (liters/h) | 63.0b | 43.6–91.1 | 53.8 | 36.8–78.5 | ||
| V/F (liters) | 288b | 193–429 | 220 | 148–325 | ||
| T1/2 (h) | 3.17b | 2.61–3.86 | 2.83 | 2.42–3.32 | 0.93b | 0.73–1.17 |
For Tmax, the median and interquartile range are reported.
Two subjects were excluded because the T1/2 could not be determined.
Fig 1.
Geometric mean plasma concentrations following a single dose of 400 mg of raltegravir in the presence and absence of steady-state ginkgo biloba (semilog scale on the inset).
Fig 2.
Individual changes in the maximum plasma concentration (top) and the area under the concentration-time curve (bottom) of raltegravir alone versus raltegravir coadministered with ginkgo biloba. (Two subjects were excluded because the T1/2 and AUC0-∞ could not be determined.)
Adverse events and safety assessments.
The study medication was generally well tolerated, and no serious adverse events were reported. There were no discontinuations due to adverse events, and all subjects completed the trial. Sixteen subjects reported a total of 32 adverse events. Five (16%) adverse events were considered possibly drug related (all were classified as grade I). Diarrhea was reported by two subjects during ginkgo biloba treatment, and one subject reported a transient headache, which was possibly related to raltegravir and/or ginkgo biloba. One subject developed a grade I triglyceride elevation after administration of raltegravir, which returned to normal within 7 days. One subject had a mild elevated gamma-glutamyltransferase (γ-GT), which remained elevated during the study.
DISCUSSION
Herb-drug interactions are an important consideration in HIV-infected patients because herbs are frequently used, often not reported, and a potential cause of drug failure. A popular herbal product among HIV-infected individuals is ginkgo biloba extract, despite a lack of evidence for effectiveness or safety within this special patient group. Because human and preclinical data were inconclusive with regard to ginkgo biloba's potential to modulate UGT and P-gp activity, a drug interaction study with raltegravir in healthy volunteers was carried out.
Steady-state ginkgo biloba increased the mean exposure to raltegravir (AUC0-∞) by 21% and the Cmax by 44%. However, the large 90% confidence interval for the GMRs partly overlaps the predefined range of 0.80 to 1.25 in the bioequivalence approach and reflects the variability in the individual changes in the AUC0-∞ and Cmax of raltegravir (25). Based on our findings, raltegravir exposure after a single dose was not clinically significantly reduced by concomitant use of ginkgo biloba extract at steady state, making it less likely that ginkgo biloba is a UGT inducer, as suggested by in vitro research (16). The observed mean increase in the Cmax by concomitant use of ginkgo biloba is more likely to be caused by a change in oral bioavailability than by inhibition of the metabolism of raltegravir, because the apparent elimination half-life of raltegravir remained unaffected. The slight increase in the raltegravir AUC0-∞ when combined with ginkgo biloba is largely due to the observed increase in the Cmax (Fig. 1). The difference in the Cmax of raltegravir alone versus raltegravir with ginkgo biloba within subjects could be (partly) due to the normal intrasubject variability in raltegravir pharmacokinetics instead of an effect caused by ginkgo biloba. It is known that raltegravir pharmacokinetics exhibits considerable intra- and intersubject variability (2, 4). A possible explanation for the increase in the Cmax and bioavailability of raltegravir when combined with ginkgo biloba could be the inhibition of P-gp by ginkgo biloba. In vitro characterization of raltegravir transport by drug transporters indicates that raltegravir is a weak P-gp substrate (20). P-gp is an active ATP-dependent efflux pump and is encoded by the ABCB1 gene. Efflux mechanisms, such as P-gp, are responsible for transporting a broad range of compounds out of the intestinal epithelial cells back into the intestinal lumen and play an important role in oral drug absorption. The effect of chronic use of ginkgo biloba extract on the pharmacokinetics of the P-gp substrate talinolol was studied in healthy volunteers. The observed increase in the Cmax by 33% and in the AUC by 21% without any significant alterations in the Tmax and T1/2 of talinolol supports our hypothesis (9, 10). The variation in change of the oral bioavailability of raltegravir and subsequent Cmax values could be a reflection of individual variation in the inhibitory potential of P-gp by ginkgo biloba. The expression and transport activities of P-gp may differ between individuals due to genetic variation in the highly polymorphic ABCB1 gene (11, 15). Therefore, the extent of the inhibition of P-gp may vary accordingly. Although inhibition of P-gp-mediated efflux of raltegravir by ginkgo biloba is an interesting hypothesis, one must be cautious in translating findings obtained from in vitro experiments directly to the clinical setting. Raltegravir transport by P-gp is not yet confirmed in human studies. There are currently no clinical data indicating that the pharmacokinetic profile of raltegravir may be affected by selective P-gp inducers or inhibitors.
