Abstract
Recently, duck hepatitis A virus genotype C (DHAV-C), a causative agent of duck viral hepatitis, has been responsible for increasing economic losses in the duck industry in China and South Korea. In this study, a real-time PCR assay targeting the 2C gene for detecting DHAV-C was developed. The assay was confirmed to be specific and sensitive, and the minimum detection limit was 3.36 × 103 copies per reaction, making this assay suitable for rapid diagnosis of DHAV-C infection from clinical samples. In addition, the dynamics of the viral loads in tissues of specific-pathogen-free (SPF) ducklings infected with DHAV-C were investigated using this method. The DHAV-C could be detected earliest in the liver within 12 h postinfection. Moreover, high viral loads were identified in the heart, liver, spleen, lung, kidney, bursa of Fabricius, thymus, pancreas, brain, and small intestine after 24 h postinfection. Taking the data collectively, the study described in this report is the first to have developed a real-time PCR method for detection of DHAV-C and thus contributes to pathogenicity research.
INTRODUCTION
Duck viral hepatitis causes a highly contagious disease in domestic ducklings characterized by rapid onset and high mortality (24). Traditionally, duck hepatitis virus (DHV) strains have been classified into three serotypes: type 1 (DHV-1), type 2 (DHV-2), and type 3 (DHV-3) (6, 13, 21). DHV-2 and DHV-3 are classified as members of Astrovirus (3, 5, 20), and DHV-1, belonging to the Picornaviridae, is widely epidemic in the duck-producing areas worldwide (1, 9, 14, 19). Recently, a type of DHV newly found in South Korea (10), Taiwan (23), and mainland China (24) has been identified also as a novel Picornaviridae virus (10, 23, 24). However, no serological relationships between the new type of DHV and the traditional DHV-1 has been found.
To better distinguish different DHVs among the members of the Picornaviridae, it was suggested that DHV in the Picornaviridae should be renamed duck hepatitis A virus (DHAV) (4). Furthermore, based on the genetic structures of DHAV, DHAV was categorized into three genotypes, genotype A (DHAV-A), genotype B (DHAV-B), and genotype C (DHAV-C), which respectively correspond to the traditional type of DHV-1, the new genotype isolated from Taiwan, and the new genotype isolated from South Korea and mainland China (4, 22). DHAV-C mainly affects ducklings within 3 weeks of birth, and its epidemiology, clinical symptoms, and pathology are very similar to those of DHAV-A, making it difficult to distinguish between these two viruses. By comparisons of the complete VP1, VP0, and VP3 nucleotide and amino acid sequences and the partial three-dimensional (3D) nucleotide sequence, the DHAVs belonging to the same genotype were clearly distinguished from those of heterologous genotypes (24). To quickly diagnose a DHAV-A infection, both conventional reverse transcription-PCR (RT-PCR) (11) and real-time PCR (25) methods have been developed. Recently, a method based on amplifying the genomic region in the 5′ untranscribed region (5′-UTR) and sequence analysis has been developed for the detection and typing of DHAV (4). To further differentiate DHAV strains for disease diagnosis, a duplex RT-PCR method for simultaneous detection of DHAV-A and DHAV-C was developed in South Korea (12).
Based on the amount of information available on the genome sequences of DHAV, we analyzed the characteristics of the structures of DHAV and found that the 2C gene could be used to clearly differentiate the different DHAV subtypes. In this study, a real-time PCR assay targeting the 2C gene was developed for detecting DHAV-C from clinical samples and for determining the dynamic distribution of DHAV-C in experimentally infected specific-pathogen-free (SPF) ducklings.
MATERIALS AND METHODS
Virus, bacteria, SPF ducklings, and clinical samples.
Seventeen clinical isolates of DHAV-C, 14 virus species, and 4 bacterial species potentially infecting ducklings were used to evaluate the specificity of the real-time PCR assay in this study (Table 1). Fifty specific-pathogen-free (SPF) 3-day-old ducklings were used to determine the virus distribution in tissues. The SPF duck eggs were purchased from the experimental animal center of Harbin Veterinary Research Institute of Chinese Academy of Agricultural Science and hatched in the incubator in our laboratory. Thirty-eight clinical liver samples, collected from sick ducklings less than 3 weeks old which were suspected to be infected by the hepatitis virus, were characterized according to the presence of liver swelling with hemorrhagic lesions from April 2007 to July 2011 in China.
