Abstract
In view of reports of colistin-induced neurotoxicity in infected patients, the aim of this study was to assess whether the integrity of the blood-brain barrier (BBB) and the brain uptake of colistin are altered in the presence of systemic Pseudomonas aeruginosa infection. Bacteremia was confirmed 8 h after intramuscular administration of P. aeruginosa ATCC 27853 to Swiss Outbred mice, at which time a single subcutaneous dose of colistin sulfate (40 mg/kg of body weight) or an intravenous dose of [14C]sucrose (2 μCi) was administered. Despite a substantial elevation in plasma levels of the proinflammatory cytokines tumor necrosis factor alpha, interleukin-1β, and interleukin-6 during bacterial infection, the brain uptake of colistin was similar between infected and noninfected mice with AUCbrain/AUCplasma (where AUCbrain is the area under the brain concentration-time curve and AUCplasma is the area under the plasma concentration-time curve) ratios of 0.023 and 0.024, respectively. Similarly, the brain-to-plasma ratios of [14C]sucrose were no different between infected and noninfected mice, consistent with a lack of effect of bacteremia on BBB integrity. To further correlate any relationship between BBB disruption and plasma levels of proinflammatory cytokines, BBB integrity, colistin brain uptake, and plasma proinflammatory cytokines were measured following the administration of Salmonella enterica lipopolysaccharide (LPS), an agent known to induce BBB disruption. Despite LPS inducing a 4-fold increase in colistin brain uptake and a significant (P < 0.05) 1.2-fold increase in [14C]sucrose BBB penetration, plasma cytokine levels were lower with LPS treatment relative to those obtained with bacterial infection with P. aeruginosa. This study demonstrates that the brain uptake of colistin is not increased in mice during P. aeruginosa-induced systemic bacteremia despite a significant increase in plasma levels of three proinflammatory cytokines.
INTRODUCTION
The spread of multidrug resistance in Gram-negative bacteria presents a critical problem to physicians attempting to treat systemic infections, in particular, those caused by Pseudomonas aeruginosa, Acinetobacter baumannii, and Klebsiella pneumoniae (28). A decreased drive for the discovery of novel antibiotics has dramatically narrowed the available therapeutic options for such infections and has led to the reappraisal of colistin (polymyxin E). However, the use of colistin waned during the 1970s due to concerns related to its adverse effects (37), including neurotoxicity, manifesting as dizziness, numbness, vertigo, and lower limb weakness (28, 34). It is still unclear whether these side effects are centrally or peripherally mediated (6); however, if the neurotoxicity induced by colistin is indeed centrally mediated, colistin or its inactive prodrug colistin methanesulfonate (CMS) would be required to cross the blood-brain barrier (BBB) following systemic administration.
The BBB, formed by the endothelial cells lining the cerebral microvessels, is the interface between the blood and the cerebral tissue and acts as a major hindrance to the movement of molecules from the bloodstream into the central nervous system (CNS) (1). The endothelial cells of these cerebral microvessels have minimal pinocytotic activity and a lack of membrane fenestrations (8). Under normal conditions, the restrictive nature of the BBB is mediated by intercellular tight junctions preventing paracellular diffusion and by various efflux transport systems limiting transcellular movement (20). In an attempt to understand the potential for colistin to traverse the BBB following systemic administration, we have previously assessed the brain uptake of this antibiotic following single and multiple injections to healthy mice, and these studies demonstrated minimal CNS penetration of colistin (18, 19). This is not surprising given that, in addition to its relatively large molecular weight (average, 1,163), the free γ-amino groups of the five α,γ-diaminobutyric acid residues in the structure give colistin multiple positive charges at physiological pH, rendering it quite hydrophilic. These physicochemical properties would therefore limit the ability of colistin to traverse the BBB via the transcellular or paracellular routes (33). However, the integrity of the BBB paracellular route is known to be perturbed in a variety of diseases, including acute bacterial infection (39), which may be a result of elevated plasma concentrations of proinflammatory cytokines (7). Indeed, previous studies have demonstrated that cytokines such as tumor necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), and interleukin-6 (IL-6) can lead to decreased expression and reorganization of tight junction proteins, resulting in BBB disruption (2, 12).
Therefore, in the presence of a bacterial infection, it is likely that colistin may penetrate the BBB due to perturbation of the paracellular route. Indeed, colistin has been reported to penetrate the blood-cerebrospinal fluid barrier in infected patients (3, 17, 25); however, whether enhanced BBB penetration of colistin would occur during a bacterial infection has not been demonstrated. As a surrogate model of bacterial infection, we have shown that systemic administration of Salmonella enterica lipopolysaccharide (LPS) to mice leads to a significant enhancement in colistin BBB transport, and this is associated with increased brain uptake of the normally impenetrable [14C]sucrose, a finding consistent with perturbation of the BBB paracellular route (19). This suggests that brain exposure to colistin could be substantially higher in infected patients during treatment and could potentially lead to centrally mediated neurotoxicity. However, whether a similar increase in brain uptake of colistin will be observed in the presence of a systemic bacterial infection (rather than following administration of LPS) remains unknown.
In order to assess the brain uptake of colistin during systemic infection, a mouse thigh infection model involving intramuscular administration of P. aeruginosa was established. During the last decade, the opportunistic P. aeruginosa (40) has developed the multidrug resistance phenotype and sometimes is only susceptible to colistin (27), which has been shown to have a cure rate of approximately 70% to infections caused by this bacterium (26, 29). Therefore, P. aeruginosa was considered a clinically relevant bacterial species to develop the bacterial thigh infection model. The impact of systemic bacterial infection on the integrity of the BBB and colistin brain uptake was then determined and related to the plasma levels of the proinflammatory cytokines TNF-α, IL-1β, and IL-6. Furthermore, we measured the plasma cytokine levels in mice pretreated with LPS from S. enterica, a treatment that we have shown leads to increased BBB paracellular permeability and increased brain uptake of colistin (19), to determine any relationship between plasma proinflammatory cytokine levels and BBB disruption.
MATERIALS AND METHODS
Bacterial strain and media.
P. aeruginosa ATCC 27853 (American Type Culture Collection, Rockville, MD) was stored in tryptone soy broth (Oxoid, Thebarton, South Australia, Australia) with 20% (vol/vol) glycerol at −80°C. The bacterial strain was subcultured onto nutrient agar plates (Media Preparation Unit, The University of Melbourne, Parkville, Australia) prior to each experiment and incubated overnight at 37°C. A colony was then selected and grown overnight in 10 ml of cation-adjusted Mueller-Hinton broth (CAMHB; Oxoid, Hampshire, England) from which the logarithmic-phase growth was obtained.
Chemicals and reagents.
