Abstract
Methicillin-resistant Staphylococcus aureus (MRSA) originated from the health care setting but is now emerging in communities without health care contact (CA-MRSA) or in livestock (LA-MRSA). The impact on the whole MRSA population was assessed in a German prospective multicenter study. Thirty-three laboratories consecutively collected up to 50 MRSA isolates from infection or carriage during two sampling periods in 2004 to 2005 and 2010 to 2011. Patient-related data were collected using a standardized questionnaire. Methicillin resistance was confirmed by the detection of mecA or its homologue mecALGA251. The spa type and major virulence factors were analyzed for each isolate. In total, 1,604 (2004 to 2005) and 1,603 (2010 to 2011) MRSA isolates were analyzed; one isolate from each sampling period harbored mecALGA251. LA-MRSA increased significantly (odds ratio [OR] = 22.67, 95% confidence interval [CI] = 8.51 to 85.49, P < 0.0005) and spread over Germany, originating from northwestern regions. Panton-Valentine leukocidin-positive CA-MRSA rose significantly, particularly in southern Germany, but the proportion in 2010 to 2011 remained low (2.7%, OR = 2.80, 95% CI = 1.54 to 5.34, P < 0.0005). The emerging MRSA clones changed the MRSA population in Germany during a 6-year period significantly. The ongoing epidemiological shift and changes of MRSA sources create a need for revision of guidelines for MRSA infection control and treatment.
INTRODUCTION
Staphylococcus aureus is a bacterium causing skin and soft tissue infection, pneumonia, meningitis, endocarditis, and osteomyelitis. The first antistaphylococcal agent was penicillin, but soon after its introduction penicillin-resistant S. aureus spread globally in the 1950s and 1960s (9). Subsequently, members of the penicillinase-stable penicillins (i.e., methicillin) became the most important antibiotic drug for the treatment of S. aureus infection. However, the first methicillin-resistant S. aureus (MRSA) isolate was detected in England in 1961, and MRSA has spread throughout the world since then (14).
In Germany, MRSA isolates were reported for the first time in the 1970s and 1980s and comprised hospital-associated MRSA (HA-MRSA) (19, 27). German HA-MRSA isolates are clonal and mainly belong to S. aureus protein A locus (spa) types t002, t003, t008, and t032, which are associated with sequence types ST5, ST225, ST8, and ST22, as determined by multilocus sequence typing (MLST) (5). Typical risk factors for HA-MRSA carriage are history of hospitalization, residence in a nursing home, skin lesions, and hemodialysis, as well as indwelling catheters and inserted or implanted foreign bodies (15).
In the 1990s, new MRSA lineages emerged in communities without risk factors for HA-MRSA and spread in different countries to various extents (9). These community-associated MRSA (CA-MRSA) lineages are associated with skin and soft tissue infections and frequently harbor the Panton-Valentine leukocidin (PVL), a pore-forming toxin which lyses human neutrophils (20). While German CA-MRSA isolates are still rare and comprise different clonal lineages, isolates from the United States are dominated by the spread of MLSTs ST1 (USA400) and ST8 (USA300), suggesting a high transmissibility and/or virulence of these clones (8, 9).
In the early 2000s, so-called livestock-associated MRSA (LA-MRSA) emerged in Dutch pig farmers, who may have acquired this new subtype (ST398) from breeding animals (28). In regions with high density of livestock farming, LA-MRSA can be imported into hospitals, causing hospital-acquired infections (13, 15, 28).
The recent detection of a mecA homologue (mecALGA251), which is hitherto undetectable by routine molecular methods, raises the question of its prevalence (11, 18).
To elucidate if and to what extent the emergence of CA-MRSA and LA-MRSA may have changed the MRSA population in Germany, we performed a prospective longitudinal study and compared genotypes and virulence factors of human MRSA isolates from 33 laboratories throughout Germany collected in 2004 to 2005 with isolates obtained in the same study centers in 2010 to 2011.
MATERIALS AND METHODS
Study design.
In total, 36 microbiological laboratories throughout Germany associated with outpatient clinics and primary to tertiary care hospitals were invited to collect prospectively MRSA isolates from routine diagnostics. In two sampling periods (1 February 2004 to 31 January 2005 and 1 February 2010 to 31 January 2011), every laboratory collected the first 50 MRSA isolates detected irrespective of the type of specimen. Only one isolate per patient was included, and the laboratories were asked to indicate whether the sample was obtained from a case associated with clinical signs of an S. aureus infection. A standardized questionnaire was completed for each isolate, including demographic data of the patient (age, gender) and sample-related data (date of sampling, sample material, in- or outpatient care, risk factors for MRSA colonization or infection, type of S. aureus infection). Samples and questionnaires were sent to the Institute of Medical Microbiology, University Hospital Münster, Münster, Germany, for molecular characterization, data entry, and analysis. Study centers which collected MRSA isolates during one sampling period only were excluded from the analysis.
Molecular characterization.
