LETTER
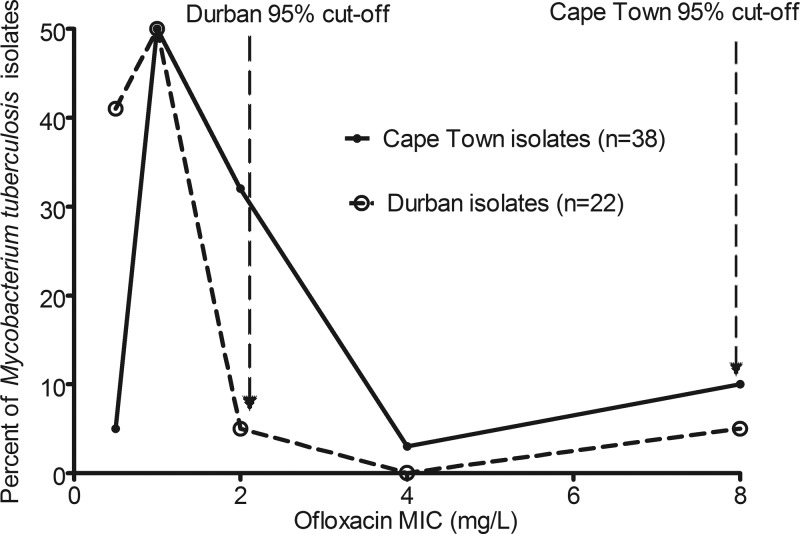
We read with interest the recent study by Chigutsa et al., especially their data on MIC distributions (2). The pivotal study by Drusano et al. (3) a decade ago in this journal set the stage for a mathematically rational way to determine susceptibility breakpoints by taking into account pharmacokinetic variability and antimicrobial pharmacokinetics and pharmacodynamics. We recently applied this to first-line antituberculosis drugs and redefined susceptibility breakpoints for rifampin, isoniazid, and pyrazinamide substantially downwards (4). Chigutsa et al. correctly applied this method to ofloxacin and revised the breakpoint from 2.0 mg/liter to 0.5 mg/liter. This is crucial to redefining extremely drug-resistant tuberculosis. We have argued that the standard approach of epidemiologic cutoff values, originating in the 1960s, has several logical flaws, one of which is that the definition of critical concentrations as inhibiting “95% of wild-type isolates” suffers from a simple biological drawback: evolution. The point of evolution is variability. Pharmacokinetic variability is but one part of this process, which brings to naught the “average” drug concentration approach implicit in epidemiologic cutoff methods. Second, one would expect, a priori, that evolution has led to a wide distribution of MICs which differ from place to place, so that 95% cutoff points in Mycobacterium tuberculosis isolates in different locales will be different. The latter view was recently panned in a letter in this journal: “wild-type isolates have similar MIC distributions regardless of geographical origin or source (human, animal, or environmental), and there is no obvious reason why M. tuberculosis should be different” (1). The ofloxacin paper (2), in our opinion, provides a dramatic rebuttal. The authors examined the pharmacokinetics of ofloxacin in two populations in South Africa: one in Durban and the other a 1.5-h flight away in Cape Town. Differences in pharmacokinetic parameters between the two groups of patients were noted, so that even between two nearby populations, pharmacokinetic variability was still encountered. Uniquely, they examined ofloxacin MICs from the patient isolates in the two places. The MIC determinations were performed in the same laboratory, using the same methodology. We used their Table 2 results to show in Fig. 1 that depending on whether you are in Durban or Cape Town, a 1.5-h flight away, the epidemiologic cutoff point leads to the absurdity of two different breakpoints. Clearly, even within the same country, MIC variability exists, as does pharmacokinetic variability, and this variability is expected to continue between different places. Then, we ask, whose collection of “wild-type” isolates and whose “average” drug concentrations should be used to calculate the correct cutoff point? The data from South Africa, together with recent clinical evidence that the new breakpoints we proposed are actually supported by clinical findings in the case of rifampin (5), despite the inconvenience that the “suggested susceptibility breakpoints split the wild-type MIC distribution” (1), mean that there no longer can be any excuses to keep the standard M. tuberculosis susceptibility breakpoints. Epidemiologic cutoff methods have served us well in the last half-century; but we should now allow them to gracefully retire.
Fig 1.
MIC distribution in two sets of Mycobacterium tuberculosis isolates from South Africa.
Footnotes
Ed. Note: The authors of the published article did not feel a reply was necessary.
REFERENCES
- 1. Angeby K, et al. 2011. Wild-type MIC distributions must be considered to set clinically meaningful susceptibility testing breakpoints for all bacterial pathogens, including Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 55:4492–4493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chigutsa E, et al. 2012. Population pharmacokinetics and pharmacodynamics of ofloxacin in South African patients with multidrug-resistant tuberculosis. Antimicrob. Agents Chemother. 56:3857–3863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Drusano GL, et al. 2001. Use of preclinical data for selection of a phase II/III dose for evernimicin and identification of a preclinical MIC breakpoint. Antimicrob. Agents Chemother. 45:13–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gumbo T. 2010. New susceptibility breakpoints for first-line antituberculosis drugs based on antimicrobial pharmacokinetic/pharmacodynamic science and population pharmacokinetic variability. Antimicrob. Agents Chemother. 54:1484–1491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Williamson DA, et al. 2012. Clinical failures associated with rpoB mutations in phenotypically occult multidrug-resistant Mycobacterium tuberculosis. Int. J. Tuberc. Lung Dis. 16:216–220 [DOI] [PubMed] [Google Scholar]