Table 1.
Reference strains and isolates used in this study for specificity testing of real-time PCR
| Species | No. | Sourcea | Results of real-time PCRb |
|---|---|---|---|
| DHAV-C strains SWUN3501 to SWUN3517 | 17 | a | + |
| DHAV-A virulent strains SWUN3520 to SWUN3522 | 3 | a | − |
| DHAV-A attenuated strain SWUN3523 | 1 | a | − |
| NDV SWUN 2690 | 1 | a | − |
| IBDV SWUN 5011 | 1 | a | − |
| GPV SWUN 5301 | 1 | a | − |
| DPV SWUN 5104 | 1 | a | − |
| REV SWUN sd0101 | 1 | a | − |
| MPV SCAU 251 | 1 | b | − |
| NGVEV SCAU 663 | 1 | b | − |
| AIV CAHEC 343 | 1 | c | − |
| DAstV-1 CAU121 | 1 | d | − |
| DAstV-2 CAU122 | 1 | d | − |
| P. multocida SWUN 0300 | 1 | a | − |
| E. coli (O46) SWUN 0314 | 1 | a | − |
| S. enteritidis SWUN 5223 | 1 | a | − |
| R. anatipestifer SWUN 0233 | 1 | a | − |
a, Southwest University for Nationalities, China; b, Sichuan Agricultural University, China; c, China Animal Health and Epidemiology Center, China; d, China Agricultural University, China.
+, positive; −, negative.
Nucleic acid extraction and cDNA synthesis.
DNA was extracted using a DNA extraction kit (TaKaRa Biotechnology, Dalian, China) according to the manufacturer's instructions. RNA was extracted using TRIzol reagent (Applied Biosystems Inc., Carlsbad, CA). The general RNA was reverse transcribed into cDNA using a Moloney murine leukemia virus (M-MLV) reverse transcription kit (TIANGEN Biotech, Beijing, China). The cDNA and DNA were stored at −20°C for further use.
Development of quantitative real-time PCR.
The design of specific oligonucleotide primers was based on sequence analysis of 20 DHAV-C 2C gene regions published in GenBank. The primer sequences were as follows: forward primer (DHAV-C FP), 5′-TCCAACAGGGTCAAAGC-3′; reverse primer (DHAV-C RP), 5′-AACTACTACAAGTCTGCCACG-3′. The primers amplified a 194-bp fragment of DHAV-C.
The results of conventional RT-PCR amplification with DHAV-C PCR products were verified by electrophoresis using a 2.0% agarose gel and then sequencing. The sequencing result was aligned with other DHAV-C sequences in GenBank. Moreover, the PCR products were detected by real-time PCR melting curve analysis using an SYBR Premix Ex Taq kit (TaKaRa) according to the manufacturer's instructions to verify the specificity of the amplification.
The conventional RT-PCR was performed with viral cDNA and the primers mentioned above. The amplicon was purified by the use of a gel extraction kit (Tiangen) according to the manufacturer's instructions. The purified cDNA fragment was inserted into a clone vector, pMD19-T (TaKaRa), and transformed into Escherichia coli TOP10 host cells (Tiangen). The recombinant plasmid was purified using a plasmid extraction reagent (Tiangen). The product was stored at −20°C for further analysis.
DHAV-C amplification by real-time PCR was performed in a 20-μl reaction mixture containing 2.0 μl of a standard DNA template, 10 μl of SYBR green I master mix, 0.4 μl (each) of the primers (10 μM), and 7.2 μl of sterilized deionized water. The reactions were carried out in a 7300 Real Time PCR system (ABI). The PCR conditions consisted of one cycle of 3 min at 95°C followed by 30 two-step cycles of 15 s at 95°C and 31 s at 60°C.
The standard DNA template was 10-fold serially diluted with sterilized deionized water to generate a standard curve, and each dilution was run in triplicate. The PCR, data acquisition, and analysis were performed using 7300 system software (ABI).
