Abstract
The steady-state concentrations of solithromycin in plasma were compared with concomitant concentrations in epithelial lining fluid (ELF) and alveolar macrophages (AM) obtained from intrapulmonary samples during bronchoscopy and bronchoalveolar lavage (BAL) in 30 healthy adult subjects. Subjects received oral solithromycin at 400 mg once daily for five consecutive days. Bronchoscopy and BAL were carried out once in each subject at either 3, 6, 9, 12, or 24 h after the last administered dose of solithromycin. Drug concentrations in plasma, ELF, and AM were assayed by a high-performance liquid chromatography-tandem mass spectrometry method. Solithromycin was concentrated extensively in ELF (range of mean [± standard deviation] concentrations, 1.02 ± 0.83 to 7.58 ± 6.69 mg/liter) and AM (25.9 ± 20.3 to 101.7 ± 52.6 mg/liter) in comparison with simultaneous plasma concentrations (0.086 ± 0.070 to 0.730 ± 0.692 mg/liter). The values for the area under the concentration-time curve from 0 to 24 h (AUC0–24 values) based on mean and median ELF concentrations were 80.3 and 63.2 mg · h/liter, respectively. The ratio of ELF to plasma concentrations based on the mean and median AUC0–24 values were 10.3 and 10.0, respectively. The AUC0–24 values based on mean and median concentrations in AM were 1,498 and 1,282 mg · h/L, respectively. The ratio of AM to plasma concentrations based on the mean and median AUC0–24 values were 193 and 202, respectively. Once-daily oral dosing of solithromycin at 400 mg produced steady-state concentrations that were significantly (P < 0.05) higher in ELF (2.4 to 28.6 times) and AM (44 to 515 times) than simultaneous plasma concentrations throughout the 24-h period after 5 days of solithromycin administration.
INTRODUCTION
Solithromycin (CEM-101) is a novel fluoroketolide agent that has demonstrated in vitro antimicrobial activity against Gram-positive and Gram-negative respiratory pathogens such as Streptococcus pneumoniae (including macrolide-lincosamide-streptogramin B [MLSB]- and ketolide-resistant subsets), Haemophilus influenzae, and Moraxella catarrhalis (4, 5, 7, 9, 10, 18, 21). Solithromycin is also active against atypical respiratory pathogens such as Mycoplasma pneumoniae, Chlamydophila pneumoniae, and Legionella pneumophila (4, 13, 18, 20). Because of this in vitro spectrum of activity, solithromycin is currently being developed as monotherapy for the treatment of community-acquired bacterial pneumonia (CABP), and a phase 2 clinical trial of solithromycin for this indication has recently been completed (20) (NCT01168713; ClinicalTrials.gov).
Information about anti-infective drug concentrations in the extracellular and intracellular compartments of the lung is essential for development of a drug for lower respiratory tract infections (1, 14). The intrapulmonary concentrations of newer macrolides (e.g., clarithromycin and azithromycin) and ketolides (e.g., telithromycin and cethromycin) have been reported to be consistently higher than those in plasma and are responsible, in part, for the effectiveness of these agents in the treatment of extracellular and intracellular pathogens associated with lower respiratory tract infections (3, 6, 14–16). The goals of this study were to determine and compare the plasma and intrapulmonary concentrations of solithromycin in healthy, nonsmoking, adult subjects after multiple daily doses of 400 mg.
(This work was presented in part at the Interscience Conference on Antimicrobial Agents and Chemotherapy, Boston, MA, September 2010.)
MATERIALS AND METHODS
Study design and subjects.
This was a phase I, multiple-dose, open-label pharmacokinetic study in 30 healthy adults, including male and female subjects. The protocol was approved by the Quorum Review and University of Illinois at Chicago Institutional Review Boards. Healthy subjects between the ages of 18 and 55 years were enrolled into this study after written informed consent was obtained. All subjects were required to have a baseline medical history, physical examination, laboratory evaluation, and electrocardiogram (ECG) within 2 weeks (screening visit) and within 3 days (baseline) prior to solithromycin administration. All subjects had a body mass index of between 18 and 32 kg/m2, inclusive. Female subjects with childbearing potential were required to use one of the protocol-defined acceptable birth control methods before and during solithromycin administration and for 30 days following the last dose of solithromycin. Male subjects were also required to use protocol-defined birth control methods and were not allowed to donate sperm for 90 days after the last dose of solithromycin.
