Abstract
The early identification of outbreaks is crucial for the control of Clostridium difficile infection. This study aimed to determine if the number of hospital-acquired C. difficile infections could be reduced by rapidly typing C. difficile strains using multiple-locus variable-number tandem-repeat analysis (MLVA) compared to typing using PCR ribotyping. A total of 16 hospitals were recruited to the study, and all periods of increased incidence (PIIs) of C. difficile infection were identified. The hospitals were randomized into two study arms, the test and the control, with all isolates typed in the test using MLVA and in the control using PCR ribotyping. Following a PII, each hospital received a structured questionnaire regarding control measures implemented or stopped prior to or following the typing results. During the study period, there were a total of 1,682 hospital-apportioned C. difficile toxin-positive cases, with 868 in the control and 814 in the test, with modeling demonstrating no differences between the two arms. A total of 245 PIIs occurred, involving 785 patients. There was a significant difference in the mean turnaround time between the ribotyping and MLVA typing (13.6 and 5.3 days, respectively [P < 0.001]). The discriminatory ability of MLVA was greater than ribotyping, with 85 outbreaks being confirmed by ribotyping and 62 by MLVA. In the test arm, 40.6% of respondents strongly agreed that the typing result had aided their management of clusters, as opposed to 9.9% in the control. The study demonstrated the utility of rapidly typing C. difficile strains, demonstrating that it aided the management of clusters, enabling effective targeting of infection control resources.
INTRODUCTION
Clostridium difficile is an important cause of hospital-acquired infection, causing a range of symptoms from diarrhea to toxic megacolon and death. In the mid-2000s, rates of C. difficile infection (CDI) rose across North America and Europe (8, 12). Due to the introduction of a number of control measures, including antibiotic stewardship, increased cleaning, and epidemiological typing of isolates, the rates have fallen (4, 5). Rapidly identifying definite transmission episodes is important for the effective control of outbreaks. Conversely, demonstrating that clusters of CDI do not constitute an outbreak can also assist management by enabling the cessation of specific infection control measures. In order to manage clusters of C. difficile infection in a timely manner, a typing technique with good discriminatory power and a rapid turnaround time is required. A variety of techniques are available, including pulsed-field gel electrophoresis (PFGE), multilocus sequencing typing (MLST), PCR ribotyping, and multiple-locus variable-number tandem-repeat analysis (MLVA) (11). All of the techniques have limitations, with PFGE being time consuming, MLST lacking the discriminatory power, and PCR ribotyping requiring the interpretation of analogue data. In the United Kingdom, a free-of-charge service has been provided by the regional public health laboratories of the Health Protection Agency (HPA) since 2007 for PCR ribotyping of all isolates from periods of increased incidence (PIIs), which is defined as two or more C. difficile toxin-positive cases in a ward within a 28-day period (9). The turnaround time for the ribotyping service is 14 days, which makes rapid infection control intervention difficult. MLVA typing provides a greater discriminatory ability and a reduced time in obtaining results compared to PCR ribotyping and has been shown to have utility in investigating clusters of isolates (13, 18).
The primary aim of this study was to determine if the number of C. difficile cases acquired in the hospital could be reduced by rapidly typing isolates using a highly discriminatory scheme compared to typing isolates using PCR ribotyping and by introducing targeted control measures. The secondary aim of this study was to establish if a rapid service could be delivered to 16 hospitals with a target turnaround time of 6 days. In addition, we obtained physician feedback on the impact typing had on the management of clusters.
MATERIALS AND METHODS
Study setting and design.
Since the introduction of national guidance in 2009 (5), all hospitals in England have routinely identified periods of increased incidence (PIIs) of CDI, defined as two or more patients developing laboratory confirmed C. difficile infection within a 28-day period in the same ward. A PII is considered closed if no more cases occur within 28 days following the last case. Hospitals within the East and West Midlands submitted fecal samples from these cases to the Public Health Laboratory, Birmingham, United Kingdom, for PCR ribotyping and MLVA typing. All typing was requested and reported using the secure Clostridium difficile ribotyping network (CDRN) website.