(i) Specificity of the real-time PCR.
Specificity of the real-time PCR was evaluated with the extracts of RNA or DNA templates from duck hepatitis A virus genotype A (DHAV-A) attenuated strain SWUN3523, duck hepatitis A virus genotype A (DHAV-A) virulent strains SWUN3520 to SWUN3522, duck astrovirus 1 (DAstV-1), duck astrovirus 2 (DAstV-2), muscovy parvovirus (MPV), gosling new type viral enteritis virus (NGVEV), avian influenza virus (AIV [H5N1]), Newcastle disease virus (NDV), infection bursal disease virus (IBDV), gosling parvovirus (GPV), duck plaque virus (DPV), reticuloendotheliosis virus (REV), Pasteurella multocida, E. coli (O46), Salmonella enteritidis, Riemerrella anatipestifer, and the allantoic fluid of a normal duck embryo.
(ii) Sensitivity of the real-time PCR.
Ten-fold serially diluted standard DNA templates were assayed by the real-time PCR to determine their sensitivity. Each dilution was also tested with the conventional RT-PCR.
(iii) Repeatability and reproducibility of the real-time PCR.
Three positive samples were used to evaluate the repeatability and reproducibility of the real-time PCR. Intra-assay variability was assessed by testing the different samples in triplicate in a single experiment. Interassay variability was determined from three independent experiments during three consecutive days using the samples mentioned above.
Detection and validation of clinical samples by the real-time PCR assay.
Thirty-eight clinical samples were tested by real-time PCR for DHAV-C. Samples negative for DHAV-C further underwent RT-PCR to detect DHAV-A (25). To validate the positive samples determined by the real-time PCR, 15 positive liver samples were used randomly for virus isolation using 10-day-old SPF duck eggs. RNA was extracted from dead bodies and allantoic fluids to detect DHAV-C by RT-PCR (12).
Detection of DHAV-C in experimentally infected ducklings by the real-time PCR.
The animal study was approved by the Ethical and Animal Welfare Committee from the Universitat Autònoma de Barcelona and followed European Union norms (Council Directive 86/609/EEC). Fifty SPF ducklings were divided into an experimental group (42 ducklings) and a control group (8 ducklings). The experimental group was subcutaneously infected with 0.2 ml of 4.73 × 104 copies of DHAV-C strain SWUN 3504. The controls were injected with the same volume of sodium chloride physiological solution. The experimental and control groups were separated in different SPF incubators, following strict biosafety controls. Three SPF ducklings were randomly selected at each time point and were euthanized to collect heart, liver, spleen, lung, kidney, thymus, bursa of Fabricius (BF), pancreas, intestine, and brain as samples at time points 1, 6, 12, 18, 24, 48, and 72 h. All these samples were tested by real-time PCR.
In order to ensure the accuracy of test results, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), stably expressed in tissues of the DHVA-C-infected duckling tested by our laboratory, was used as the reference gene to normalize the results of viral loads detected by the real-time PCR assay. The primers and conditions of a real-time PCR assay for detecting mRNA expression of GAPDH were as follows: the forward primer (GAPDH FP) (5′-CACAGCCACACACGAAGACA-3′) and reverse primer (GAPDH RP) (5′-CCTTAGCCAGCCCCAGTAGA-3′) were used to amplify a 107-bp fragment of duck GAPDH gene (GenBank accession no. AY436595). The amplification system contained the following components: 2.0 μl of standard DNA template, 12.5 μl of SYBR green I master mix, 0.5 μl (each) of the primers (10 μM), and 9.5 μl of sterilized deionized water. The PCR conditions consisted of one cycle of 5 min at 95°C followed by 40 two-step cycles of 15 s at 95°C and 30 s at 60°C.
Data analysis.