Exclusion criteria included evidence of or a history of clinically significant medical disorders or concurrent infection, any condition possibly affecting oral drug absorption, history of postantibiotic colitis, drug or alcohol abuse within the past 2 years, allergy, hypersensitivity, or serious adverse reactions to any macrolide, azalide, or ketolide antibiotics, and the presence of any clinically significant baseline laboratory abnormalities, a positive urine drug screen, a positive serum pregnancy test, or QTc of greater than 450 ms for males and 470 ms for females. Subjects must not have had a history of tobacco use (defined as smoking or use of snuff chewing tobacco or other nicotine or nicotine-containing products) during the 12 months before screening procedures. Subjects could not have received a prescription drug or herbal supplements within 14 days before the first dose of solithromycin or have received nonprescription medications, vitamins, or dietary supplements within 7 days of the first dose of solithromycin unless prior approval was granted by the investigators and sponsor. Subjects could not have consumed alcohol within 72 h of receiving solithromycin or have a positive alcohol test at the time of prestudy screening or baseline laboratory testing. Grapefruit, grapefruit juices, or juices containing grapefruit could not be consumed within 7 days prior to the first dose of solithromycin. Subjects could not have donated blood or experienced significant blood loss within 56 days of the screening visit, had a hemoglobin level of <12 g/dl, or donated plasma within 7 days of the first dose of solithromycin. Subjects with positive tests for human immunodeficiency virus, hepatitis B virus surface antigen, or anti-hepatitis C virus antibody were excluded. Subjects could not have received an investigational drug or participated in another research study within 30 days of the first dose of solithromycin.
Each subject received 400 mg of oral solithromycin (two 200-mg capsules) administered once daily for a total of 5 days. Each dose was taken with 240 ml of drinking water at a consistent time under fasting conditions and under direct observation at the study site. Blood samples were collected to measure drug concentrations in plasma just before each dose administration on study days 1 to 5 and at 1, 2, 3, 4, 6, 9, 12, and 24 h following the last dose of solithromycin. Each subject had a single standardized bronchoscopy and bronchoalveolar lavage (BAL) in the outpatient facility at either 3, 6, 9, 12, or 24 h following the last dose of solithromycin.
A blood sample to determine plasma urea concentration was obtained just prior to the scheduled bronchoscopy. Aliquots of BAL fluid were obtained to determine urea concentrations in BAL fluid and cell count with differential. Standardized bronchoscopy and BAL procedures and sampling preparation methods for the collection of plasma and intrapulmonary samples have been previously described (6, 15, 16).
Solithromycin concentration determination.
Determination of solithromycin and its metabolites in plasma, BAL fluid supernatant, and cell pellet were assayed using high-performance liquid chromatography-tandem mass spectrometry methods at MicroConstants, Inc. (San Diego, CA) (report numbers MC10B-0025 and MC10B-0026).
A total of 404 plasma samples were assayed between 3 February 2010 and 24 February 2010. All of the samples were analyzed in seven analytical runs. Forty-two samples were reassayed to evaluate the incurred sample reproducibility (ISR) of previously analyzed samples.
Plasma standard curves were linear (r2 = 1.00) from 10.0 to 20,000 ng/ml for solithromycin. The coefficients of variation for quality control (QC) plasma samples at 30, 300, and 16,000 ng/ml of solithromycin were 6.10%, 3.35%, and 4.37%, respectively. The accuracy for plasma QC determinations of solithromycin ranged from 0.667 to 3.13%. The lower limit of detection in plasma was 10 ng/ml for solithromycin. All of the samples selected for reproducibility had a percent difference between the original and ISR values within 20.0% for solithromycin.
A total of 30 BAL fluid samples were assayed for solithromycin. The standard curve for solithromycin in BAL fluid was linear (r2 ≥ 0.999) over the concentration range of 0.100 to 200 ng/ml. The accuracy ranged from −10.6% to 12.0%. The lower limit of detection of solithromycin in epithelial lining fluid (ELF) samples was 0.100 ng/ml.
A total of 30 cell pellet samples were assayed for solithromycin on the same day. The standard curve for solithromycin in cell pellet was linear (r2 ≥ 0.999) over the concentration range of 1.00 to 1,000 ng/ml. The accuracy ranged from −3.5% to 10.0%. The lower limit of detection for solithromycin in cell pellet samples was 1.00 ng/ml.
Urea concentration determination.
Concentrations of urea in plasma and BAL fluid supernatant were determined using the urea assay kit from BioChain (Hayward, CA) at MicroConstants, Inc. (San Diego, CA) (report number MC10B-0026). The assay employed a colorimetric technique where color development proportional to the quantity of bound urea was measured using an absorbance microplate reader monitoring at 520 and 430 nm. All standards, QCs, and samples were analyzed in duplicate.
The assay was linear (r2 ≥ 0.999) for concentrations of urea in plasma and BAL fluid ranging from 2.50 to 50.0 mg/dl and 0.150 to 2.50 mg/dl, respectively. The accuracy ranges for all plasma and ELF urea determinations were −4.40 to 14.0% and −10.0 to 2.00%, respectively. The coefficients of variation for QC samples at 29.3 and 41.2 mg/dl of plasma urea were 3.81% and 2.47%, respectively. For urea in BAL fluid, the coefficients of variation for QC samples at 0.3, 0.75, and 2.00 mg/dl were 1.05%, 1.03%, and 0.724%, respectively. The accuracy ranges for all plasma and BAL fluid determinations of urea were −3.07 to −8.50% and −3.60 to 7.67%, respectively.