Colistin sulfate was purchased from Zhejiang Shenghua Biok Biology Co., Ltd. (EP5 grade; Zhejiang, China). Lipopolysaccharide from S. enterica serotype Typhimurium was obtained from Sigma-Aldrich (Castle Hill, New South Wales, Australia). [14C]sucrose was obtained from American Radiolabeled Chemicals (St. Louis, MO). Solid-phase extraction (SPE) cartridges (C18 Sep-Pak, 100 mg) were purchased from Waters (Milford, MA). Cyclophosphamide was obtained from Baxter Healthcare Pty. Ltd. (Old Toongabbie, New South Wales, Australia). Glycerol was purchased from Ajax Finechem (Seven Hills, New South Wales, Australia). All other reagents were of analytical and/or high-performance liquid chromatography (HPLC) grade, and water was obtained from a Millipore purification system (Millipore Corporation, Billerica, MA).
Animal studies.
Animal experiments were approved by the Monash Institute of Pharmaceutical Sciences Animal Ethics Committee and were performed in accordance with the Australian National Health and Medical Research Council guidelines for the care and use of animals for scientific purposes. Male Swiss Outbred mice (6 to 8 weeks of age, 25 to 30 g) were used in all studies. Mice had free access to food and water during all experimental periods.
Induction of systemic infection in neutropenic and nonneutropenic mice.
Systemic bacterial infection was induced through the development of a thigh infection as described previously, with minor modifications (13). The thigh infection was induced in both neutropenic and nonneutropenic mice to elucidate whether the presence of neutrophils (and the associated inflammatory cascade) was essential for any bacterium-induced BBB disturbance. To induce neutropenia in mice, cyclophosphamide was injected intraperitoneally 4 days (at a dose of 150 mg/kg of body weight) and 1 day (at a dose of 100 mg/kg) prior to the bacterial inoculation. On the day of inoculation, neutropenic mice were anesthetized with isoflurane by inhalation and then a 50-μl suspension of 1-h logarithmic-phase P. aeruginosa (approximately 5 × 105 CFU in CAMHB) or CAMHB was injected into each posterior thigh muscle. The initial inocula were quantified by viable counting using a Synbiosis ProtoCOL colony counter (Don Whitley Scientific Ltd., England). At 4, 8, and 13 h after inoculation, mice were anesthetized by isoflurane and blood was aseptically collected by cardiac puncture (n = 4 at each time point) and subjected to viable counting to determine the number of bacterial cells in the blood. To induce bacteremia in nonneutropenic mice, a 50-μl suspension of 1-h logarithmic-phase P. aeruginosa (approximately 5 × 107 CFU reconstituted in saline) or saline was injected into each posterior thigh muscle following anesthesia. Blood samples were collected at 1, 2, 4, and 8 h after inoculation (n = 4 at each time point) to determine at what time point bacteremia had been achieved. The blood samples collected from both neutropenic and nonneutropenic mice at each time point were serially diluted in sterile saline and plated on nutrient agar plates (which were incubated overnight at 37°C), followed by counting of bacterial colonies.
Effect of systemic infection on brain uptake of colistin.
An aliquot (200 μl) of colistin sulfate solution (equivalent to 40 mg/kg in saline) filtered through a 0.22-μm membrane (Millipore, Carrigtwohill, Country Cork, Ireland) was subcutaneously administered in the interscapular region of neutropenic or nonneutropenic mice 8 h following bacterial inoculation, as this was the time demonstrated to result in reproducible bacteremia (see Results). At various times (0.5, 1, 2, or 4 h) after administration of colistin sulfate, mice (n = 4 at each time point) were anesthetized with isoflurane, blood was collected by cardiac puncture, and the whole brain was removed following cervical dislocation. Plasma and brain samples were then stored at −20°C until analyzed by HPLC using an assay previously developed and validated in our laboratory (18). The standards used for quantitating colistin in mouse plasma and brain homogenate were prepared in noninfected matrices from mice. However, to ensure that the presence of bacteria in plasma and brain samples did not affect the ability to quantitate colistin, quality control (QC) samples of colistin (n = 4) were also prepared in plasma and brain obtained from mice treated with cyclophosphamide and P. aeruginosa. The peak areas from low and high QC samples were compared to standards prepared in noninfected matrices, and precision and accuracy were calculated.
Following determination of the colistin concentrations in brain homogenate and plasma, a brain/plasma (B:P) concentration ratio of colistin was calculated at each postdose time point. In addition to discrete B:P ratios, the area under the plasma concentration-time curve from zero time to 4 h (AUCplasma), the area under the corresponding brain concentration-time curve (AUCbrain), and their associated variances were determined by Bailer's approach using the linear trapezoidal rule (4), which was previously validated using WinNonlin (version 4.0; Pharsight Corporation, Mountain View, CA).
Effect of LPS on brain uptake of colistin.
Given that LPS (S. enterica) is known to induce BBB disruption, the following component of the study was included as a positive control. In brief, mice were administered three intraperitoneal injections (200 μl) of 0.9% (wt/vol) saline (control) or LPS (S. enterica) at 0, 6, and 24 h as described previously (19). An aliquot (200 μl) of a colistin sulfate solution (40 mg/kg in saline) was then administered subcutaneously to mice 4 h after the third saline or LPS dose. At 0.5 h after the administration of colistin, plasma and brain samples (n = 4) were harvested, and concentrations of colistin in brain homogenate and plasma were determined by HPLC to obtain a B:P ratio.
Assessment of BBB integrity.
The brain uptake of [14C]sucrose was assessed to detect whether the paracellular integrity of the BBB was compromised in infected (both neutropenic and nonneutropenic) and LPS-treated mice (as a positive control). At 8 h after the initial bacterial or saline inoculation or 4 h after the third LPS or saline dose, mice were intravenously administered a 50-μl solution of [14C]sucrose (2 μCi in saline) (n = 6 per group). Plasma and brain samples were collected 5 min postdose, and the radioactivity in plasma and brain was determined using liquid scintillation counting (Tri-Carb 2800 TR; Perkin Elmer, Boston, MA) as described previously (19).
Measurement of plasma cytokine concentrations.
Concentrations of TNF-α, IL-1β, and IL-6 were determined in the plasma obtained from nonneutropenic mice 8 h after the initial bacterial or saline inoculation or 4 h after the third LPS or saline dose, in an attempt to correlate BBB disruption to plasma proinflammatory cytokine levels. Plasma (50 μl) was added to the wells of mouse cytokine 96-well kits (Ready-Set-Go!; eBioscience, San Diego, CA), and absorbance was recorded at 450 nm with a FLUOstar Optima microplate reader (BMG Labtech, Mount Eliza, Victoria, Australia). The endogenous components within plasma did not interfere with the assay, as no absorbance was obtained following incubation with plasma alone. Concentrations of the three cytokines in each plasma sample were calculated by comparison to a standard curve developed with known concentrations of each cytokine. The quantification range for the cytokines ranged between 8 and 1,000 pg/ml. Plasma samples containing cytokine concentrations exceeding 1,000 pg/ml were diluted in blank plasma and reassayed.