Quantitative real-time PCR data were analyzed with 7300 system software. Linear regression analysis was used to compare the threshold cycle colonization density data (ΔCT) from the DHAV-C samples (ΔCTsample = CTDHAV-C sample − CTGAPDH sample) to the data from the calibration sample (ΔCTcalibration = CTDHAV-C calibration − CTGAPDH calibration). The relative colonization density data were quantified as the ΔΔCT determined by comparisons between the DHAV-C samples and calibration samples (ΔΔCT = ΔCTsample − ΔCTcalibration), and the relative quantifications of the virus load were assessed by the 2−ΔΔCT method (15).
RESULTS
Specificity of primers.
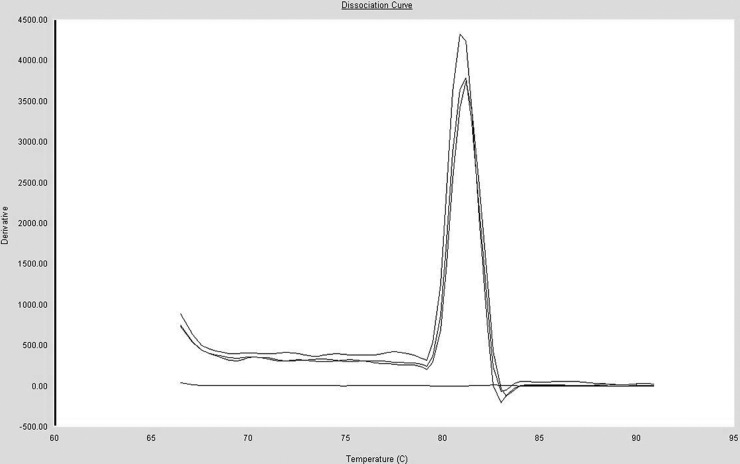
The conventional RT-PCR performed with primers (DHAV-C FP and DHAV-C RP) amplified the expected 194-bp fragment from DHAV-C, as observed by 2.0% gel electrophoresis. The alignment showed that the fragment sequence was 100% identical to those of the 2C genes of all the 20 genomes of DHAV-C strains in GenBank. The melting curves of DHAV-C displayed a single specific peak with a melting temperature (Tm) value at 81.0 ± 0.4°C, suggesting a single product (Fig. 1).
Fig 1.
Melting curve of the DHAV-C PCR products. The melting curve analysis showed a single specific peak, suggesting there were not nonspecific amplification and primer dimers.
Standard curves of the real-time PCR.
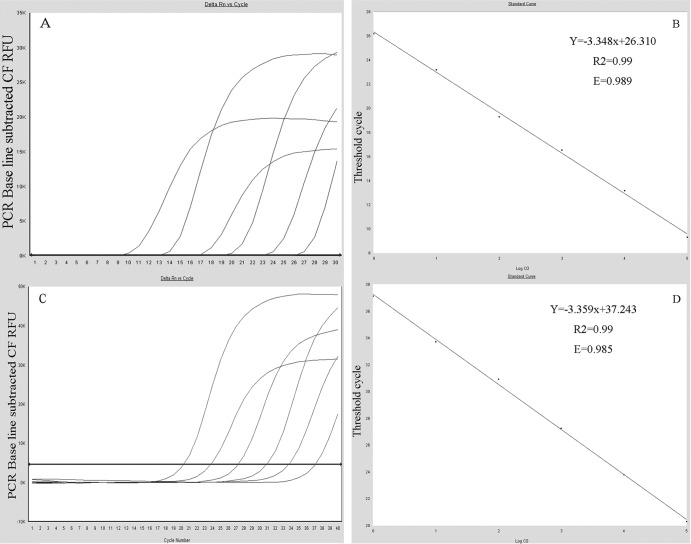
The 10-fold serially diluted standard plasmid DNAs of DHAV-C and GAPDH generated two typical standard curves. The standard curves for DHAV-C and GAPDH were generated with a range of 3.36 × 103 to 3.36 × 108 gene copies per reaction and 8.32 × 102 to 8.32 × 107 gene copies per reaction (Fig. 2), respectively. Both the DHAV-C and GAPDH assays were linear over a 106 dilution range of template DNA. The R2 value for both was 0.99, and the reaction efficiencies were 98.9% and 98.5%, respectively. The cDNA copy numbers of DHAV-C and GAPDH for unknown samples were quantified using the equations YDHAV-C = −3.348XDHAV-C + 26.310 and YGAPDH = −3.359XGAPDH + 37.243, where Y is the threshold cycle and X is the log of the starting quantity.