Isolates were confirmed to be S. aureus by detection of the S. aureus-specific thermostable nuclease gene (nuc), and methicillin resistance was confirmed by mecA PCR (2). A PCR targeting the mecA homologue mecALGA251 was used for those isolates which were mecA negative but showed phenotypic methicillin resistance (18). Genes encoding the toxic shock syndrome toxin (tst), enterotoxins (sea, seb, sec, sed, see, seg, seh, sei, sej), exfoliative toxins (eta, etb, etd), and the epidermal cell differentiation inhibitors (edin-A, edin-B, edin-C) were detected by multiplex PCRs (1, 3, 29, 30). Subtypes of the accessory gene regulator (agr I to IV) were detected by PCR (26). Sequence-based typing of the hypervariable region of the S. aureus protein A locus (spa typing) was performed for each isolate (21). Related spa types were clustered in spa clonal complexes (spa-CC) using the BURP (based upon repeat pattern) algorithm and applying the default parameters (x = 5, y = 4) as implemented in Ridom StaphType 2.2.1 (21). Multilocus sequence typing was performed for new spa types which were among the 10 most frequent spa types (10).
Statistical analyses.
Data were analyzed with the software “R,” version 2.13.1 (http://cran.r-project.org), and the package epicalc. Continuous variables were compared using Student's t test. Categorical variables were compared using the χ2 test and the odds ratio (OR) and its 95% confidence interval (95% CI). The significance level was 0.05. Missing values were removed from the statistical analyses.
RESULTS
Study population.
All 36 invited study centers participated in the first sampling period, 2004 to 2005, and 33 centers also took part in the second sampling period, 2010 to 2011. The three centers which did not contribute during the second period were excluded from the analysis. The majority of isolates were collected in northern, western, and southern Germany. While the mean ages and the proportions of females did not differ between the first and second sampling periods, there was a significant increase in patients with risk factors for MRSA colonization in 2010 to 2011 (Table 1).
Table 1.
Characteristics of MRSA patients in 2004 to 2005 and 2010 to 2011
| Parameter | Resulta for MRSA patients from: |
OR (95% CI) | P | |
|---|---|---|---|---|
| 2004–2005 (n = 1,604) | 2010–2011 (n = 1,603) | |||
| Mean age (yr) ± SD | 64.9 ± 18.9 | 65.3 ± 20.4 | NA | 0.65 |
| Proportion female | 42.4 (680) | 42.8 (671) | 1.01 (0.88–1.17) | 0.84 |
| Risk factors for HA-MRSA | ||||
| History of MRSA infection/colonization | 22.4 (219) | 30.2 (219) | 1.5 (1.2–1.88) | <0.0005 |
| Hemo-/peritoneal dialysis in the past 12 mo | 7.6 (63) | 11.5 (59) | 1.58 (1.06–2.33) | 0.16 |
| Surgery in the past 12 mo | 52.6 (442) | 54.9 (307) | 1.1 (0.88–1.37) | 0.40 |
| Hospitalization in the past 12 mo | 75.9 (635) | 82.8 (512) | 1.54 (1.17–2.02) | 0.001 |
| Residence in a day care or rehabilitation center | 24.7 (198) | 30 (137) | 1.31 (1.00–1.71 | 0.04 |
| MRSA infection | ||||
| Sepsis (foreign body associated) | 3.2 (51) | 2.3 (37) | 0.72 (0.46–1.13) | 0.13 |
| Foreign body-associated infection | 2.1 (33) | 1.6 (26) | 0.78 (0.45–1.36) | 0.36 |
| Pneumonia | 7.5 (120) | 3.6 (57) | 0.46 (0.32–0.64) | <0.0005 |
| Abscess | 1.4 (22) | 1.7 (27) | 1.23 (0.67–2.28) | 0.47 |
| Meningitis | 0.06 (1) | 0 (0) | 0 (0–39.02) | 1 |
| Surgical site infection | 5.9 (94) | 3.1 (49) | 0.51 (0.35–0.73) | <0.0005 |
| Skin and soft tissue infection | 21.1 (339) | 12.4 (199) | 0.53 (0.43–0.64) | <0.0005 |
| Urinary tract infection | 3.2 (51) | 2.1 (33) | 0.64 (0.40–1.02) | 0.05 |
| Osteomyelitis | 1.3 (21) | 0.3 (4) | 0.19 (0.05–0.56) | 0.001 |
| MRSA asymptomatic colonization | 36.7 (589) | 30.1 (483) | 1.35 (1.16–1.56) | <0.0005 |
| Careb | ||||
| Outpatient department | 19.5 (312) | 20.8 (333) | 0.92 (0.77–1.10) | 0.35 |
| Intensive care unit | 19.2 (308) | 15.5 (249) | 1.29 (1.07–1.56) | 0.006 |
| Dialysis department | 0 (0) | 0.6 (10) | 0 (0–0.44) | <0.0005 |
| Hemato-oncology department | 2.1 (33) | 1.2 (19) | 1.75 (0.96–3.27) | 0.05 |
| General care wards | 59.3 (951) | 56.2 (901) | 1.13 (0.98–1.31) | 0.08 |
Values are % (no.) except for ages.
For 91 persons in 2010 to 2011, the type of care was not reported.
MRSA typing.
In total, 3,207 MRSA isolates were collected during the first (n = 1,604) and the second (n = 1,603) sampling periods. Of these, 1,236 isolates derived from infections and 1,072 from asymptomatic carriers. A clear attribution to “infection” or “asymptomatic carriage” was not possible for 899 isolates. All isolates were nuc positive and—with two exceptions—harbored the classical mecA gene. One isolate in each sampling period harbored mecALGA251 (spa type t843). The first isolate was recovered from sputum of a patient with S. aureus pneumonia, and the second isolate was isolated from a skin/mucosal swab for MRSA colonization of a patient who suffered from chronic respiratory tract disease.