Data analysis.
All data are presented as means ± standard deviations (SD), unless otherwise stated. Student's t test was used to examine the difference between two groups, whereas bacterial colony numbers in infected animals (using viable counts on a linear scale) were compared using a one-way analysis of variance (ANOVA) followed by a Newman-Keuls multiple-comparisons test (PASW Statistics for Windows, version 17.0; Chicago, IL). A P value of <0.05 was considered to be a significant difference. When comparing AUCplasma and AUCbrain between noninfected and infected mice, a z test was used to test significant differences as proposed by Bailer (4). A z value of >1.96 was considered to be a significant difference between groups.
RESULTS
Bacterial burden in blood after inoculation of neutropenic and nonneutropenic mice.
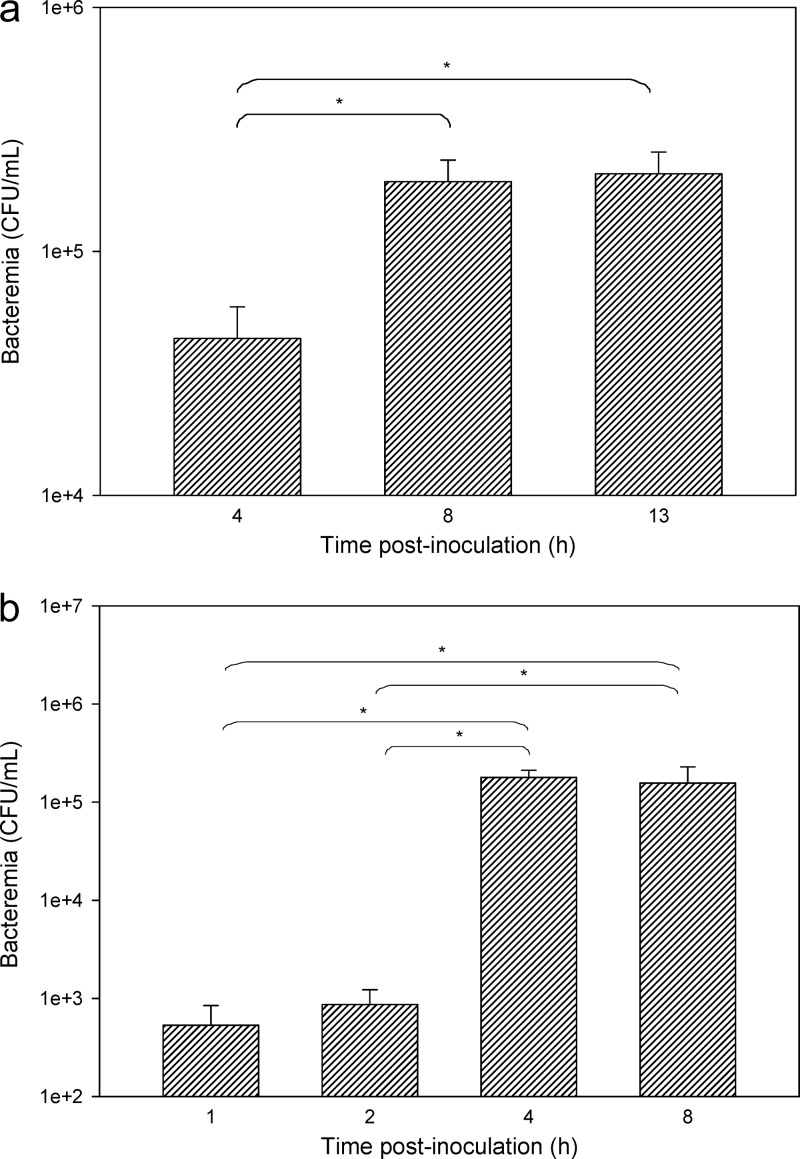
At 4 h after inoculating neutropenic mice with P. aeruginosa (5 × 105 CFU), bacteremia was relatively low (less than 4.65 log10 CFU/ml of blood), and it was not until 8 h postinoculation that bacteremia appeared to be reproducibly established with blood levels of 5.05 to 5.38 log10 CFU/ml (Fig. 1a). There was no significant difference in the level of bacteremia between 8 and 13 h (P > 0.05). For this reason, all subsequent brain uptake experiments in neutropenic mice were conducted 8 h following bacterial inoculation or saline administration. When the same inoculum of P. aeruginosa was injected into nonneutropenic mice, no bacteremia was detectable, and therefore, the inoculum was increased to approximately 5 × 107 CFU. As shown in Fig. 1b, bacteremia was minimal at 1 and 2 h postinoculation (less than 2.95 log10 CFU/ml), whereas at 4 h postinoculation, blood levels reached a plateau of 4.90 to 5.48 log10 CFU/ml, which is similar to the level observed in neutropenic mice (albeit at a lower inoculum). At 8 h postinoculation, there was no significant difference in bacteremia relative to that at 4 h (P > 0.05); however, in order to keep a consistent time exposure between neutropenic and nonneutropenic studies, all brain uptake assessments in nonneutropenic mice were also undertaken at 8 h postinoculation.
Fig 1.
Bacteremia (CFU/ml) at various time points following intramuscular administration of P. aeruginosa to neutropenic Swiss Outbred mice (a) and nonneutropenic Swiss Outbred mice (b). Data are presented as means ± standard errors of the means (SEM) (n = 4). *, P < 0.05 (using a one-way ANOVA).
Brain uptake of colistin following systemic infection or LPS treatment.
To ensure that P. aeruginosa inoculation or cyclophosphamide treatment did not interfere with HPLC analysis of colistin in plasma and brain homogenate, the HPLC methods developed previously (18) were revalidated using plasma and brain from bacteria-inoculated neutropenic mice. No changes in colistin chromatographic peak shapes or retention times were observed in colistin-spiked plasma or brain homogenate obtained from mice pretreated with cyclophosphamide and inoculated with bacteria. Using standard-curve solutions of colistin prepared in plasma and brain homogenate from noninfected mice, the precision and accuracy values for QC samples prepared in plasma and brain homogenate from infected mice were within acceptable ranges (as shown in Table 1). These results indicated that plasma and brain from noninfected mice were suitable to establish standard curves to calculate the concentrations of colistin in samples from infected animals.
Table 1.