Fig 2.
Standard curve and amplification curves. Standard curves (based on plasmid DNA) indicating the linearity and efficiency of the reactions are shown. The results of serial 10-fold dilutions of standard DHAV-C (A) and GAPDH (C) from 3.36 × 103 to 3.36 × 108 gene copies per reaction and 8.32 × 102 to 8.32 × 107 gene copies per reaction were determined by real-time PCR, respectively. Linearity of two assays of DHAV-C (B) and GAPDH (D) spanned 6 orders of magnitude. The slope of the curve (logarithmic dilution versus threshold cycle) and the intercept are given in the equation in the figure. In the linear equation, the X represents the log of the starting quantity and the Y represents the threshold cycle. R2, correlation coefficient; E, efficiency.
Specificity of the real-time PCR.
All 17 DHV-C strains were able to detect the DHAV-C RNA, while other non-DHAV-C viruses and bacteria, including 2 negative-control samples, presented no amplification by the real-time PCR. The results are shown in Table 1.
Sensitivity of the real-time PCR.
The detection limit of the real-time PCR developed in this study was about 3.36 × 103 copies per reaction for DHAV-C cDNA. The conventional RT-PCR was performed with the same primers as were used for real-time PCR, and the detection limit was about 3.36 × 104 copies per reaction for DHAV-C cDNA. Thus, the sensitivity of the real-time PCR was 10 times higher than that of the conventional RT-PCR.
Repeatability and reproducibility test.
The interassay and intra-assay coefficients of variation (CVs) for the detection of the three positive samples were calculated to be within the ranges of 1.02% to 1.77% and 0.15% to 2.0%, respectively, for viral cDNA (Table 2).
Table 2.
Repeatability and reproducibility of the assay for selected samples containing DHAV-Ca
| Species | Intra-assay result (CT) |
SD | % CV | Interassay result (CT) |
SD | % CV | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample 1 | Sample 2 | Sample 3 | Mean | Sample 1 | Sample 2 | Sample 3 | Mean | |||||
| SWUN3501 | 13.48 | 13.51 | 13.52 | 13.50 | 0.021 | 0.15 | 13.50 | 13.73 | 13.26 | 13.50 | 0.26 | 1.74 |
| SWUN3502 | 15.50 | 15.67 | 15.54 | 15.57 | 0.089 | 0.57 | 15.57 | 15.37 | 15.26 | 15.40 | 0.16 | 1.02 |
| SWUN3503 | 17.04 | 16.40 | 16.56 | 16.67 | 0.333 | 2.0 | 16.67 | 16.83 | 17,25 | 16.92 | 0.30 | 1.77 |
All repeat assays were performed at the same time and under the same conditions. SD, standard deviation; CV, coefficient of variation.
Detection and validation of clinical sample results.
Twenty-two (57.9%) DHAV-C RNAs were identified from a total of 38 clinical samples by real-time PCR detection. Moreover, other, non-DHAV-C RNAs (16/38 [42.1%]) were further identified in samples as DHAV-A positive. The results of virus isolation from 15 DHAV-C-positive samples were all confirmed as positive by the real-time PCR assay.
Dynamic and virus titer results in experimentally infected ducklings.
The DHAV-C was successfully used to artificially infect SPF ducklings in this experiment. Clinical symptoms were observed at 24 h postinfection, and the SPF ducklings began to die at 32 h postinfection, with pathological changes typical of duck hepatitis.
As shown in Fig. 3, DHAV-C was first detected in the liver at 12 h postinfection. DHAV-C could be detected in the heart, liver, spleen, kidney, and intestine tissues at 18 h postinfection. Viral cDNA was detected in all samples of the infected ducklings from 24 h postinfection. Furthermore, high levels of virus titers were detected in all samples from 24 to 72 h postinfection. The positive detection rates of the virus are shown in Table 3.
Fig 3.