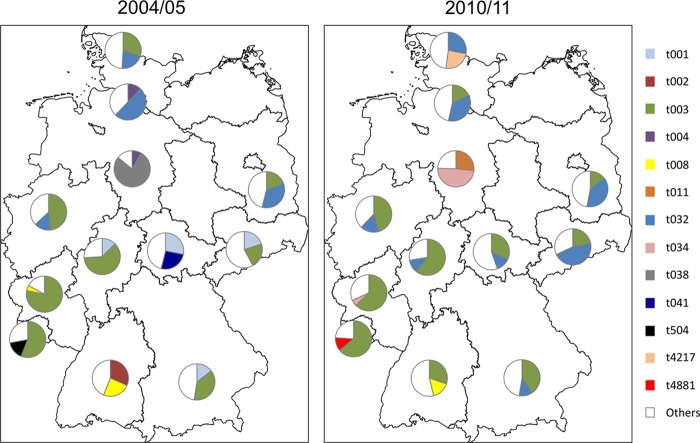
Isolates of the first sampling period comprised 127 different spa types (two MRSA isolates [0.1%] were nontypeable), and isolates of the second period were associated with 236 different spa types (nine MRSA isolates [0.6%] were nontypeable) (see Table S1 in the supplemental material). The distributions of spa types stratified in spa-CCs in isolates from infection and carriage were similar. The proportion of classical HA-MRSA spa types (t003 and t032) did not change between 2004 to 2005 and 2010 to 2011 (Table 2). It is noteworthy that spa type t008 has mainly spread in southern Germany and decreased slightly in 2010 to 2011 (Table 2; Fig. 1). Isolates belonging to spa type t008 and t002 were predominant in southwest Germany in 2004 to 2005 (Fig. 1). Isolates of spa type t002 decreased in the whole country from 6.86% (n = 110) to 3.18% (n = 51) in 2010 to 2011. Isolates belonging to spa type t008 decreased from 7.48% (n = 120) in 2004 to 2005 to 4.49% (n = 72) in 2010 to 2011 (Table 2). Similarly, some of the most prevalent spa types in southeast Germany (t001), northern Germany (t004), Lower Saxony (t038), and central Germany (t041) were no longer predominant in 2010 to 2011 (Fig. 1). In 2010 to 2011, new MRSA isolates with spa types that are infrequent so far emerged in northern Germany (t4217, spa-CC 032, ST22; n = 12) and in southwest Germany (t4881, spa-CC 003, ST225; n = 6) (Fig. 1; see Table S1 in the supplemental material).
Table 2.
Most frequent spa types of MRSA isolates from 2004 to 2005 and 2010 to 2011
| Rank | 2004–2005 |
2010–2011 |
||||||
|---|---|---|---|---|---|---|---|---|
| spa type | MLST(s)a | Frequency [% (no.)] | Cumulative frequency (%) | spa type | MLST(s)a | Frequency, % (no.) | Cumulative frequency (%) | |
| 1 | t003 | ST5, ST225 | 40.15 (644) | 40.15 | t003 | ST5, ST225 | 39.86 (639) | 39.86 |
| 2 | t032 | ST22 | 15.59 (250) | 55.74 | t032 | ST22 | 15.53 (249) | 55.4 |
| 3 | t008 | ST8, ST247, ST250, ST254 | 7.48 (120) | 63.22 | t008 | ST8, ST247, ST250, ST254 | 4.49 (72) | 59.88 |
| 4 | t002 | ST5, ST231 | 6.86 (110) | 70.08 | t002 | ST5, ST231 | 3.18 (51) | 63.06 |
| 5 | t001 | ST5, ST222, ST228 | 5.93 (95) | 76.01 | t034 | ST398b | 2.56 (41) | 65.62 |
| 6 | t004 | ST45 | 4.05 (65) | 80.06 | t011 | ST398b | 2.37 (38) | 67.99 |
| 7 | t041 | ST111, ST228 | 1.50 (24) | 81.56 | t045 | ST5, ST225 | 1.75 (28) | 69.74 |
| 8 | t038 | ST45 | 1.00 (16) | 82.56 | t014 | ST672, ST225c | 1.31 (21) | 71.05 |
| 9 | t022 | ST22 | 0.69 (11) | 83.25 | t8374 | ST22d | 1.19 (19) | 72.24 |
| 10 | t023 | ST22 | 0.69 (11) | 83.94 | t022 | ST22 | 1.19 (19) | 73.43 |
| >10 | Others | —e | 16.03 (258) | 100 | Others | — | 26.58 (426) | 100 |
| Total | 100 (1,604) | 100 (1,603) | ||||||
Associated multilocus sequence types as published on http://spaserver.ridom.de.
Previously published (15).
Previously published (23).
This study.
—, not determined.
Fig 1.
Distribution of the two most prevalent spa types of methicillin-resistant Staphylococcus aureus in Germany. Pie charts show the average prevalence in each federal state.