Precision and accuracy values of QC samplesa
| QC sample type | Target colistin concn in matrices from infected mice | Measured colistin concnb | Precision (%) | Accuracy (%) |
|---|---|---|---|---|
| Plasma | 1.25 | 1.26 ± 0.044 | 3.2 | 100.8 |
| 10.00 | 10.9 ± 1.46 | 13.4 | 109.2 | |
| Brain homogenate | 0.19 | 0.184 ± 0.025 | 13.4 | 96.8 |
| 3.00 | 3.17 ± 0.084 | 2.6 | 105.7 |
Samples were prepared in plasma (μg/ml; n = 4) and brain homogenate (μg/g; n = 4) obtained from infected neutropenic mice and compared to a standard curve prepared in matrices obtained from noninfected mice.
Data are presented as means ± SD.
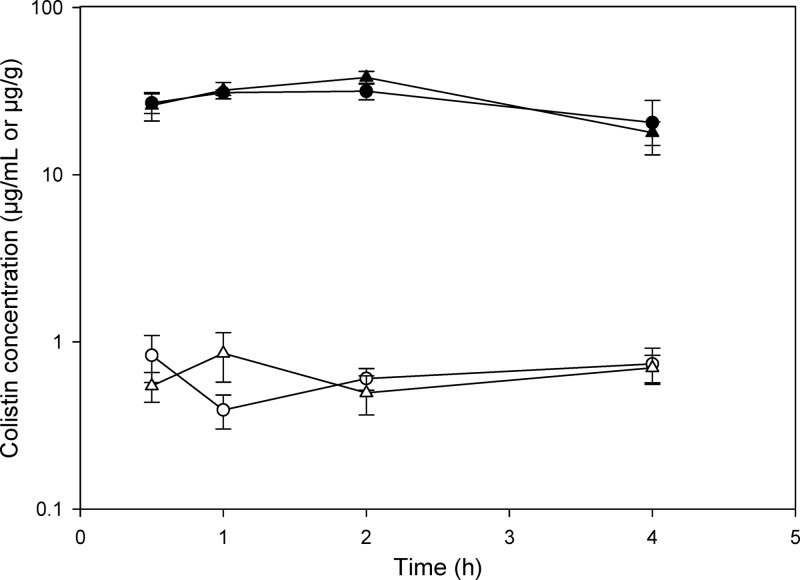
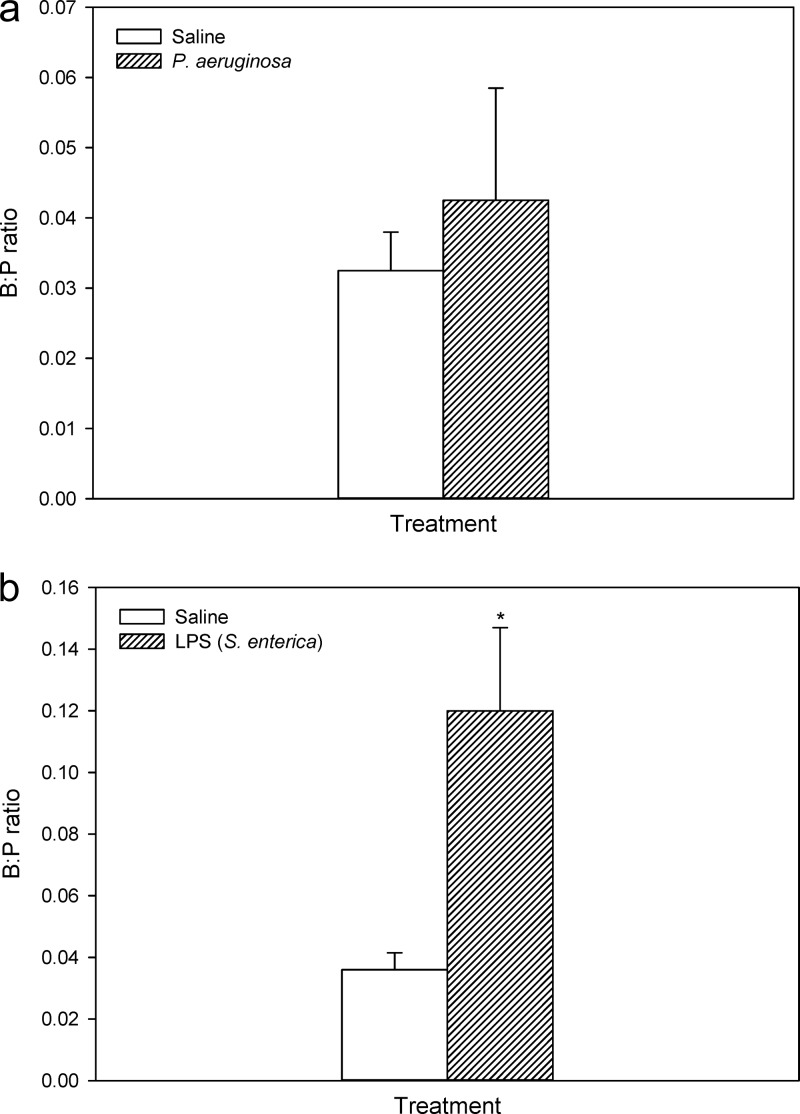
All mice tolerated the current single subcutaneous dose of colistin with no observed toxicity over the experimental period. The plasma and brain concentrations of colistin in infected and noninfected neutropenic mice are shown in Fig. 2. Plasma concentrations of colistin were not significantly affected by inoculation with P. aeruginosa, with AUCplasma values of infected and noninfected mice being 118.6 ± 11.6 and 108.3 ± 11.5 μg · h/ml, respectively (z = 0.20). Similarly, the brain homogenate concentrations of colistin were not different between infected and noninfected animals over the 4-h postdose period, with AUCbrain values of 2.74 ± 0.21 and 2.58 ± 0.28 μg · h/g, respectively (z = 0.45). Similarly, when colistin was administered to nonneutropenic animals, there was no significant difference in the B:P ratio of colistin between infected and noninfected mice at 0.5 h postadministration (Fig. 3a), with B:P ratios of 0.043 ± 0.032 (infected mice) and 0.032 ± 0.011 (noninfected mice). Given that there was no difference in the brain uptake of colistin at this single time point, no further mice were dosed to obtain a concentration-time profile similar to that obtained for neutropenic mice (Fig. 2). However, in mice treated with LPS from S. enterica, the B:P ratio of colistin at 0.5 h postadministration was 0.12 ± 0.053 (Fig. 3b), which was significantly higher than that of saline-treated mice (0.036 ± 0.011), confirming that when the BBB is perturbed, the brain uptake of colistin is indeed enhanced.
Fig 2.
Plasma (μg/ml) and brain (μg/g) concentrations of colistin after subcutaneous administration of colistin sulfate (40 mg/kg) to neutropenic Swiss Outbred mice 8 h after inoculation with P. aeruginosa (∼5 × 105 CFU/thigh) or saline. The data indicate plasma (▲) and brain (△) from infected mice and plasma (●) and brain (○) from noninfected mice and are presented as means ± SEM (n = 4).
Fig 3.