It is well known that the pharmacokinetics of raltegravir displays large intersubject variability, which was observed in our study, as well (CV in AUC0-∞, 66%) (2, 4). Contributing factors, in general, are differences in absorption due to food intake or pH effects, genetic polymorphisms associated with altered UGT1A1 activity, and potential drug interactions. In this study, raltegravir was administered in a fasted state to minimize intersubject variability. Nevertheless, differences in gastric pH and therefore absorption of raltegravir probably led to variability in pharmacokinetic parameters between subjects, and maybe within subjects, as well. Individuals with decreased UGT1A1 expression caused by UGT1A1*28 polymorphism (approximately 7 to 19% of the Caucasian population is homozygous for UGT1A1*28) are known to have moderately elevated plasma levels of raltegravir. However, this increase in the plasma level is not considered to be of clinical importance (14, 27). Pharmacogenetic testing was not performed in our study, and UGT1A1*28 polymorphism might have contributed to the intersubject variability, as well. In two subjects, the elimination half-life of raltegravir could not be determined because of secondary peaks in the plasma concentration-time curve. These secondary peaks are frequently observed in pharmacokinetic studies of raltegravir and can be attributed to either delayed absorption or enterohepatic circulation, which is not uncommon for UGT substrates (4, 6). The pharmacokinetic parameters observed in this study were compared with data from Wenning et al., as these healthy subjects (all with the UGT1A1*1/*1 genotype) were exposed to similar study conditions, i.e., a single intake of a 400-mg raltegravir tablet on an empty stomach (27). No major differences between our data and these historical controls could be observed.
The combined use of chronic ginkgo biloba and a single dose of raltegravir was well tolerated. No serious events were reported during the trial. The reported adverse events related to the study medication were mild and transient. In clinical practice, raltegravir is well tolerated, with no dose-related toxicities indentified so far. Given that there have been no acute safety findings associated with peak raltegravir concentrations, the somewhat higher Cmax values for raltegravir when coadministered with ginkgo biloba compared with intake of raltegravir alone are not expected to lead to any meaningful clinically significant safety concerns.
Our study was undertaken in healthy volunteers, limiting our interpretation in HIV-infected individuals with concomitant medication and comorbidity. Because of the inconsistencies and controversies in the literature regarding the exact action of ginkgo biloba extract on metabolizing enzymes or drug transporters and the variation in effects seen in this study, it is not possible to draw any definite conclusions. However, the study does provide data to support the idea that raltegravir can be used safely for the management of HIV infection when taken in combination with ginkgo biloba. No decrease in the AUC0-∞ of raltegravir was observed, and the increases in the maximum plasma concentrations are not considered to be of clinical importance, due to the normal intersubject variability of raltegravir pharmacokinetics and the good safety profile of raltegravir.
ACKNOWLEDGMENTS
This study was supported by the Investigator Initiated Study Program of Merck (37252).
D.B. has received honoraria for serving on advisory boards, speaker's fees, and grants for clinical research from Merck, the manufacturer of raltegravir.
We thank the healthy volunteers for participating in this trial and the study personnel at the Clinical Research Centre Nijmegen for their help in conducting the study.