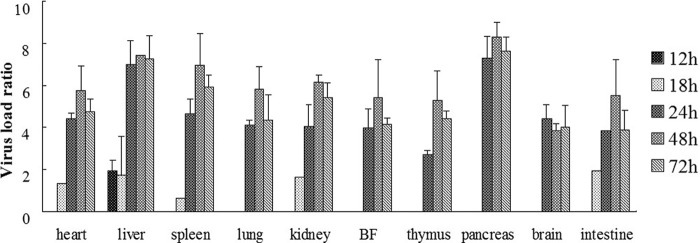
Virus titer of DHAV-C in the internal organs of experimentally infected ducklings. Seven organs were obtained from the experimentally infected ducklings, and viral loads were quantified by the real-time PCR at 1 to 72 h postinfection. One of the spleen samples at 18 h generated the lowest normalized CT values (ΔCT), and this was used to calibrate the data. The calibrated quantification ratios assessed by 2−ΔΔCT were converted into logarithmic values, and the results are shown in histograms. All histograms are arranged according to the time after infection (i.e., 12 to 72 h). The virus titer was not detected at 1 and 6 h postinfection.
Table 3.
The rates of detection of DHAV-C in different organs of experimentally infected SPF ducklings
| Organ | No. of positive real-time PCR results/total. no. of results at indicated time (h) |
||||||
|---|---|---|---|---|---|---|---|
| 1 | 6 | 12 | 18 | 24 | 48 | 72 | |
| Brain | 0 | 0 | 0 | 0 | 3/3 | 3/3 | 3/3 |
| Bursa of Fabricius | 0 | 0 | 0 | 0 | 3/3 | 3/3 | 3/3 |
| Heart | 0 | 0 | 0 | 1/3 | 3/3 | 3/3 | 3/3 |
| Intestine | 0 | 0 | 0 | 1/3 | 3/3 | 3/3 | 3/3 |
| Kidney | 0 | 0 | 0 | 1/3 | 3/3 | 3/3 | 3/3 |
| Lung | 0 | 0 | 0 | 0 | 3/3 | 3/3 | 3/3 |
| Liver | 0 | 0 | 3/3 | 2/3 | 3/3 | 3/3 | 3/3 |
| Pancreas | 0 | 0 | 0 | 0 | 3/3 | 3/3 | 3/3 |
| Spleen | 0 | 0 | 0 | 1/3 | 3/3 | 3/3 | 3/3 |
| Thymus | 0 | 0 | 0 | 0 | 3/3 | 3/3 | 3/3 |
DISCUSSION
The epidemiology, clinical symptoms, and pathological changes of DHAV-C infection are very similar to those of DHAV-A infections, making molecular biological methods necessary to distinguish DHAV-C infection from other DHV infections (8, 16). DHAV strains were categorized into three distinct genetic groups based on the phylogenetic analysis of complete VP1, VP0, and VP3 and partial 3D sequences (24). To screen the molecular targets for detecting DHAV-C, we analyzed 42 genome sequences of DHAV, including 20 DHAV-C, 20 DHAV-A, and 2 DHAV-B sequences published in GenBank. The analysis of phylogenetic relationships of the 2C genes of three different genotypes of DHAV showed that the three genetic groups of DHAV could be clearly distinguished based on 2C nucleotide sequences. The result was in agreement with the analysis of phylogenetic relationships based on other regions of the DHAV genome (24). However, the mutation rate of 2C gene nucleotides was significantly lower than that of other regions in the DHAV-C genome, making this gene a potential target for molecular detection of DHAV-C. Based on the bioinformation analysis described above, a real-time PCR assay was established for detecting DHAV-C in this study. This assay was sensitive and specific, enabling it to be used a practical technique for rapid diagnosis and pathogen monitoring of DHAV-C infection.