Brain/plasma (B:P) ratios of colistin following administration to nonneutropenic Swiss Outbred mice inoculated with saline or P. aeruginosa (∼5 × 107 CFU/thigh) (a) and Swiss Outbred mice administered saline or LPS from S. enterica (3 mg/kg at 0, 6, and 24 h) (b). Data are presented as means ± SEM (n = 4). *, P < 0.05 relative to saline-administered mice (using Student's t test).
BBB integrity following systemic infection or LPS treatment.
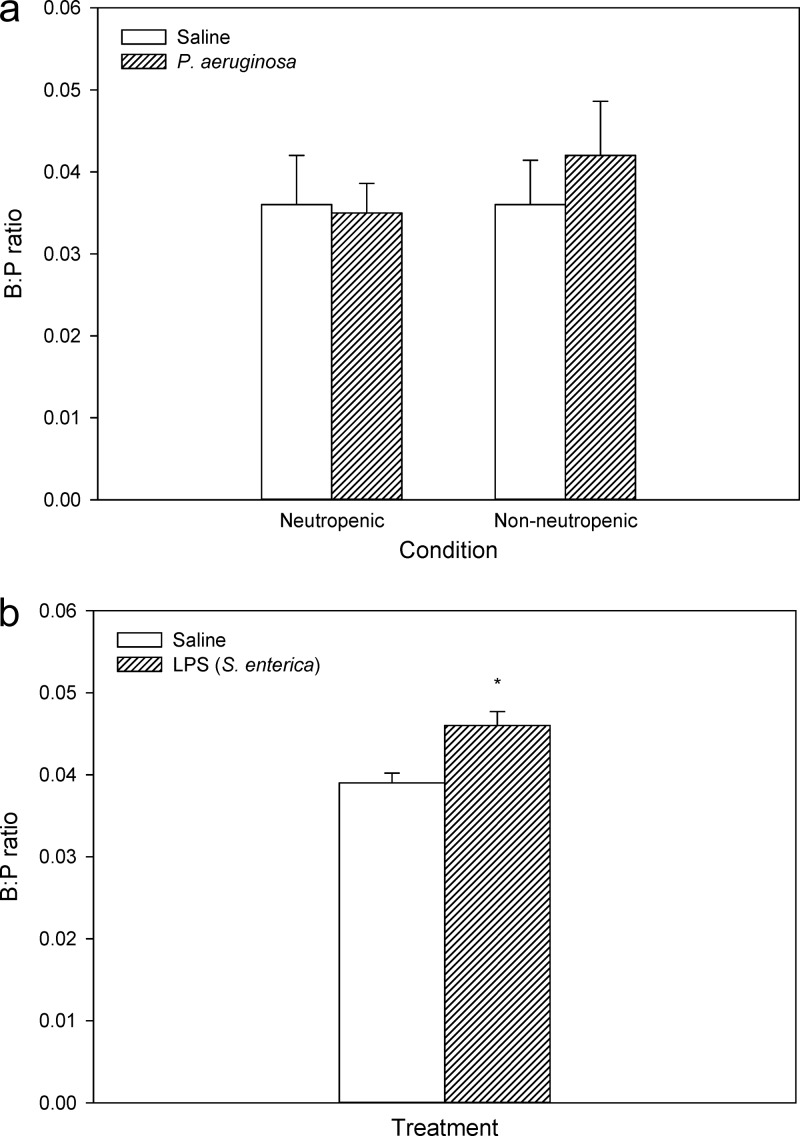
The B:P ratio of the BBB paracellular route marker, [14C]sucrose, was determined in mice during bacteremia to confirm a lack of paracellular dysfunction, as suggested by the lack of difference in colistin brain uptake between noninfected and infected mice. In both neutropenic and nonneutropenic mice, the average B:P ratios of [14C]sucrose were not significantly different between infected and noninfected mice (Fig. 4a), suggesting that even though bacteremia was present, the tight junctions at the BBB were still functionally intact. In contrast, a small but significant difference in [14C]sucrose brain uptake was observed between saline- and LPS-treated mice with B:P ratios of 0.046 ± 0.0035 (LPS treated) and 0.039 ± 0.0024 (saline treated) (Fig. 4b).
Fig 4.
Brain/plasma (B:P) ratios of [14C]sucrose following administration to neutropenic or nonneutropenic Swiss Outbred mice inoculated with saline or P. aeruginosa (∼5 × 105 to 5 × 107 CFU/thigh) (a) and Swiss Outbred mice administered saline or LPS from S. enterica (3 mg/kg at 0, 6, and 24 h) (b). Data are presented as means ± SEM (n = 6). *, significant (P < 0.05) difference between saline and LPS-treated mice (using Student's t test).
Plasma cytokine levels following systemic infection or LPS treatment.
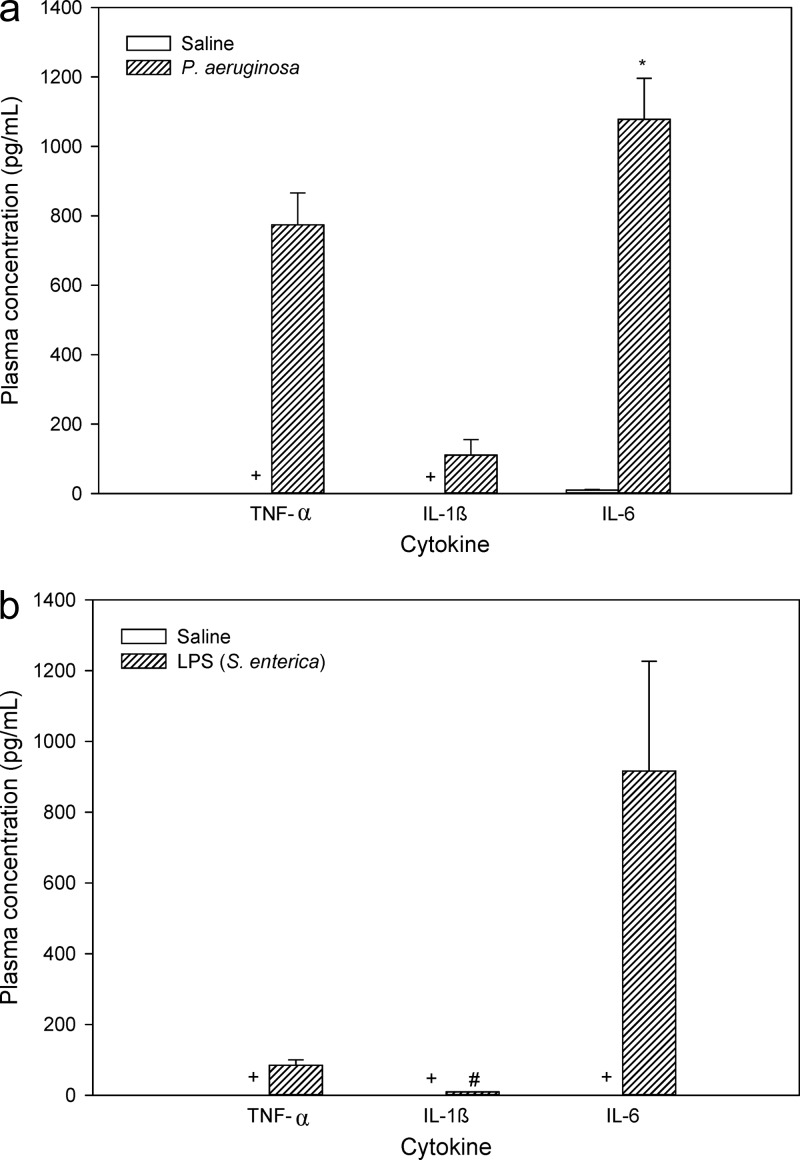
Plasma concentrations of TNF-α, IL-1β, and IL-6 in infected and noninfected nonneutropenic mice are shown in Fig. 5a. The values of all three cytokines were very low in noninfected mice, with TNF-α and IL-1β levels being below the limit of quantitation of the assay (i.e., 8 pg/ml). After inoculation with P. aeruginosa, concentrations of cytokines were substantially higher with values of 774.1 ± 184.4 pg/ml for TNF-α, 110.7 ± 88.4 pg/ml for IL-1β, and 1078.3 ± 235.5 pg/ml for IL-6, consistent with the proinflammatory mediators having been activated during systemic bacterial infection. Studies to measure the plasma cytokine levels in neutropenic mice were not conducted, as results from nonneutropenic mice were more likely to resemble the clinical setting.
