Abstract
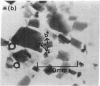
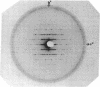
Preliminary x-ray diffraction data from single crystals of the MoFe proteins of nitrogenase from Clostridium pasteurianum and Azotobacter vinelandii have been obtained. Both protein crystals belong to the P2(1) space group. The MoFe protein crystals from C. pasteurianum and A. vinelandii diffract to angles corresponding to resolutions as great as 2.4 A and 3.0 A, respectively. The cell dimensions of the MoFe protein crystals from the two species have been determined from precession photographs.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dalton H., Mortenson L. E. Dinitrogen (N 2 ) fixation (with a biochemical emphasis). Bacteriol Rev. 1972 Jun;36(2):231–260. doi: 10.1128/br.36.2.231-260.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis L. C., Henzl M. T., Burris R. H., Orme-Johnson W. H. Iron-sulfur clusters in the molybdenum-iron protein component of nitrogenase. Electron paramagnetic resonance of the carbon monoxide inhibited state. Biochemistry. 1979 Oct 30;18(22):4860–4869. doi: 10.1021/bi00589a014. [DOI] [PubMed] [Google Scholar]
- Kurtz D. M., Jr, McMillan R. S., Burgess B. K., Mortenson L. E., Holm R. H. Identification of iron-sulfur centers in the iron-molybdenum proteins of nitrogenase. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4986–4989. doi: 10.1073/pnas.76.10.4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews B. W. Solvent content of protein crystals. J Mol Biol. 1968 Apr 28;33(2):491–497. doi: 10.1016/0022-2836(68)90205-2. [DOI] [PubMed] [Google Scholar]
- Mortenson L. E. Purification of nitrogenase from Clostridium pasteurianum. Methods Enzymol. 1972;24:446–456. doi: 10.1016/0076-6879(72)24090-3. [DOI] [PubMed] [Google Scholar]
- Mortenson L. E., Thorneley R. N. Structure and function of nitrogenase. Annu Rev Biochem. 1979;48:387–418. doi: 10.1146/annurev.bi.48.070179.002131. [DOI] [PubMed] [Google Scholar]
- Mortenson L. E., Zumpft W. G., Palmer G. Electron paramagnetic resonance studies on nitrogenase. 3. Function of magnesium adenosine 5'-triphosphate and adenosine 5'-diphosphate in catalysis by nitrogenase. Biochim Biophys Acta. 1973 Feb 22;292(2):422–435. doi: 10.1016/0005-2728(73)90048-0. [DOI] [PubMed] [Google Scholar]
- Münck E., Rhodes H., Orme-Johnson W. H., Davis L. C., Brill W. J., Shah V. K. Nitrogenase. VIII. Mössbauer and EPR spectroscopy. The MoFe protein component from Azotobacter vinelandii OP. Biochim Biophys Acta. 1975 Jul 21;400(1):32–53. doi: 10.1016/0005-2795(75)90124-5. [DOI] [PubMed] [Google Scholar]
- Shah V. K., Brill W. J. Nitrogenase. IV. Simple method of purification to homogeneity of nitrogenase components from Azotobacter vinelandii. Biochim Biophys Acta. 1973 May 30;305(2):445–454. doi: 10.1016/0005-2728(73)90190-4. [DOI] [PubMed] [Google Scholar]
- Smith B. E., Lowe D. J., Bray R. C. Studies by electron paramagnetic resonance on the catalytic mechanism of nitrogenase of Klebsiella pneumoniae. Biochem J. 1973 Oct;135(2):331–341. doi: 10.1042/bj1350331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann R., Münck E., Brill W. J., Shah V. K., Henzl M. T., Rawlings J., Orme-Johnson W. H. Nitrogenase X: Mössbauer and EPR studies on reversibly oxidized MoFe protein from Azotobacter vinelandii OP. Nature of the iron centers. Biochim Biophys Acta. 1978 Dec 20;537(2):185–207. doi: 10.1016/0005-2795(78)90504-4. [DOI] [PubMed] [Google Scholar]
- Zumft W. G., Palmer G., Mortenson L. E. Electron paramagnetic resonance studies on nitrogenase. II. Interaction of adenosine 5'-triphosphate with azoferredoxin. Biochim Biophys Acta. 1973 Feb 22;292(2):413–421. doi: 10.1016/0005-2728(73)90047-9. [DOI] [PubMed] [Google Scholar]