Calculation of the volume of ELF and the concentrations of drug.
The apparent volume of ELF (VELF) in BAL fluid was determined with the urea dilution method as previously described by Rennard and colleagues (12). The VELF was determined from the equation VELF = VBAL × ureaBAL/ureaplasma, where VBAL is the volume of aspired BAL fluid, ureaBAL is the concentration of urea in BAL fluid, and ureaplasma is the concentration of urea in plasma.
The concentration of solithromycin in ELF (ABXELF) was determined as ABXELF = ABXBAL × VBAL/VELF, where ABXBAL is the measured concentration of solithromycin in BAL fluid.
The concentration of solithromycin in alveolar macrophages (ABXAM) was determined as ABXAM = ABXpellet/VAC, where ABXpellet and VAC are the measured concentration of drug and volume of alveolar cells in the cell suspension, respectively. A differential cell count of the BAL fluid was carried out, and the percentage of macrophages and monocytes was determined. A mean macrophage cell volume of 2.42 μl/106 cells was used in the calculations for VAC (3).
Pharmacokinetic analysis.
Noncompartmental analyses (WinNonlin, version 5.2; Pharsight Corporation, Cary, NC) were used to generate pharmacokinetic parameters of each subject for solithromycin in plasma. Reported parameters included peak plasma concentration (Cmax), time to Cmax (Tmax), and elimination half-life (t1/2). The area under the plasma concentration-time curve over 24 h (AUC0–24) after the last dose on day 5 was calculated with the linear-log trapezoidal method.
The ratios of ELF and alveolar macrophage (AM) concentrations to the simultaneous plasma concentrations were calculated for each subject and summarized for each group at each sampling time. The AUC0–24 values for plasma, ELF, and AM were calculated with the linear trapezoidal method using the mean and median concentrations of solithromycin at the bronchopulmonary sampling times (e.g., 3, 6, 9, 12, and 24 h). The concentrations at the 24-h sampling time were also used as a time zero value for determining AUC0–24. Solithromycin penetration was estimated from the ratios of the AUC0–24 for ELF or AM to the AUC0–24 in plasma.
Statistical analysis.
All statistical analyses were carried out using PC SAS, version 9.1. (SAS Institute, Inc., Cary, NC). A test for normality was carried out using the Shapiro-Wilks test. If a measurement variable met the normality assumption of an analysis of variance (ANOVA), then the generalized linear model (GLM) was used. Otherwise the Kruskal-Wallis test (nonparametric analogue of an one-way ANOVA) was used. Comparisons of demographic and baseline characteristics between sampling times were carried out using an ANOVA. Comparisons between sampling times were carried out using a contrast statement within PROC GLM. Comparisons between concentrations within each sampling time were carried out using PROC TTEST. A P value of <0.05 was considered statistically significant.
Laboratory and safety assessment.
Safety was monitored by clinical laboratory tests (serum chemistry, hematology, coagulation, and urinalysis), physical examination, standard 12-lead ECGs, vital signs, and monitoring of adverse events. The investigators assessed subjects for the occurrence of adverse events throughout the study. An adverse event was defined as any untoward, undesired, unplanned clinical event in the form of signs, symptoms, disease, or laboratory or physical observations, regardless of causal relationship.
RESULTS
Subjects.
A total of 31 subjects were enrolled in this study. One of the subjects was withdrawn because of a treatment-emergent adverse event (upper respiratory tract infection) unrelated to solithromycin. Since this event occurred prior to bronchoscopy, the subject was replaced, and 30 healthy adult subjects (6 per BAL sampling time) completed the study and were included in the final pharmacokinetic analysis. Table 1 lists the demographic characteristics and the total cell count and percentage of macrophages obtained from BAL fluid for each sampling time. The only notable differences across sampling times for demographic characteristics were gender (12- and 24-h sampling times were 50% male, compared to 100% male at other sampling times) and percentage of macrophages in BAL fluid (the value at the 12-h sampling time was significantly [P < 0.05] lower than those at the 3-h and 6-h sampling times).
Table 1.