The proportion of PVL-positive isolates belonging to spa types associated with the classical PVL-positive European CA-MRSA clone (t002, t044) increased from 0.4% (n = 6) in 2004 to 2005 to 0.6% (n = 9) in 2010 to 2011. Similarly, spa types of PVL-positive isolates associated with the USA300 clone (t008) increased from 0.1% (n = 2) in 2004 to 2005 to 0.6% (n = 10) in 2010 to 2011. In total, CA-MRSA isolates rose significantly, particularly in southern Germany (OR = 2.80, 95% CI = 1.54 to 5.34, P < 0.0005).
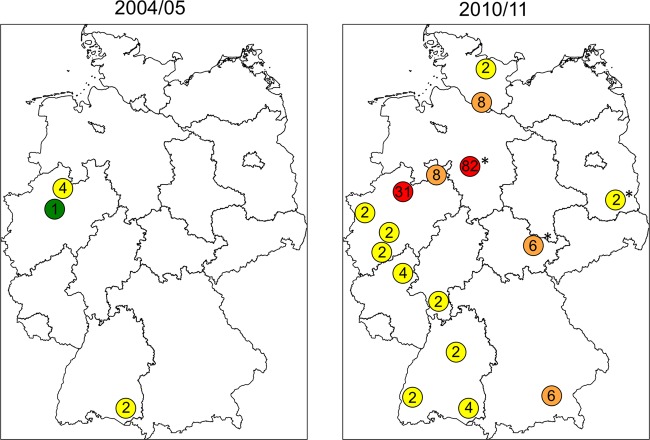
The MRSA isolates associated with spa-CC011 (CC011 is indicative of ST398) belong to t034 and t011 and related spa types according to the BURP analysis (t108, t1451, t2576, t4395t, t8377). Isolates belonging to these spa types increased significantly from 0.3% (n = 4) in 2004 to 2005 to 5.4% (n = 86) in 2010 to 2011 (OR = 22.67, 95% CI = 8.51 to 85.49, P < 0.0005). In 2004 to 2005 these spa types, which are indicative of the LA-MRSA clonal lineage ST398, were—with one exception (Ravensburg, southwestern Germany)—found only in western Germany. These isolates were restricted to spa types t011 (Münster and Ravensburg) and t108 (Bochum). In 2010 to 2011 spa-CC011-associated spa types were detected all over Germany, but still with highest prevalence in northwestern Germany (Fig. 2). Association of these MRSA isolates with infection was reported only for isolates from 2010 to 2011; cases included skin and soft tissue infection (n = 4), foreign body infection (n = 1), sepsis (n = 1), pneumonia (n = 1), surgical site infection (n = 1), and endocarditis (n = 1). No or unknown association with infection was reported for 77 MRSA spa-CC011 isolates, which were isolated from urine (n = 2), sputum (n = 3), or skin/mucosal swabs (n = 72).
Fig 2.
Distribution and spread of livestock-associated methicillin-resistant Staphylococcus aureus in Germany in 2004 to 2005 and 2010 to 2011. The numbers indicate the prevalence (%) of LA-MRSA isolates in the respective study centers (green, 1%; yellow, 2 to 5%; orange, 6 to 10%; red, >10%). Asterisks indicate that only one study center reported from the region.
In 2004 to 2005 the agr II subtype was predominant (62.2%, n = 998) followed by agr I (34.5%, n = 553), agr III (1.8%, n = 28), and agr IV (1%, n = 16). Similarly, agr II was the most frequent subtype in 2010 to 2011 (54.3%, n = 870), followed by agr I (43.4%, n = 696) and agr III (2.2%, n = 35). In 2010 to 2011, agr IV was not detected.
MRSA exotoxin characterization.
Overall, 16 (1%) and 44 (2.7%) PVL-positive MRSA isolates were found in 2004 to 2005 and 2010 to 2011, respectively (Table 3). Taking into account the results of both sampling periods, they were isolated from skin and soft tissue infections (26.7%, n = 16), abscesses (15%, n = 9), pneumonia and postsurgical wound infections (3.3%, n = 2, each), and urinary tract infections (1.7%, n = 1). For 16.7% (n = 10), no association with infection was reported, and for 28.3% (n = 17) PVL-positive isolates, the association with an infection was unclear. These isolates were derived from skin/mucosal swabs (52.9%, n = 9), aspirates/secretions (23.4%, n = 4), wounds (17.7%, n = 3), and other specimens (5.9%, n = 1). In 2004 to 2005, PVL-positive MRSA isolates were more frequently isolated from skin/mucosal swabs than in 2010 to 2011 (75.0% versus 36.7%).
Table 3.