Footnotes
Published ahead of print 16 July 2012
REFERENCES
- 1. Abad MJ, Bedoya LM, Bermejo P. 2010. An update on drug interactions with the herbal medicine Ginkgo biloba. Curr. Drug Metab. 11:171–181 [DOI] [PubMed] [Google Scholar]
- 2. Arab-Alameddine M, et al. 2012. Population pharmacokinetic analysis and pharmacogenetics of raltegravir in HIV-positive and healthy individuals. Antimicrob. Agents Chemother. 56:2959–2966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Birks J, Grimley EV, Van Dongen M. 2009. Ginkgo biloba for cognitive impairment and dementia. Cochrane Database Syst. Rev. 1:3120. [DOI] [PubMed] [Google Scholar]
- 4. Brainard DM, Wenning LA, Stone JA, Wagner JA, Iwamoto M. 2011. Clinical pharmacology profile of raltegravir, an HIV-1 integrase strand transfer inhibitor. J. Clin. Pharmacol. 51:1376–1402 [DOI] [PubMed] [Google Scholar]
- 5. Burger D, et al. 2011. The International Interlaboratory Quality Control Program for Measurement of Antiretroviral Drugs in Plasma: a global proficiency testing program. Ther. Drug Monit. 33:239–243 [DOI] [PubMed] [Google Scholar]
- 6. Burger DM. 2010. Raltegravir: a review of its pharmacokinetics, pharmacology and clinical studies. Expert Opin. Drug Metab. Toxicol. 6:1151–1160 [DOI] [PubMed] [Google Scholar]
- 7. Diletti E, Hauschke D, Steinijans VW. 1991. Sample size determination for bioequivalence assessment by means of confidence intervals. Int. J. Clin. Pharmacol. Ther. Toxicol. 29:1–8 [PubMed] [Google Scholar]
- 8. European AIDS Clinical Society 2011. European guidelines for treatment of HIV infected adults in Europe, version 6.0, October. European AIDS Clinical Society, Paris, France: http://www.europeanaidsclinicalsociety.org/images/stories/EACS-Pdf/EACSGuidelines-v6.0-English.pdf [Google Scholar]
- 9. Fan L, et al. 2009. Effect of Schisandra chinensis extract and Ginkgo biloba extract on the pharmacokinetics of talinolol in healthy volunteers. Xenobiotica 39:249–254 [DOI] [PubMed] [Google Scholar]
- 10. Fan L, et al. 2009. Effects of Ginkgo biloba extract ingestion on the pharmacokinetics of talinolol in healthy Chinese volunteers. Ann. Pharmacother. 43:944–949 [DOI] [PubMed] [Google Scholar]
- 11. Fellay J, et al. 2002. Response to antiretroviral treatment in HIV-1-infected individuals with allelic variants of the multidrug resistance transporter 1: a pharmacogenetics study. Lancet 359:30–36 [DOI] [PubMed] [Google Scholar]
- 12. Hicks C, Gulick RM. 2009. Raltegravir: the first HIV type 1 integrase inhibitor. Clin. Infect. Dis. 48:931–939 [DOI] [PubMed] [Google Scholar]
- 13. Kassahun K, et al. 2007. Metabolism and disposition in humans of raltegravir (MK-0518), an anti-AIDS drug targeting the HIV-1 integrase enzyme. Drug Metab. Dispos. 35:1657–1663 [DOI] [PubMed] [Google Scholar]
- 14. Kiang TK, Ensom MH, Chang TK. 2005. UDP-glucuronosyltransferases and clinical drug-drug interactions. Pharmacol. Ther. 106:97–132 [DOI] [PubMed] [Google Scholar]
- 15. Leschziner GD, Andrew T, Pirmohamed M, Johnson MR. 2007. ABCB1 genotype and PGP expression, function and therapeutic drug response: a critical review and recommendations for future research. Pharmacogenomics J. 7:154–179 [DOI] [PubMed] [Google Scholar]