Characteristics of study subjects receiving oral solithromycin at 400 mg once daily for 5 daysa
| Sampling time (h)b | Sexc (n) | Age (yr) | Height (cm) | Wt (kg) | Body mass index (kg/m2) | Total cell count in BAL fluid (cells/mm3) | Macrophages (%) |
|---|---|---|---|---|---|---|---|
| 3 | M (6) | 36 ± 10 | 177 ± 7 | 83.7 ± 10.5 | 26.9 ± 3.0 | 182 ± 106 | 85 ± 9 |
| 6 | M (6) | 38 ± 6 | 175 ± 8 | 83.8 ± 7.3 | 27.3 ± 4.1 | 215 ± 76 | 83 ± 3 |
| 9 | M (6) | 32 ± 4 | 178 ± 8 | 87.6 ± 7.2 | 27.4 ± 2.1 | 163 ± 58 | 82 ± 6 |
| 12 | M (3), F (3) | 32 ± 11 | 165 ± 8 | 81.5 ± 15.5 | 26.2 ± 4.6 | 218 ± 276 | 61 ± 22d |
| 24 | M (3), F (3) | 30 ± 11 | 169 ± 10 | 74.4 ± 16.3 | 25.7 ± 3.0 | 332 ± 514 | 74 ± 15 |
Data are expressed as mean ± SD except for sex.
Six subjects per sampling time.
M, males; F, females.
The percentage of macrophages in BAL fluid at the 12-h sampling time was statistically significantly (P < 0.05) lower than those at the 3-h and 6-h sampling times.
Solithromycin was well tolerated, and no serious adverse events were observed during the study. Seventeen of 31 subjects (54.8%) experienced a treatment-emergent adverse event during the study. Fourteen subjects (45.2%) reported one or more probably or possibly drug-related adverse effects. The most frequently reported adverse events (≥10% of subjects) included headache (6 subjects), diarrhea (4 subjects), and nausea (4 subjects). All other adverse events (abdominal pain, dizziness, and fungal infection) occurred in one or two subjects. The severity of all adverse events was considered to be mild in intensity except for one subject who had moderate chest pain related to the bronchoscopy procedure. Mild, transient, and reversible elevations of alanine aminotransferase (all ≤1.5 times the upper limit of normal) were observed in three subjects on day 6. None of these elevations was considered clinically significant.
Pharmacokinetics.
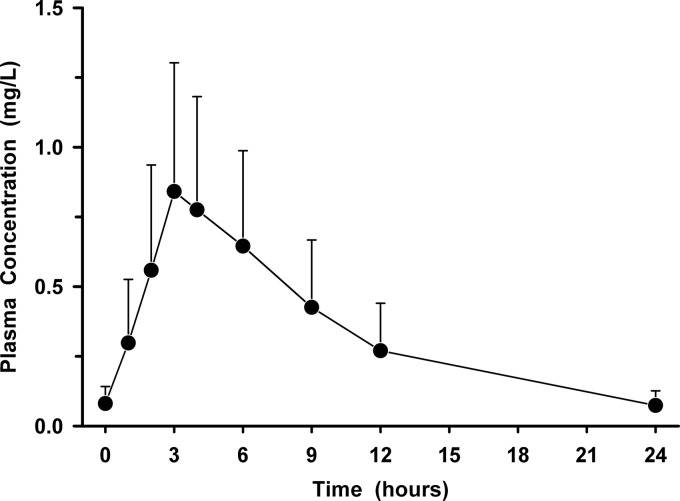
The mean (± standard deviation [SD]) plasma concentration-versus-time profile for solithromycin is presented in Fig. 1. The mean (± SD) pharmacokinetic parameters of solithromycin in plasma included a Cmax of 0.899 ± 0.459 mg/liter, Tmax of 3.57 ± 1.10 h, AUC0–24 of 7.92 ± 4.39 mg · h/liter, and t1/2 of 5.60 ± 1.20 h. The mean (± SD) plasma solithromycin concentrations before the administration of the second, third, fourth, and fifth doses were 0.029 ± 0.017 mg/liter, 0.47 ± 0.036 mg/liter, 0.078 ± 0.054 mg/liter, and 0.081 ± 0.062 mg/liter, respectively.
Fig 1.
Mean (± SD) plasma concentration-versus-time profile of solithromycin before and after the fifth oral dose of 400 mg once daily.
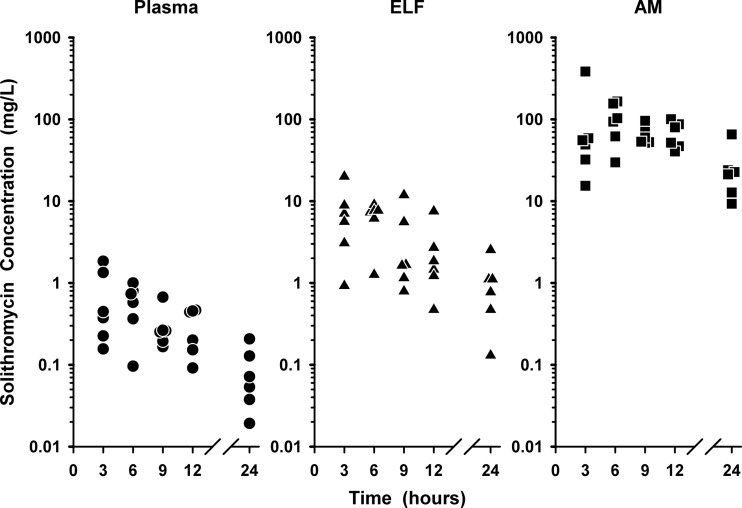
The individual plasma, ELF, and AM concentrations of solithromycin during the 24-h interval following the last dose are shown in Fig. 2. Once-daily oral dosing of solithromycin at 400 mg produced steady-state concentrations that were higher in ELF (2.4 to 28.6 times) and AM (44 to 515 times) than simultaneous plasma concentrations throughout the 24-h period after the last dose of solithromycin on the fifth day. The concentrations of solithromycin in AM were statistically (P < 0.05) higher than the concentrations in plasma and ELF at all sampling times.