Virulence factors of German MRSA isolates from 2004 to 2005 and 2010 to 2011
| Virulence factor | % (no.) of MRSA isolates froma: |
OR (95% CI) | P | |
|---|---|---|---|---|
| 2004–2005 | 2010–2011 | |||
| lukS-PV/lukF-PV | 1.0 (16) | 2.7 (44) | 2.80 (1.54–5.34) | <0.0005 |
| tst | 9.6 (154) | 0.9 (14) | 0.08 (0.04–0.14) | <0.0005 |
| sea | 13.6 (218) | 6.6 (105) | 0.45 (0.35–0.57) | <0.0005 |
| seb | 0.5 (8) | 0.4 (7) | 0.88 (0.27–2.77) | 0.8 |
| sec | 13.3 (213) | 20.1 (322) | 1.64 (1.35–1.99) | <0.0005 |
| sed | 56.2 (901) | 49.9 (800) | 0.78 (0.67–0.9) | <0.0005 |
| seg | 88.5 (1,419) | 85.0 (1,363) | 0.74 (0.60–0.91) | 0.004 |
| seh | 0.3 (5) | 0.7 (11) | 2.21 (0.71–8.13) | 0.13 |
| sei | 90.2 (1,447) | 85.2 (1,366) | 0.63 (0.50–0.78) | <0.0005 |
| sej | 45.0 (721) | 47.5 (762) | 1.11 (0.96–1.28) | 0.14 |
| eta | 0.4 (6) | 0 (0) | 0 (0–0.85) | 0.03 |
| etb | 0.1 (2) | 0 (0) | 0 (0–5.33) | 0.5 |
| etd | 1.0 (16) | 0.5 (8) | 0.5 (0.18–1.24) | 0.1 |
| edin-A | 0 (0) | 0.06 (1) | Infb (0.03–Inf) | 0.5 |
| edin-B | 0.9 (14) | 0.5 (8) | 0.57 (0.21–1.46) | 0.2 |
| hlg | 99.8 (1,600) | 100 (1,603) | Inf (0.66–Inf) | 0.13 |
Percentages are given as relation to the respective evaluable questionnaire data.
Inf, infinity.
The risk factors history of hospitalization and MRSA detection in specimens obtained more than 48 h after admission were significantly less frequently reported for PVL-positive MRSA isolates than for PVL-negative isolates (Table 4). Furthermore, the mean number of risk factors for health care-associated MRSA (HA-MRSA) colonization in persons with PVL-positive isolates was 0.7 (range, 0 to 4) compared to 1.2 (range, 0 to 6) for persons with PVL-negative isolates (P = 0.001). Only the risk factor proportion of persons who had hemodialysis or peritoneal dialysis in the past 12 months was more prevalent in the PVL-positive group (Table 4).
Table 4.
Risk factors for HA-MRSA colonization in PVL-positive isolates
| Risk factor | % (no.) of patients with: |
OR (95% CI) | P | |
|---|---|---|---|---|
| PVL-positive MRSA | PVL-negative MRSA | |||
| History of MRSA colonization | 20 (6) | 25.9 (432) | 0.72 (0.24–1.82) | 0.47 |
| MRSA detection >48 h after admission | 27.6 (8) | 52.1 (875) | 0.35 (0.13–0.83) | 0.01 |
| Dialysis in the past 12 mo | 13 (3) | 9.0 (119) | 1.51 (0.28–5.21) | 0.46 |
| Surgery in the past 12 mo | 42.9 (9) | 53.7 (740) | 0.65 (0.24–1.68) | 0.32 |
| Hospitalization in the past 12 mo | 50 (11) | 79.3 (1,136) | 0.26 (0.1–0.67) | 0.001 |
| Residence in a nursing home or rehabilitation center in the past 12 mo | 14.3 (3) | 26.8 (332) | 0.45 (0.09–1.57) | 0.32 |
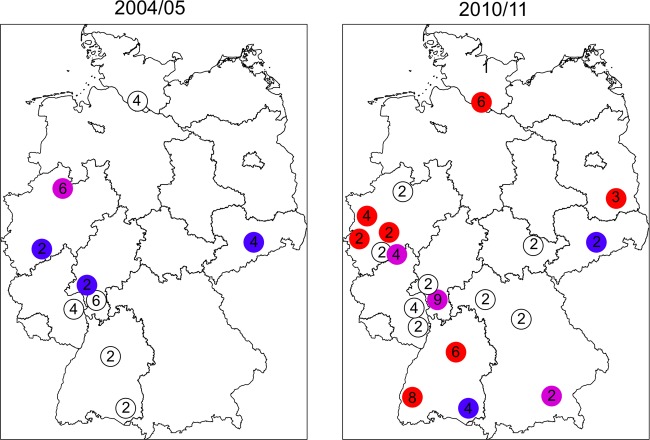
PVL-positive isolates from 2004 to 2005 belonged to spa types/STs t044/ST80 (n = 5), t003/ST5 (n = 2), and t008/ST8 (n = 2), followed by t001, t002, t019, t032, t041, t808, and t322 (n = 1 each) (Fig. 3). In 2010 to 2011, PVL-positive isolates belonging to spa types/STs t008/ST8 (n = 10), t044/ST80 (n = 5), t002/ST5 (n = 4), and t318/ST30 (n = 4) became the most prevalent, followed by t437 (n = 3); t003, t019, t203, and t4741 (n = 2 each); and t005, t016, t045, t657, t692, t791, t967, t2518, t7656, and t8376 (n = 1 each). PVL-positive MRSA isolates were present in four major spa-CCs (spa-CC003, -008, -012, and -032).
Fig 3.
Distribution and spread of PVL-positive methicillin-resistant Staphylococcus aureus in Germany in 2004 to 2005 and 2010 to 2011. The numbers indicate the proportions (%) of PVL-positive MRSA isolates in the respective study centers. Colors indicate detection of ST8 (red), ST80 (blue), ST8 and ST80 (violet), and non-ST8/non-ST80 (white).
DISCUSSION
This is the first systematic prospective and nationwide multicenter study analyzing the molecular background and exotoxin equipment of MRSA isolates in Germany. Main findings are an increasing proportion of LA-MRSA and PVL-positive MRSA isolates between 2004 to 2005 and 2010 to 2011.