In this study, 38 clinical liver samples, which were collected from April 2007 to July 2011 in China from sick ducklings suspected of duck hepatitis virus infection, were tested by the real-time PCR assay. Interestingly, all 22 samples collected from September 2009 to July 2011 were determined as representing DHAV-C infection. Nevertheless, the 16 liver samples collected before September 2009 were negative for DHAV-C and were later identified as representing DHAV-A infection. No coinfections by both DHAV-A and DHAV-C were found in this study. The results presented above suggest that DHAV-C is a recent epidemic DHV strain in China, which is consistent with reports about the epidemiology of DHV in other regions of China (2, 7), although the number of samples investigated in this study was limited. In recent years, the spread of DHAV-C has represented a serious threat and has had a significant economic impact on the duck industry in China. Moreover, the report of the prevalence of DHAV-C in South Korea showed that DHAV-C has spread in Asia (12). Hence, the development of rapid methods for detection of the strain from clinical samples is important for pathogenic diagnosis and disease control.
The dynamic quantitative detection of viral loads in tissues is useful for understanding the pathogenicity of viruses and determination of the target tissues collected for clinical virus detection (17, 18). The results showed that high DHAV-C loads were detected in various tissues and organs at 24 h postinfection and suggested that DHAV-C is a pantropic virus and causes extensive tissue damage in ducklings. In this study, the DHAV-C was first detected in liver, and persistence in this organ at high levels suggests that the liver is an ideal target organ for detecting this virus. In addition, the viral loading in the brain clearly showed that the DHAV-C could directly invade the central nervous system of ducklings, which also explained the presence of neurologic signs, including ataxia and opisthotonos, in the sick ducklings infected by DHAV-C. Interestingly, no obviously visible pathological change was observed in immune organs, but presence of the virus could be determined in the thymus, bursa of Fabricius, and spleen, which suggests that the immune function of duckling might be affected by DHAV-C. Collectively, the data in this report provide a valuable basis for researching pathogenicity and for prevention and control of DHAV.
In conclusion, a real-time PCR assay for detecting DHAV-C was developed in this study. The assay could be applied as a rapid, sensitive, and specific molecular tool for diagnosis of DHAV-C infection and for epidemiological surveys.
ACKNOWLEDGMENTS
We gratefully acknowledge Anchun Chen (Sichuan Agricultural University, China) for kindly presentation of muscovy parvovirus and gosling new type viral enteritis virus. We also thank Hanchun Yang and Dabin Zhang (China Agricultural University, China) for kindly presentation of duck astrovirus 1 and duck astrovirus.
This work was supported by funding from the National Hightech R&D Program (863 Program-2012AA101304) and by Veterinary Medicine Discipline Program of Southwest University for Nationalities (2011XWD-S0906).
Footnotes
Published ahead of print 1 August 2012
REFERENCES
- 1. Asplin FD. 1965. Duck hepatitis: vaccination against two serological types. Vet. Rec. 77:1529–1530 [PubMed] [Google Scholar]
- 2. Fan SC, et al. 2009. Isolation and characterization of a new serotype of duck hepatitis virus. Chin. J. Prev. Vet. Med. 31:770–775 (In Chinese.) [Google Scholar]
- 3. Fauquet CM, Mayo A, Maniloff J, Desselberger U, Ball LA. 2005. Virus taxonomy: VIIIth report of the International Committee on Taxonomy of Viruses. Elsevier Academic Press, San Diego, CA [Google Scholar]
- 4. Fu Y, et al. 2008. Molecular detection and typing of duck hepatitis A virus directly from clinical specimens. Vet. Microbiol. 131:247–257 [DOI] [PubMed] [Google Scholar]