Fig 5.
Plasma concentrations (pg/ml) of TNF-α, IL-1β, and IL-6 from nonneutropenic mice (a) 8 h after inoculation with saline or P. aeruginosa (∼5 × 107 CFU/thigh) and (b) 4 h after the last dose of LPS (S. enterica) or saline. Data are presented as means ± SEM (n = 4). *, P < 0.05 between bacteria-inoculated and saline-treated animals (using Student's t test); +, plasma levels were below 8 pg/ml; #, n = 2 (as the remaining replicates were below 8 pg/ml).
The levels of TNF-α, IL-1β, and IL-6 were also determined in plasma of saline- and LPS-treated mice (Fig. 5b), given that S. enterica LPS induced BBB disruption, therefore allowing for a better correlation to be made between BBB disruption and plasma cytokine levels. The plasma levels of these cytokines from LPS-treated mice were 84.8 ± 14.9 pg/ml for TNF-α, 9.3 pg/ml for IL-1β (the SD value could not be calculated, as concentrations of only two samples were above 8 pg/ml), and 916.5 ± 310.4 pg/ml for IL-6. The concentrations of cytokines in saline-treated mice were in all cases below the limit of quantitation of the assays. While statistical analysis could not be conducted, given that saline-treated mice exhibited nonquantifiable plasma cytokine levels, it was obvious that plasma concentrations of these three cytokines were higher after LPS treatment. In addition, the TNF-α plasma levels in infected mice were substantially higher than the corresponding levels in LPS-treated mice (P < 0.05), even though BBB disruption and enhanced colistin brain uptake were only observed in LPS-treated mice, suggesting a lack of correlation between BBB disruption and plasma levels of this cytokine.
DISCUSSION
As a consequence of the development of antibiotic resistance, there has been increasing clinical use of the cationic polypeptide antibiotic colistin for the treatment of Gram-negative bacterial infections (24, 31). Associated with this clinical use are reports of neurotoxicity, suggesting that colistin has the potential to permeate the BBB. We have demonstrated that colistin exhibits minimal penetration across the healthy BBB; however, it has the ability to traverse the BBB during systemic inflammation due to LPS-mediated perturbation of the paracellular route (19). To reflect the clinical scenario more closely, the impact of systemic infection with P. aeruginosa on the brain uptake of colistin was assessed in a mouse model. The strain of P. aeruginosa selected to induce bacteremia (ATCC 27853) was initially isolated from a patient and has been widely used to determine the antimicrobial activities of various antibiotics (15, 35), and therefore, it was considered clinically relevant for this study.
It has been shown that inoculation of mice with Escherichia coli and Streptococcus pneumoniae at sepsis-inducing doses leads to BBB disruption, which was demonstrated by brain access of the impermeable horseradish peroxidase (38). Although a different bacterial strain, an increased BBB permeability and enhancement in colistin brain uptake was therefore expected following inoculation with P. aeruginosa. Interestingly, despite a high level of bacteremia similar to that achieved following inoculation with E. coli and S. pneumoniae (38), the plasma and brain concentrations of colistin did not differ between P. aeruginosa-infected and noninfected mice. Moreover, the B:P ratios of the BBB paracellular route marker, [14C]sucrose, were not different between the two groups, suggesting that the tight junctions were not disturbed following P. aeruginosa inoculation. However, administration of LPS from S. enterica led to an increase in the brain uptake of both colistin and [14C]sucrose, demonstrating that our model was able to discriminate between an intact and disturbed BBB, suggesting that the observations with P. aeruginosa were indicative of a negative impact on BBB dynamics. Although colistin is administered parenterally as its inactive prodrug CMS (5), we did not assess the impact of bacterial infection on the brain uptake of CMS. Due to stability issues of CMS and its ability to convert to colistin in vivo (22, 23), it would be difficult to accurately quantify the brain uptake of CMS in vivo and, furthermore, whether the brain uptake of CMS is altered during bacterial infection.
The lack of effect of P. aeruginosa on BBB integrity and colistin brain uptake was first assessed in neutropenic animals, whose immune system and ability to produce proinflammatory cytokines may be affected (9). Cytokines are proinflammatory mediators of the immune system that are released in order to respond to acute infection. These mediators are known to modify BBB permeability during neuroinflammatory disease states, such as multiple sclerosis, meningitis, and encephalitis, and this is associated with their ability to affect the expression and function of interendothelial tight junction proteins, including occludin and zonula occludens-1 (21, 30, 41). Various in vitro studies have demonstrated that proinflammatory mediators such as TNF-α, IL-1β, and IL-6 are able to cause BBB paracellular dysfunction by altering the cytoskeleton structure of endothelial cells (12). It has also been demonstrated that direct administration of such cytokines in vivo could lead to BBB disruption (2, 36). Given the role of proinflammatory cytokines in mediating BBB disruption, and the altered inflammatory response of neutropenic mice, it was considered that the neutropenic state may have prevented the ability for bacteremia to induce BBB dysfunction. For this reason, we then developed a bacterial infection model in nonneutropenic (i.e., noncyclophosphamide-treated) mice to determine whether the presence of neutrophils (and the subsequent altered release of proinflammatory cytokines) would lead to BBB dysfunction and enhanced colistin brain uptake. Such a scenario is also more likely to be representative of that observed in otherwise healthy patients who have contracted a bacterial infection. Not surprisingly, a higher inoculum of P. aeruginosa was required to induce bacteremia in nonneutropenic mice. However, similar to that observed in neutropenic mice, no significant difference in the brain uptake of colistin or [14C]sucrose was found between infected and noninfected mice, suggesting that the paracellular route was not disrupted in the infected mice. These studies suggested that, even in the presence of an intact immune system, bacteremia induced by P. aeruginosa was not able to induce BBB dysfunction.