We detected an increased proportion of patients with risk factors for HA-MRSA colonization. The higher proportion of patients who had been hospitalized in the past 12 months in 2010 to 2011 may reflect the 8.3% increase of inpatient cases in German hospitals from 2004 (20,365 cases per 100,000 inhabitants) to 2010 (22,057 cases per 100,000 inhabitants) (24). In contrast, the increased proportion of patients with a history of MRSA infection/colonization in 2010 to 2011 does not reflect the stable or decreasing proportion of MRSA infection/colonization in recent years (19.5 to 21.2% between 2004 and 2009) (17, 22).
The vast majority of MRSA isolates harbored the classical mecA gene; only two isolates (0.06%) with the mecA homologue mecALGA251 were collected in 2004 to 2005 and 2010 to 2011. Similarly low rates of mecALGA251-positive MRSA isolates were also reported in retrospective analyses of German strain collections (6, 18). The two isolates from our study belonged to spa type t843, which is the most frequent spa type in mecALGA251-positive isolates from cows and humans in Great Britain and Denmark (11). While the proportion of mecALGA251 MRSA isolates seemed to increase in Denmark from 2008 (0.12%) to 2010 (0.67%), we observed a continuously low prevalence (0.06%) (11). There is evidence, at least for one isolate, which was isolated from a pneumonia patient, that MRSA harboring mecALGA251 can cause clinical infection.
The major MRSA lineages in Germany, which are traditionally named after the region of their first occurrence, comprise the Rhine-Hessen MRSA (t002/ST5 and t003/ST225), the Barnim MRSA (t032/ST22), and the northern German MRSA clone (t008/ST8). The proportion of the classical HA-MRSA lineages (t003/ST225 and t032/ST22) was stable during the study period (Table 2), which is consistent with recent reports (5). The origin of the emerging MRSA spa type t4217/ST22 in northern Germany in 2010 to 2011 is unclear as this spa type is not associated with published outbreaks in this area and as its occurrence cannot be explained by a hypothetical cross-border transfer from Denmark. Similarly, the source of MRSA t4881/ST225 in southwest Germany (Fig. 1) is unclear. A cross-border import of these isolates from France is unlikely, as the major French MRSA clones belong to t008/ST8, t024/ST8, and t777/ST5 (12). The t4881 clone might have evolved from t504, which was the second most common spa type in this region in 2004 to 2005. Since MRSA t504 (26-17-20-17-12) and t4881 (26-17-20-17-13-17-17-16) have similar repeat patterns, a microevolution from t504 to t4881 is possible, although deletions of spa repeats are more frequent than duplications in spa type alterations (4).
Importantly, spa types associated with spa-CC011 (t011, t034) are presumptively LA-MRSA within the MLST clonal complex 398. While LA-MRSA isolates were initially detected in commercially raised pigs, their spread to other animals (cattle, chicken, horses, and pets) as well as farmers, veterinarians, and other exposed persons has been reported (7). Here, we show a significantly increasing prevalence of LA-MRSA-associated spa types from 0.3% (2004 to 2005) to 5.4% (2010 to 2011) (28). However, the prevalence of LA-MRSA was particularly high in northwestern Germany, which is characterized by a high density of livestock production (Lower Saxony, North Rhine-Westphalia). Proportions of up to 20% of all MRSA isolates have been reported from “pig-dense” regions, consistent with our results (16). However, the high prevalence in Lower Saxony (82%) (Fig. 2) might be questionable, as only one laboratory reported results from this region.
CA-MRSA isolates frequently harbor PVL-encoding genes and are highly prevalent in the United States but are still rare in Germany. In our comparative analysis, we showed that the proportion of PVL-positive isolates among MRSA isolates has significantly increased between 2004 to 2005 (1.0%) and 2010 to 2011 but that the proportion remains low (2.7%) (Table 3). While the European CA-MRSA clone (t044, ST80 [17]) was the most prevalent PVL-positive MRSA clone in 2004 to 2005, the USA300 clone (t008, ST8 [17]) became predominant in 2010 to 2011. Interestingly, in both sampling periods increasing high proportions of PVL-positive MRSA isolates were found in the cities of Hamburg (2004 to 2005, 2.0%; 2010 to 2011, 6.1%) and Frankfurt am Main (2004 to 2005, 6.5%; 2010 to 2011, 8.8%). These metropolitan areas have a high population density and are international aviation hubs. Several studies have shown that PVL-positive MRSA and methicillin susceptible S. aureus can be imported and spread through international travel (25, 31).
The different proportions of genes encoding members of the pyrogenic toxin superantigen (PTSAg) family in 2004 to 2005 compared to 2010 to 2011 might be due to changes in dominating clonal lineages. In particular, the decrease of tst and enterotoxin gene-harboring strains might be explained by the advent of LA-MRSA CC398 isolates which only sporadically harbor these PTSAgs.
Our study comprises nationwide data from laboratories which perform microbiological diagnostics for outpatient care and for primary to tertiary care hospitals and includes isolates from carriage and infection. Since the same laboratories participated in both sampling periods, we controlled for the geographical bias (12). As we included only consecutively collected samples, we ruled out a reporting bias.