- 5. Gough RE, Collins MS, Borland E, Keymer LF. 1984. Astrovirus-like particles associated with hepatitis in ducklings. Vet. Rec. 114:279. [DOI] [PubMed] [Google Scholar]
- 6. Haider SA, Calnek BW. 1979. In vitro isolation, propagation, and characterization of duck hepatitis virus type III. Avian Dis. 23:715–729 [PubMed] [Google Scholar]
- 7. He RY, Yu M, Zhang YL, Zhang DY, Cao ZX, Zhang GH. 2010. Epidemiological investigation and genetic variation in VP1 gene of duck hepatitis virus isolates from in southwestern China in 2007-2009. Chin. J. Anim. Infect. Dis. 18:7–15 (In Chinese.) [Google Scholar]
- 8. Kim MC, et al. 2009. Development of duck hepatitis A virus type 3 vaccine and its use to protect ducklings against infections. Vaccine 27:6688–6694 [DOI] [PubMed] [Google Scholar]
- 9. Kim MC, et al. 2006. Molecular analysis of duck hepatitis virus type 1 reveals a novel lineage close to the genus Parechovirus in the family Picornaviridae. J. Gen. Virol. 87:3307–3316 [DOI] [PubMed] [Google Scholar]
- 10. Kim MC, et al. 2007. Recent Korean isolates of duck hepatitis virus revealed the presence of a new geno- and serotype when compared to duck hepatitis virus type 1 type strains. Arch. Virol. 152:2059–2072 [DOI] [PubMed] [Google Scholar]
- 11. Kim MC, et al. 2007. Development of one-step reverse transcriptase-polymerase chain reaction to detect duck hepatitis virus type 1. Avian Dis. 51:540–545 [DOI] [PubMed] [Google Scholar]
- 12. Kim MC, Kwon YK, Joh SJ, Kwon JH, Lindberg AM. 2008. Differential diagnosis between type-specific duck hepatitis virus type 1 (DHV-1) and recent Korean DHV-1-like isolates using a multiplex polymerase chain reaction. Avian Pathol. 37:171–177 [DOI] [PubMed] [Google Scholar]
- 13. Levine PP, Fabricant J. 1950. A hitherto-undescribed virus disease of ducks in North America. Cornell Vet. 40:71–76 [Google Scholar]
- 14. Levine PP, Hofstad MS. 1945. Duck disease investigation. Annu. Rep. New York State Vet. Coll. 1945:55–56 [Google Scholar]
- 15. Liu J, et al. 2011. Dynamic distribution and tissue tropism of classical swine fever virus in experimentally infected pigs. Virol. J. 8:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu M, et al. 2011. Goose haemorrhagic hepatitis caused by a new subtype duck hepatitis type 1 virus. Vet. Microbiol. 152:280–283 [DOI] [PubMed] [Google Scholar]
- 17. Miao LF, Zhang CF, Chen CM, Cui SJ. 2009. Real-time PCR to detect and analyze virulent PPV loads in artificially challenged sows and their fetuses. Vet. Microbiol. 138:145–149 [DOI] [PubMed] [Google Scholar]
- 18. Qi X, et al. 2009. Replication kinetics of duck virus enteritis vaccine virus in ducklings immunized by the mucosal or systemic route using real-time quantitative PCR. Res. Vet. Sci. 86:63–67 [DOI] [PubMed] [Google Scholar]
- 19. Tauraso NM, Coghill GE, Klutch MJ. 1969. Properties of the attenuated vaccine strain of duck hepatitis virus. Avian Dis. 13:321–329 [PubMed] [Google Scholar]
- 20. Todd D, et al. 2009. Identification of chicken enterovirus-like viruses, duck hepatitis virus type 2 and duck hepatitis virus type 3 as astroviruses. Avian Pathol. 38:21–30 [DOI] [PubMed] [Google Scholar]
- 21. Toth TE. 1969. Studies of an agent causing mortality among ducklings immune to duck virus hepatitis. Avian Dis. 13:834–846 [PubMed] [Google Scholar]
- 22. Tseng CH, Knowles NJ, Tsai HJ. 2007. Molecular analysis of duck hepatitis virus type 1 indicates that it should be assigned to a new genus. Virus Res. 123:190–203 [DOI] [PubMed] [Google Scholar]
- 23. Tseng CH, Tsai HJ. 2007. Molecular characterization of a new serotype of duck hepatitis virus. Virus Res. 126:19–31 [DOI] [PubMed] [Google Scholar]
- 24. Wang L, Pan M, Fu Y, Zhang D. 2008. Classification of duck hepatitis virus into three genotypes based on molecular evolutionary analysis. Virus Genes 37:52–59 [DOI] [PubMed] [Google Scholar]
- 25. Yang M, Cheng A, Wang M, Xing H. 2008. Development and application of a one-step real-time Taqman RT-PCR assay for detection of duck hepatitis virus type1. J. Virol. Methods 153:55–60 [DOI] [PubMed] [Google Scholar]