To ensure that there was indeed an inflammatory response induced by bacteremia, and to examine possible relationships between inflammatory mediators and BBB disruption, the plasma levels of TNF-α, IL-1β, and IL-6 were measured in infected and noninfected mice. For all three cytokines, the concentration in plasma was higher in the infected mice than in noninfected mice. Despite the higher plasma cytokine levels, the BBB integrity was not affected during bacterial infection, suggesting that there was no clear correlation between plasma cytokine levels and BBB dysfunction, at least for the three cytokines examined. To further confirm this lack of correlation, we measured the plasma cytokine levels in mice having a disturbed BBB (i.e., mice treated with LPS from S. enterica). This showed that even though we observed a significant increase in colistin and [14C]sucrose brain uptake after LPS treatment, plasma cytokine levels were actually lower than those observed following bacterial infection (where there was no BBB disruption). These data confirmed that for these three plasma cytokines, there did not appear to be a direct link between plasma cytokine levels and the extent of BBB disruption.
There are various reasons as to why there appeared to be differences between the BBB-disrupting effects of our two treatment paradigms. First, it is important to note that bacterial infection with P. aeruginosa led to a larger proinflammatory response (relative to S. enterica LPS administration), yet the BBB-disrupting effects of LPS appeared greater. This suggests that there may be a direct effect of LPS species on the BBB, independent of the release of plasma cytokines, as has been suggested elsewhere (16). Alternatively, given that the levels of cytokines released in these studies were well below those previously shown to induce BBB disruption both in vitro and in vivo (10–12, 36, 38), it is possible that a more representative correlation may be observed if higher bacterial inocula of P. aeruginosa were administered. Indeed, the levels of TNF-α and IL-6 released in patients with nonbacterially induced acute pancreatitis (where enhanced BBB permeability was detected) were substantially higher than the levels observed in our studies following P. aeruginosa infection (14). It should also be noted that we only measured the levels of IL-1β, IL-6, and TNF-α, and it is possible that other cytokines such as gamma interferon (IFN-γ) or IL-10 may also play an important role in BBB disruption (32). It is also possible that P. aeruginosa ATCC 27853 may be less virulent (and BBB disruptive) than other P. aeruginosa strains or other clinically relevant bacterial strains. The impact of such strains on BBB disruption, and on the brain uptake of colistin (and other therapeutic agents), warrants further investigation. Nevertheless, we have demonstrated that the brain uptake of colistin does not appear to be substantially affected during bacteremia induced with this clinically relevant strain. We have also confirmed that such an enhancement in the brain uptake of colistin is possible following administration of LPS from S. enterica, which suggests that the CNS exposure of this antibiotic may be increased during infection with other bacterial strains.
These studies have demonstrated that, despite increasing the plasma concentrations of three proinflammatory cytokines, induction of bacteremia by P. aeruginosa did not alter the BBB paracellular route or the ability of colistin to enter the brain parenchyma following systemic administration. Furthermore, a direct relationship between plasma levels of TNF-α, IL-1β, and IL-6 and BBB disruption was not evident in vivo, in contrast to that which has been suggested from in vitro studies.
ACKNOWLEDGMENTS
Roger L. Nation and Jian Li are supported by research grants from the National Institute of Allergy and Infectious Diseases (R01A107896 and R01A1079330). Jian Li is an Australian NHMRC Senior Research Fellow.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
Footnotes
Published ahead of print 30 July 2012
REFERENCES
- 1. Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. 2010. Structure and function of the blood-brain barrier. Neurobiol Dis. 37:13–25 [DOI] [PubMed] [Google Scholar]
- 2. Abraham CS, Deli MA, Joo F, Megyeri P, Torpier G. 1996. Intracarotid tumor necrosis factor-alpha administration increases the blood-brain barrier permeability in cerebral cortex of the newborn pig: quantitative aspects of double-labelling studies and confocal laser scanning analysis. Neurosci. Lett. 208:85–88 [DOI] [PubMed] [Google Scholar]
- 3. Antachopoulos C, et al. 2010. Serum and cerebrospinal fluid levels of colistin in pediatric patients. Antimicrob. Agents Chemother. 54:3985–3987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bailer AJ. 1988. Testing for the equality of area under the curves when using destructive measurement techniques. J. Pharmacokinet. Biopharm. 16:303–309 [DOI] [PubMed] [Google Scholar]
- 5. Bergen PJ, Li J, Rayner CR, Nation RL. 2006. Colistin methanesulfonate is an inactive prodrug of colistin against Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 50:1953–1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Beringer P. 2001. The clinical use of colistin in patients with cystic fibrosis. Curr. Opin. Pulm. Med. 7:434–440 [DOI] [PubMed] [Google Scholar]
- 7. Capaldo CT, Nusrat A. 2009. Cytokine regulation of tight junctions. Biochim. Biophys. Acta 1788:864–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Coisne C, Engelhardt B. 2011. Tight junctions in brain barriers during central nervous system inflammation. Antioxid. Redox Signal. 15:1285–1303 [DOI] [PubMed] [Google Scholar]
- 9. Daley JM, Ivanenko-Johnston T, Reichner JS, Albina JE. 2005. Transcriptional regulation of TNF-alpha production in neutropenia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 288:R409–R412 [DOI] [PubMed] [Google Scholar]
- 10. Deli MA, et al. 1995. Exposure of tumor necrosis factor-alpha to luminal membrane of bovine brain capillary endothelial cells cocultured with astrocytes induces a delayed increase of permeability and cytoplasmic stress fiber formation of actin. J. Neurosci. Res. 41:717–726 [DOI] [PubMed] [Google Scholar]
- 11. Desai TR, Leeper NJ, Hynes KL, Gewertz BL. 2002. Interleukin-6 causes endothelial barrier dysfunction via the protein kinase C pathway. J. Surg. Res. 104:118–123 [DOI] [PubMed] [Google Scholar]