Fig 2.
Individual concentrations of solithromycin in plasma, epithelial lining fluid (ELF), and alveolar macrophages (AM) at 3, 6, 9, 12, and 24 h after the fifth oral dose of 400 mg once daily. The y axis is in the log scale.
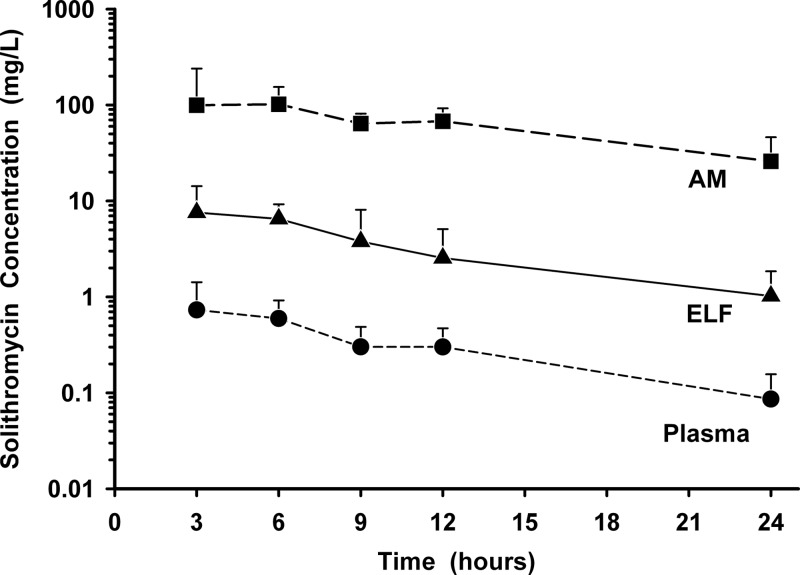
The mean (± SD) concentrations of solithromycin in plasma, ELF, and AM after 5 days of oral dosing are reported in Table 2 and displayed in Fig. 3. The highest mean concentrations for ELF occurred at the 3-h sampling time, compared to 6 h for AM concentrations. The mean ratios of ELF and AM concentrations to simultaneous plasma concentrations for solithromycin during the 24-hour period after drug administration ranged from 8.8 to 14.0 and 132 to 345, respectively.
Table 2.
Solithromycin concentrations in plasma, ELF, and AM at time of bronchoscopy and BALa
| Sampling time (h) | Solithromycin concn (mg/liter) in: |
||
|---|---|---|---|
| Plasma | ELF | AM | |
| 3 | 0.730 ± 0.692b | 7.58 ± 6.69b | 99.4 ± 140.8d,e |
| 6 | 0.595 ± 0.325c | 6.50 ± 2.73c | 101.7 ± 52.6d,e |
| 9 | 0.301 ± 0.185 | 3.78 ± 4.32 | 64.1 ± 17.2d,e |
| 12 | 0.300 ± 0.171b | 2.54 ± 2.55b | 67.8 ± 24.6d,e |
| 24 | 0.086 ± 0.070c | 1.02 ± 0.83c | 25.9 ± 20.3d,e |
Data are expressed as mean ± SD for 6 subjects at each sampling time.
Differences between 3 h and 12 h were significant (P < 0.05) for the same body fluid.
Differences between 6 h and 24 h were significant (P < 0.05) for the same body fluid.
Differences between AM and plasma were significant (P < 0.05) during this sampling time.
Differences between AM and ELF were significant (P < 0.05) during this sampling time.
Fig 3.
Mean (± SD) concentration-versus-time profiles of solithromycin in plasma, epithelial lining fluid (ELF), and alveolar macrophages (AM) at 3, 6, 9, 12, and 24 h after the fifth oral dose of 400 mg once daily. The y axis is in the log scale.