- 16. Li L, Stanton JD, Tolson AH, Luo Y, Wang H. 2009. Bioactive terpenoids and flavonoids from Ginkgo biloba extract induce the expression of hepatic drug-metabolizing enzymes through pregnane X receptor, constitutive androstane receptor, and aryl hydrocarbon receptor-mediated pathways. Pharm. Res. 26:872–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Littlewood RA, Vanable PA. 2008. Complementary and alternative medicine use among HIV-positive people: research synthesis and implications for HIV care. AIDS Care 20:1002–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mohamed ME, Frye RF. 2011. Effects of herbal supplements on drug glucuronidation. Review of clinical, animal, and in vitro studies. Planta Med. 77:311–321 [DOI] [PubMed] [Google Scholar]
- 19. Mohamed ME, Frye RF. 2010. Inhibition of intestinal and hepatic glucuronidation of mycophenolic acid by Ginkgo biloba extract and flavonoids. Drug Metab. Dispos. 38:270–275 [DOI] [PubMed] [Google Scholar]
- 20. Moss DM, et al. 2011. Raltegravir is a substrate for SLC22A6: a putative mechanism for the interaction between raltegravir and tenofovir. Antimicrob. Agents Chemother. 55:879–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Panel on Antiretroviral Guidelines for Adults and Adolescents 14 October2011. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services, Washington, DC: http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf [Google Scholar]
- 22. Robertson SM, et al. 2008. Effect of Ginkgo biloba extract on lopinavir, midazolam and fexofenadine pharmacokinetics in healthy subjects. Curr. Med. Res. Opin. 24:591–599 [DOI] [PubMed] [Google Scholar]
- 23. Schouten J, Cinque P, Gisslen M, Reiss P, Portegies P. 2011. HIV-1 infection and cognitive impairment in the cART era: a review. AIDS 25:561–575 [DOI] [PubMed] [Google Scholar]
- 24. Snitz BE, et al. 2009. Ginkgo biloba for preventing cognitive decline in older adults: a randomized trial. JAMA 302:2663–2670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. U.S. Food and Drug Administration 2010. Guidance for industry. Statistical approaches to establishing bioequivalence. http://www.fda.gov/downloads/drugs/guidancecomplianeregulatoryinformation/guidances/ucm070244.pdf U.S. Food and Drug Administration, Washington, DC [Google Scholar]
- 26. van den Bout-van den Beukel CJ, Koopmans PP, van der Ven A, De Smet PA, Burger DM. 2006. Possible drug-metabolism interactions of medicinal herbs with antiretroviral agents. Drug Metab. Rev. 38:477–514 [DOI] [PubMed] [Google Scholar]
- 27. Wenning LA, et al. 2009. Pharmacokinetics of raltegravir in individuals with UGT1A1 polymorphisms. Clin. Pharmacol. Ther. 85:623–627 [DOI] [PubMed] [Google Scholar]
- 28. Wiegman DJ, Brinkman K, Franssen EJ. 2009. Interaction of Ginkgo biloba with efavirenz. AIDS 23:1184–1185 [DOI] [PubMed] [Google Scholar]
- 29. Williams JA, et al. 2002. Differential modulation of UDP-glucuronosyltransferase 1A1 (UGT1A1)-catalyzed estradiol-3-glucuronidation by the addition of UGT1A1 substrates and other compounds to human liver microsomes. Drug Metab. Dispos 30:1266–1273 [DOI] [PubMed] [Google Scholar]
- 30. Yang CY, et al. 2006. Marked decrease of cyclosporin bioavailability caused by coadministration of ginkgo and onion in rats. Food Chem. Toxicol. 44:1572–1578 [DOI] [PubMed] [Google Scholar]
- 31. Zhou S, Lim LY, Chowbay B. 2004. Herbal modulation of P-glycoprotein. Drug Metab. Rev. 36:57–104 [DOI] [PubMed] [Google Scholar]