One limitation of our study is the underrepresentation of isolates from the eastern part of Germany, especially from the capital of Berlin, the inclusion of which might have added important information regarding the prevalence of PVL-positive isolates in urban areas. Second, we did not assess the contact to animals as a risk factor for MRSA carriage or infection in 2004 to 2005, because there was only a limited awareness of this MRSA reservoir in that time.
In conclusion, the emergence of novel MRSA clonal lineages highlights the possibility of substantial changes in MRSA epidemiology within relatively short time periods.
Supplementary Material
ACKNOWLEDGMENTS
The following persons (in alphabetical order) in Germany were participants of the study group: M. Abele-Horn, Würzburg; Aepfelbacher, Hamburg; F. Albert, Erlangen; A. Anders, Bochum; W. Bär and B. Beyreiß, Cottbus; G. Bierbaum, Bonn; U. Bührlen, Stuttgart; K. H.-U. Borg, Hamburg; Claußen, Hannover; C. Diaz, Cologne; A. Ditzen, Dresden; M. Dobonici, Ludwigshafen; U. Eigner, Heidelberg; H. Erichsen, Moers; A. Fahr, Heidelberg; P. Finzer, Moers; U. Frank, Freiburg; C. Freytag, Bad-Oeynhausen; M. Frosch, Würzburg; G. Funke, Ravensburg; S. Gatermann, Bochum; J. Geisen, Cologne; W. Hell, Koblenz; M. Herrmann, Homburg; U. Höffler, Ludwigshafen; E. Jacobs, Dresden; B. Jansen, Mainz; D. Jonas, Freiburg; M. Kaase, Bochum; M. Kaulfers and J. K. Knobloch, Hamburg; M. Kresken and B. Körber-Irrgang, Rheinbach; M. van der Linden, Aachen; A. Lommel and C. Lücking, Ludwigshafen; D. Mack, Hamburg; S. Müller, Würzburg; L. von Müller, Homburg; A. Müller-Chorus, Bochum; K. Noldt, Hamburg; W. Pfister, Jena; T. Regnath, W. Reiter, Stuttgart; J. Rissland, Koblenz; A. Roggenkamp, Münich; U. Rohr, Bochum; E. Rosenthal, Wiesbaden; S. Schade and S. Scherpe, Hamburg; T. Schmidt-Wieland, Ravensburg; C. Schoerner, Erlangen; S. Schubert, Kiel; R. Schwarz, Cologne; K. Schwegmann, Hannover; H. Seifert, Cologne; V. Simon, Münich; E. Straube, Jena; M. Trautmann, Stuttgart; U. Ullmann, Kiel; U. Vogel, Würzburg, M. Vogt, Koblenz; H. von Wulffen, Hamburg; T. Wichelhaus, Frankfurt; M. L. Wimmer-Dahmen, Mönchengladbach; J. Wüllenweber, Homburg; B. Würstl, Münich; W. Kalka-Moll, Mönchengladbach; S. Monecke, Dresden; R. R. Reinert, Aachen; B. Zöllner, Moers.
This work was supported and performed under the auspices of the Paul Ehrlich Gesellschaft für Chemotherapie (K.B. and C.V.E.), working group Staphylococcal infections, and in part supported by grants to R.K., A.W.F., G.P., and K.B. (01KI1014A) within the MedVet-Staph project by the Bundesministerium für Bildung und Forschung.
Footnotes
Published ahead of print 18 July 2012
Supplemental material for this article may be found at http://jcm.asm.org/.
REFERENCES
- 1. Becker K, et al. 2003. Prevalence of genes encoding pyrogenic toxin superantigens and exfoliative toxins among strains of Staphylococcus aureus isolated from blood and nasal specimens. J. Clin. Microbiol. 41:1434–1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Becker K, et al. 2006. Does nasal cocolonization by methicillin-resistant coagulase-negative staphylococci and methicillin-susceptible Staphylococcus aureus strains occur frequently enough to represent a risk of false-positive methicillin-resistant S. aureus determinations by molecular methods? J. Clin. Microbiol. 44:229–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Becker K, Roth R, Peters G. 1998. Rapid and specific detection of toxigenic Staphylococcus aureus: use of two multiplex PCR enzyme immunoassays for amplification and hybridization of staphylococcal enterotoxin genes, exfoliative toxin genes, and toxic shock syndrome toxin 1 gene. J. Clin. Microbiol. 36:2548–2553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boye K, Westh H. 2011. Variations in spa types found in consecutive MRSA isolates from the same patients. FEMS Microbiol. Lett. 314:101–105 [DOI] [PubMed] [Google Scholar]
- 5. Cuny C, Layer F. 2011. Auftreten und Verbreitung von MRSA in Deutschland 2010. Epidemiologisches Bull. 26:233–241 [Google Scholar]
- 6. Cuny C, Witte W. 2011. Auftreten von MRSA mit negativem Nachweis für mecA (PCR) und Penicillin-Bindeprotein PBP2a (Agglutinationstest). Epidemiologisches Bull. 38:351–352 [Google Scholar]