- 12. de Vries HE, et al. 1996. The influence of cytokines on the integrity of the blood-brain barrier in vitro. J. Neuroimmunol. 64:37–43 [DOI] [PubMed] [Google Scholar]
- 13. Dudhani RV, et al. 2010. Elucidation of the pharmacokinetic/pharmacodynamic determinant of colistin activity against Pseudomonas aeruginosa in murine thigh and lung infection models. Antimicrob. Agents Chemother. 54:1117–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Farkas G, et al. 1998. Experimental acute pancreatitis results in increased blood-brain barrier permeability in the rat: a potential role for tumor necrosis factor and interleukin 6. Neurosci. Lett. 242:147–150 [DOI] [PubMed] [Google Scholar]
- 15. Giacometti A, Cirioni O, Barchiesi F, Fortuna M, Scalise G. 1999. In-vitro activity of cationic peptides alone and in combination with clinically used antimicrobial agents against Pseudomonas aeruginosa. J. Antimicrob. Chemother. 44:641–645 [DOI] [PubMed] [Google Scholar]
- 16. He F, et al. 2011. RhoA and NF-kappaB are involved in lipopolysaccharide-induced brain microvascular cell line hyperpermeability. Neuroscience 188:35–47 [DOI] [PubMed] [Google Scholar]
- 17. Jiménez-Mejías ME, et al. 2002. Cerebrospinal fluid penetration and pharmacokinetic/pharmacodynamic parameters of intravenously administered colistin in a case of multidrug-resistant Acinetobacter baumannii meningitis. Eur. J. Clin. Microbiol. Infect. Dis. 21:212–214 [DOI] [PubMed] [Google Scholar]
- 18. Jin L, Li J, Nation RL, Nicolazzo JA. 2009. Brain penetration of colistin in mice assessed by a novel high-performance liquid chromatographic technique. Antimicrob. Agents Chemother. 53:4247–4251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jin L, Li J, Nation RL, Nicolazzo JA. 2011. Impact of p-glycoprotein inhibition and lipopolysaccharide administration on blood-brain barrier transport of colistin in mice. Antimicrob. Agents Chemother. 55:502–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jong A, Huang SH. 2005. Blood-brain barrier drug discovery for central nervous system infections. Curr. Drug Targets Infect. Disord. 5:65–72 [DOI] [PubMed] [Google Scholar]
- 21. Leib SL, Leppert D, Clements J, Tauber MG. 2000. Matrix metalloproteinases contribute to brain damage in experimental pneumococcal meningitis. Infect. Immun. 68:615–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li J, Milne RW, Nation RL, Turnidge JD, Coulthard K. 2003. Stability of colistin and colistin methanesulfonate in aqueous media and plasma as determined by high-performance liquid chromatography. Antimicrob. Agents Chemother. 47:1364–1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li J, et al. 2004. Pharmacokinetics of colistin methanesulphonate and colistin in rats following an intravenous dose of colistin methanesulphonate. J. Antimicrob. Chemother. 53:837–840 [DOI] [PubMed] [Google Scholar]
- 24. Li J, et al. 2006. Colistin: the re-emerging antibiotic for multidrug-resistant Gram-negative bacterial infections. Lancet Infect. Dis. 6:589–601 [DOI] [PubMed] [Google Scholar]
- 25. Markantonis SL, et al. 2009. Penetration of colistin into cerebrospinal fluid. Antimicrob. Agents Chemother. 53:4907–4910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Markou N, et al. 2003. Intravenous colistin in the treatment of sepsis from multiresistant Gram-negative bacilli in critically ill patients. Crit. Care. 7:R78–R83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mastoraki A, et al. 2008. Pseudomonas aeruginosa susceptible only to colistin in intensive care unit patients. Surg. Infect. (Larchmt.) 9:153–160 [DOI] [PubMed] [Google Scholar]
- 28. Michalopoulos A, Falagas ME. 2008. Colistin and polymyxin B in critical care. Crit. Care Clin. 24:377–391 [DOI] [PubMed] [Google Scholar]
- 29. Michalopoulos AS, Tsiodras S, Rellos K, Mentzelopoulos S, Falagas ME. 2005. Colistin treatment in patients with ICU-acquired infections caused by multiresistant Gram-negative bacteria: the renaissance of an old antibiotic. Clin. Microbiol. Infect. 11:115–121 [DOI] [PubMed] [Google Scholar]
- 30. Morgan L, et al. 2007. Inflammation and dephosphorylation of the tight junction protein occludin in an experimental model of multiple sclerosis. Neuroscience 147:664–673 [DOI] [PubMed] [Google Scholar]
- 31. Nation RL, Li J. 2009. Colistin in the 21st century. Curr. Opin. Infect. Dis. 22:535–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Oshima T, et al. 2001. Interferon-gamma and interleukin-10 reciprocally regulate endothelial junction integrity and barrier function. Microvasc. Res. 61:130–143 [DOI] [PubMed] [Google Scholar]
- 33. Pajouhesh H, Lenz GR. 2005. Medicinal chemical properties of successful central nervous system drugs. NeuroRx 2:541–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Reed MD, Stern RC, O'Riordan MA, Blumer JL. 2001. The pharmacokinetics of colistin in patients with cystic fibrosis. J. Clin. Pharmacol. 41:645–654 [DOI] [PubMed] [Google Scholar]
- 35. Ryge TS, Frimodt-Moller N, Hansen PR. 2008. Antimicrobial activities of twenty lysine-peptoid hybrids against clinically relevant bacteria and fungi. Chemotherapy 54:152–156 [DOI] [PubMed] [Google Scholar]
- 36. Saija A, et al. 1995. Systemic cytokine administration can affect blood-brain barrier permeability in the rat. Life Sci. 56:775–784 [DOI] [PubMed] [Google Scholar]
- 37. Spapen H, Jacobs R, Van Gorp V, Troubleyn J, Honore PM. 2011. Renal and neurological side effects of colistin in critically ill patients. Ann. Intensive Care 1:14–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tsao N, Hsu HP, Wu CM, Liu CC, Lei HY. 2001. Tumour necrosis factor-alpha causes an increase in blood-brain barrier permeability during sepsis. J. Med. Microbiol. 50:812–821 [DOI] [PubMed] [Google Scholar]
- 39. Tunkel AR, Rosser SW, Hansen EJ, Scheld WM. 1991. Blood-brain barrier alterations in bacterial meningitis: development of an in vitro model and observations on the effects of lipopolysaccharide. In Vitro Cell. Dev. Biol. 27A:113–120 [DOI] [PubMed] [Google Scholar]
- 40. Vance RE, Hong S, Gronert K, Serhan CN, Mekalanos JJ. 2004. The opportunistic pathogen Pseudomonas aeruginosa carries a secretable arachidonate 15-lipoxygenase. Proc. Natl. Acad. Sci. U. S. A. 101:2135–2139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wosik K, et al. 2007. Angiotensin II controls occludin function and is required for blood brain barrier maintenance: relevance to multiple sclerosis. J. Neurosci. 27:9032–9042 [DOI] [PMC free article] [PubMed] [Google Scholar]