The AUC0–24 values based on mean and median ELF concentrations were 80.3 and 63.2 mg · h/liter, respectively. The ratios of ELF to plasma concentrations based on the mean and median AUC0–24 values were 10.3 and 10.0, respectively. The AUC0–24 values based on mean and median AM concentrations were 1,498 and 1,282 mg · h/liter, respectively. The ratios of AM to plasma concentrations based on the mean and median AUC0–24 values were 193 and 202, respectively.
DISCUSSION
The pharmacokinetics of orally administered solithromycin in healthy subjects have recently been reported (17). Following escalating single doses (50 to 1,600 mg), Cmax and measurements of AUC increased in a greater-than-dose-proportional manner up to 1,200 mg. The mean elimination half-life also increased from 3.16 to 7.42 h over the dosing range evaluated. Administration of a high-fat meal was shown to not affect the oral bioavailability of solithromycin. Moderate amounts of accumulation (1.7 to 2.2 times greater on day 7 than on day 1) were observed after multiple daily doses (200, 400, and 600 mg).
The observed plasma concentrations in our study are similar to those obtained with 400 mg in the multiple-oral-dose pharmacokinetic study of solithromycin (17). The mean (± SD) plasma Cmax of solithromycin in that study was 1.09 ± 0.52 mg/liter (at a mean Tmax of 4.00 h), whereas our observed Cmax was 0.90 ± 0.46 mg/liter (at a mean Tmax of 3.57 h). Similarly, the reported trough plasma concentration (Cmin) at day 5 was 0.118 ± 0.099 mg/liter, versus our observed value of 0.085 ± 0.065 mg/liter. The observed AUC0–24 values in both studies were similar (7.47 ± 1.60 mg · h/liter versus 7.92 ± 4.39 mg · h/liter), with higher variability observed in our study. These differences are small and most likely can be explained by differences in the number of blood sample collection times (extensive [n = 17] versus limited [n = 9]) and the duration of dosing (7 versus 5 days).
This study is the first report of the intrapulmonary disposition of solithromycin following multiple oral doses of 400 mg once daily. The ELF concentrations of solithromycin were significantly higher than concurrent plasma concentrations during the 24 h after administration of the last oral dose (Fig. 3). The mean values of solithromycin in ELF ranged from 1.02 to 7.58 mg/liter, with individual ELF concentrations greater than 0.5 mg/liter and 1.0 mg/liter in 90% and 80% of subjects, respectively. The mean ratio of ELF to plasma concentrations of solithromycin from individual subjects was 11.9 (range, 2.4 to 24.5). This average compares very favorably to the AUC0–24 ratios of ELF to plasma concentrations based on mean and median concentration values (10.3 and 10.0, respectively).
The ratio of AUC0–24 to MIC (AUC0–24/MIC) has been suggested as the pharmacokinetic-pharmacodynamic parameter that best correlates with the efficacy of macrolides, azalides, and ketolides (6, 14–16). Preliminary results from Andes et al. suggested that ELF and unbound plasma AUC0–24/MIC ratios were the most predictive parameters of efficacy (r2 = 0.85) in a neutropenic mouse lung infection model for Streptococcus pneumoniae (2). The ELF AUC0–24/MIC ratios associated with a net bacterial stasis and a 1-log and 2-log CFU reduction from baseline were 1.26, 15.1, and 59.8, respectively. Farrell et al. have reported in vitro activity of solithromycin against Streptococcus pneumoniae (MIC90 = 0.25 mg/liter; MIC range, ≤0.008 to 0.5 mg/liter), including 18 strains of serogroup 19A (MIC90 = 0.5 mg/liter; MIC range, 0.06 to 0.5 mg/liter) (4). Combining these pharmacodynamic concepts and MIC90 values with our observed mean ELF AUC0–24 value (80.3 mg · h/liter), the estimated AUC0–24/MIC90 ratios in ELF would be 320 and 160 for Streptococcus pneumoniae. Such high intrapulmonary AUC0–24/MIC ratios in ELF of healthy adult subjects provide support for the once-daily dosing regimen of oral solithromycin at 400 mg for the treatment of susceptible extracellular pathogens associated with lower respiratory tract infections.
The mean values of solithromycin in AM ranged from 25.9 to 101.7 mg/liter, with 90% of individual AM concentrations being greater than 20 mg/liter (Fig. 2). The observed concentrations in AM were significantly higher than concurrent concentrations in plasma and ELF at all sampling times (Fig. 3). The ratio of AM to plasma concentrations in individual subjects ranged from 44 to 515 (mean, 245). The AM concentrations are comparable to or higher than those reported for other macrolides, azalides, and ketolides (6, 14–16). In addition, solithromycin has potent in vitro activity against intracellular respiratory pathogens, including Legionella pneumophila (MIC90, ≤0.015 mg/liter), Chlamydophila pneumoniae (MIC90, 0.25 mg/liter), and Mycoplasma pneumoniae (MIC90, 0.000125 mg/liter) (4, 12, 17, 18). In macrophages, solithromycin is approximately 100-fold more potent than azithromycin against phagocytized Legionella pneumophila, a lysosomal compartment intracellular pathogen (8). While no pharmacokinetic-pharmacodynamic predictive relationship has been established for the efficacy of these agents against intracellular pathogens, the extremely high concentrations in AM and established intracellular pharmacological properties (8) may be predictive of the potency and efficacy against intracellular pathogens.