- 7. Cuny C, et al. 2009. Nasal colonization of humans with methicillin-resistant Staphylococcus aureus (MRSA) CC398 with and without exposure to pigs. PLoS One 4:e6800 doi:10.1371/journal.pone.0006800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. David MZ, Daum RS. 2010. Community-associated methicillin-resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clin. Microbiol. Rev. 23:616–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Deleo FR, Otto M, Kreiswirth BN, Chambers HF. 2010. Community-associated meticillin-resistant Staphylococcus aureus. Lancet 375:1557–1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Enright MC, Day NP, Davies CE, Peacock SJ, Spratt BG. 2000. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J. Clin. Microbiol. 38:1008–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. García-Álvarez L, et al. 2011. Meticillin-resistant Staphylococcus aureus with a novel mecA homologue in human and bovine populations in the UK and Denmark: a descriptive study. Lancet Infect. Dis. 11:595–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Grundmann H, et al. 2010. Geographic distribution of Staphylococcus aureus causing invasive infections in Europe: a molecular-epidemiological analysis. PLoS Med. 7:e1000215 doi:10.1371/journal.pmed.1000215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hartmeyer GN, Gahrn-Hansen B, Skov RL, Kolmos HJ. 2010. Pig-associated methicillin-resistant Staphylococcus aureus: family transmission and severe pneumonia in a newborn. Scand. J. Infect. Dis. 42:318–320 [DOI] [PubMed] [Google Scholar]
- 14. Jevons MP. 1961. “Celbenin”-resistant Staphylococci. BMJ 1:124–125 [Google Scholar]
- 15. Köck R, et al. 2009. Prevalence and molecular characteristics of methicillin-resistant Staphylococcus aureus (MRSA) among pigs on German farms and import of livestock-related MRSA into hospitals. Eur. J. Clin. Microbiol. Infect. Dis. 28:1375–1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Köck R, et al. 2011. Characteristics of hospital patients colonized with livestock-associated meticillin-resistant Staphylococcus aureus (MRSA) CC398 versus other MRSA clones. J. Hosp. Infect. 79:292–296 [DOI] [PubMed] [Google Scholar]
- 17. Köck R, et al. 2011. The epidemiology of methicillin-resistant Staphylococcus aureus (MRSA) in Germany. Dtsch. Arztebl. Int. 108:761–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kriegeskorte A, et al. 2012. Human MRSA isolates with novel genetic homolog, Germany. Emerg. Infect. Dis. 18:1016–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lenz W, Eilers E, Lehmacher U. 1988. Characterization of methicillin-resistant Staphylococcus aureus strains isolated from 1974 to 1983 in West Germany with respect to the results of lysotyping. Zentralbl. Bakteriol. Mikrobiol. Hyg. 268:277–293 [PubMed] [Google Scholar]
- 20. Löffler B, et al. 2010. Staphylococcus aureus Panton-Valentine leukocidin is a very potent cytotoxic factor for human neutrophils. PLoS Pathog. 6:e1000715 doi:10.1371/journal.ppat.1000715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mellmann A, et al. 2008. Characterization of clonal relatedness among the natural population of Staphylococcus aureus strains by using spa sequence typing and the BURP (based upon repeat patterns) algorithm. J. Clin. Microbiol. 46:2805–2808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Meyer E, Schwab F, Schroeren-Boersch B, Gastmeier P. 2011. Increasing consumption of MRSA-active drugs without increasing MRSA in German ICUs. Intensive Care Med. 37:1628–1632 [DOI] [PubMed] [Google Scholar]
- 23. Nienhoff U, et al. 2009. Transmission of methicillin-resistant Staphylococcus aureus strains between humans and dogs: two case reports. J. Antimicrob. Chemother. 64:660–662 [DOI] [PubMed] [Google Scholar]
- 24. Statistisches Bundesamt 2012. Gesundheit. Grunddaten der Krankenhäuser. 2010. Fachserie 12 Reihe 6.1.1. Statistisches Bundesamt, Wiesbaden, Germany [Google Scholar]
- 25. Stenhem M, et al. 2010. Imported methicillin-resistant Staphylococcus aureus, Sweden. Emerg. Infect. Dis. 16:189–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. von Eiff C, Friedrich AW, Peters G, Becker K. 2004. Prevalence of genes encoding for members of the staphylococcal leukotoxin family among clinical isolates of Staphylococcus aureus. Diagn. Microbiol. Infect. Dis. 49:157–162 [DOI] [PubMed] [Google Scholar]
- 27. Witte W, Nguyen VD, Dunnhaupt K. 1986. Methicillin-resistant Staphylococcus aureus (MRSA) in the German Democratic Republic. Incidence and strain-characteristics. J. Hyg. Epidemiol. Microbiol. Immunol. 30:185–193 [PubMed] [Google Scholar]
- 28. Wulf M, Voss A. 2008. MRSA in livestock animals—an epidemic waiting to happen? Clin. Microbiol. Infect. 14:519–521 [DOI] [PubMed] [Google Scholar]
- 29. Yamaguchi T, et al. 2001. Complete nucleotide sequence of a Staphylococcus aureus exfoliative toxin B plasmid and identification of a novel ADP-ribosyltransferase, EDIN-C. Infect. Immun. 69:7760–7771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yamaguchi T, et al. 2002. Identification of the Staphylococcus aureus etd pathogenicity island which encodes a novel exfoliative toxin, ETD, and EDIN-B. Infect. Immun. 70:5835–5845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zanger P, et al. 2012. Import and spread of Panton-Valentine leukocidin-positive Staphylococcus aureus through nasal carriage and skin infections in travelers returning from the tropics and subtropics. Clin. Infect. Dis. 54:483–492 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.