The ELF and AM penetration ratios of solithromycin reported throughout this paper were based on total (bound plus unbound) plasma drug concentrations. Protein binding of solithromycin has been assessed by equilibrium dialysis using spiked solithromycin concentrations of 0.075, 0.75, and 7.5 mg/liter (data on file at Cempra Pharmaceuticals, Inc., correspondence from 11 June 2012). The extent of protein binding in human plasma ranged from 78% to 84% and does not appear to be concentration dependent. Obviously, our penetration ratios for ELF and AM would be even greater if unbound plasma concentrations were used.
The mechanisms for how macrolide, azalide, or ketolide agents achieve higher concentrations in ELF and AM than in plasma have not been well described. It has been suggested that methodological issues such as cell lysis, urea dilution, and/or contamination from blood or cells may be responsible for increased concentrations of these anti-infective agents in ELF (14). In addition, uptake by phagocytes such as human polymorphonuclear neutrophils and trapping of these basic compounds in the acidic compartments (i.e., lysosomes) have provided a reasonable rationale for higher intracellular concentrations and accumulation of these agents in AM. Several recent reports have explored whether drug transporters influence the uptake of macrolides in ELF and AM (11, 19). Based on in vitro transport experiments with Calu-3 cell monolayers and cultured AM (NR8383 cells), Togami et al. suggested that MDR1 transporters on alveolar epithelial cells were responsible for an increased penetration of clarithromycin and azithromycin from blood to ELF and that ATP-dependent, active-transport systems increased the uptake of these agents by AM (19). Peters et al. speculated that rifampin influenced the expression of nuclear pregnane X receptor (PXR) and the activity of drug transporters (i.e., ATP-binding cassette [ABC] family and organic anion-transporting polypeptides [OATPs]) in alveolar epithelial and bronchoalveolar lavage fluid cells, resulting in an increased ELF distribution of clarithromycin in foals (11). It is likely that a complex interplay between uptake carriers and efflux transporters influences the intrapulmonary penetration of agents such as macrolides and solithromycin. Further studies are needed to determine the mechanisms, localization, and regulatory elements of pulmonary penetration of these agents.
In summary, the results of this study provide important information on the time course and magnitude of extracellular and intracellular concentrations of solithromycin in the lung. Oral administration of solithromycin at 400 mg produced concentrations that were higher in ELF (2.4 to 28.6 times) and AM (44 to 515 times) than simultaneous plasma concentrations throughout the 24-h period after 5 days of once-daily dosing. The ratios of ELF to plasma concentrations and of AM to plasma concentrations based on the mean AUC0–24 values were 10.3 and 193, respectively. The in vitro activity against common typical and atypical pathogens and the sustained concentrations in ELF and AM suggest that solithromycin has the potential to be a useful antibacterial agent for the treatment of community-acquired bacterial pneumonia. Further studies are needed to determine the clinical significance of high intrapulmonary concentrations and clinical outcomes of solithromycin in the treatment of lower respiratory tract infections.
ACKNOWLEDGMENTS
We greatly appreciate the statistical assistance of Kaisheng Fan and Pharm-Olam International.
This study was supported by Cempra Pharmaceuticals.
Regarding conflicts of interest, K.A.R. and M.H.G. have been consultants to Cempra Pharmaceuticals. J.G.S. is a former employee of Cempra Pharmaceuticals. K.C. and P.F. are current employees of Cempra Pharmaceuticals.
Footnotes
Published ahead of print 16 July 2012
REFERENCES
- 1. Ambrose PG, Bhavnani SM, Ellis-Grosse EJ, Drusano GL. 2010. Pharmacokinetic-pharmacodynamic considerations in the design of hospital-acquired or ventilator-acquired bacterial pneumonia studies: look before you leap! Clin. Infect. Dis. 51(Suppl. 1):S103–S110 [DOI] [PubMed] [Google Scholar]
- 2. Andes DR, et al. 2010. Pharmacokinetic-pharmacodynamic analysis of CEM-101 against Streptococcus pneumoniae using data from a murine-lung infection model, abstr. A1-688. Abstr. 50th Intersci. Conf. Antimicrob. Agents Chemother American Society for Microbiology, Washington, DC [Google Scholar]
- 3. Baldwin DR, Wise R, Andrews JM, Ashby JP, Honeybourne D. 1990. Azithromycin concentrations at the sites of pulmonary infection. Eur. Respir. J. 3:886–890 [PubMed] [Google Scholar]
- 4. Farrell DJ, et al. 2010. Antimicrobial chacterisation of CEM-101 activity against respiratory tract pathogens, including multidrug-resistant pneumococcal serogroup 19A isolates. Int. J. Antimicrob. Agents 35:537–543 [DOI] [PubMed] [Google Scholar]
- 5. Farrell DJ, Castanheira M, Sader HS, Jones RN. 2010. The in vitro evaluation of solithromycin (CEM-101) against pathogens isolated in the United States and Europe (2009). J. Infect. 61:476–483 [DOI] [PubMed] [Google Scholar]
- 6. Gotfried MH, Danziger LH, Rodvold KA. 2003. Steady-state plasma and bronchopulmonary characteristics of clarithromycin extended-release tablets in normal healthy adults. J. Antimicrob. Chemother. 52:450–456 [DOI] [PubMed] [Google Scholar]
- 7. Jones RN, Ross JE, Rhomberg PR, Quality Control Working Group 2010. MIC quality control guidelines and disk diffusion test optimization for CEM-101, a novel fluoroketolide. J. Clin. Microbiol. 48:1470–1473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lemaire S, Van Bambeke F, Tulkens PM. 2009. Cellular accumulation and pharmacodynamic evaluation of the intracellular activity of CEM-101, a novel fluoroketolide, against Staphylococcus aureus, Listeria monocytogenes, and Legionella pneumophila in human THP-1 macrophages. Antimicrob. Agents Chemother. 53:3734–3743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McGhee P, et al. 2010. In vitro activity of CEM-101 against Streptococcus pneumoniae and Streptococcus pyogenes with defined macrolide resistance mechanisms. Antimicrob. Agents Chemother. 54:230–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pereira D, Fernandes P. 2011. Synthesis and antibacterial activity of novel 4-aryl-[1,2,3]-triazole containing macrolides. Bioorg. Med. Chem. Lett. 21:510–513 [DOI] [PubMed] [Google Scholar]
- 11. Peters J, et al. 2011. Oral absorption of clarithromycin is nearly abolished by chronic comedication of rifampicin in foals. Drug Metabol. Disp. 39:1643–1649 [DOI] [PubMed] [Google Scholar]
- 12. Rennard SI, et al. 1986. Estimation of volume of epithelial lining fluid recovered by lavage using urea as marker of dilution. J. Appl. Physiol. 60:532–538 [DOI] [PubMed] [Google Scholar]
- 13. Roblin PM, Kohlhoff SA, Parker C, Hammerschlag MR. 2010. In vitro activity of CEM-101, a new fluoroketolide antibiotic, against Chlamydia trachomatis and Chlamydia (Chlamydophila) pneumoniae. Antimicrob. Agents Chemother. 54:1358–1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rodvold KA, George JM, Yoo L. 2011. Penetration of anti-infective agents into pulmonary epithelial lining fluid: focus on antibacterial agents. Clin. Pharmacokinet. 50:637–664 [DOI] [PubMed] [Google Scholar]
- 15. Rodvold KA, Danziger LH, Gotfried MH. 2003. Steady-state plasma and bronchopulmonary concentrations of intravenous levofloxacin and azithromycin in healthy adults. Antimicrob. Agents Chemother. 47:2450–2457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rodvold KA, Gotfried MH, Danziger LH, Servi RJ. 1997. Intrapulmonary steady-state concentrations of clarithromycin and azithromycin in healthy adult volunteers. Antimicrob. Agents Chemother. 41:1399–1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Still JG, et al. 2011. Pharmacokinetics of solithromycin (CEM-101) after single or multiple oral doses and effects of food on single-dose bioavailability in healthy adult subjects. Antimicrob. Agents Chemother. 55:1997–2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sutcliffe JA. 2011. Antibiotics in development targeting protein synthesis. Ann. N. Y. Acad. Sci. 1241:122–152 [DOI] [PubMed] [Google Scholar]
- 19. Togami K, Chono S, Morimoto K. 2011. Distribution characteristics of clarithromycin and azithromycin, macrolide antimicrobial agents used for treatment of respiratory infections, in lung epithelial lung fluid and alveolar macrophages. Biopharm. Drug Metabol. 32:389–397 [DOI] [PubMed] [Google Scholar]
- 20. Waites KB, Crabb DM, Duffy LB. 2009. Comparative in vitro susceptibilities of human mycoplasmas and ureaplasmas to a new investigational ketolide, CEM-101. Antimicrob. Agents Chemother. 53:2139–2141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Woosley LN, Castanheira M, Jones RN. 2010. CEM-101 activity against Gram-positive organisms. Antimicrob. Agents Chemother. 54:2182–2187 [DOI] [PMC free article] [PubMed] [Google Scholar]