During the period of May 2010 to June 2011, 16 hospitals (9 large hospitals and 7 small/medium hospitals) from the East and West Midlands were randomized into the control or test arm with randomization stratified by hospital size. Hospitals continued to identify PIIs and submit fecal samples for typing, with no changes to the service offered to hospitals within the control arm. Samples from hospitals within the test arm were typed using a modified MLVA protocol, and results were reported in real time using secure e-mail addresses.
Each hospital followed their own protocol for the identification of C. difficile toxin-positive patients and their own infection control procedures for CDI, which were captured using questionnaires at the start of the study.
Following cessation of the study, the isolates from the control arm were also typed using MLVA and the isolates from the test arm were ribotyped to compare the results of the two typing methods.
Laboratory procedures and reporting.
All samples were cultured using the alcohol shock procedure with an equal amount of feces and ethanol being vortexed and then left at room temperature for 30 min. Two drops of the suspension was placed on a C. difficile selective agar plate (Oxoid, United Kingdom) and incubated anaerobically at 37°C for 48 h. An individual colony displaying typical morphology of C. difficile was picked for DNA extraction using the Chelex method and subcultured to blood agar to check for purity (15). Ribotyping was carried out as described previously on all isolates (15). For MLVA, 12 variable-number tandem-repeat (VNTR) loci (A6Cd, B7Cd, C6Cd, E7Cd, G8Cd, CDR5, CDR60, CD9, CD12, CD14, CD19, and CD44) were amplified by PCR as described previously by Manzoor et al., with the product size being determined using a 3130xl DNA sequencer (Applied Biosystems) (13). Analysis was undertaken using BioNumerics software v4.61, and the summed tandem-repeat differences (STRD) from all loci were calculated using the Manhattan coefficient. A PII was confirmed as an outbreak by ribotyping if two or more isolates had the same ribotype and by MLVA if the difference between the STRD of two or more isolates was ≤4 (5). The report of the MLVA data included an interpretative statement of the results.
Data collection.
Prior to the commencement of the study, a questionnaire was sent to all participating hospitals to establish the method of C. difficile testing, the isolation policy within the hospital, details of antibiotic policies, cleaning regimes, and basic demographics. Following the reporting of results from a PII, a short data collection form was sent to each hospital to establish if, following the notification of the results, either additional infection control measures had been introduced or measures that had been introduced previously had been stopped. In addition, users were asked to evaluate whether they considered that the typing result had aided their management of clusters.
For each hospital, the number of hospital-apportioned C. difficile reports, defined as patients who are in-patients and have had a specimen taken at an acute hospital two or more days after the date of admission as reported to the mandatory Department of Health surveillance scheme, was obtained (www.hpa.org.uk).
Outcome measures and statistical analysis.
The primary outcome was the number of hospital-apportioned cases of C. difficile at each hospital as reported to the mandatory reporting scheme. A negative binomial regression model was constructed to compare the number of hospital-apportioned cases in the test and control arms of the study. The model included the logarithm of the number of beds in the hospital as an offset variable to compensate for the different hospital sizes. All statistical analyses were conducted using R (version 2.12.1).
RESULTS
During the study period, there were a total of 1,682 hospital-apportioned C. difficile toxin-positive cases from the 16 hospitals, with 868 in the control and 814 in the test arm. The number of cases per hospital ranged from 32 to 246 (Table 1). Modeling of the differences in the number of hospital-apportioned C. difficile toxin-positive cases between the control and the test arm using negative binomial regression with the logarithm of the number of beds in the hospital as an offset variable indicated that there were no statistically significant differences between the two arms.
Table 1.
Data from each hospital, detailing the number of hospital-apportioned C. difficile cases, the number of PIIs, and the number of PIIs confirmed as outbreaks by the two typing methodsa
| Hospital | Study arm | No. of mandatory reported hospital-apportioned C. difficile cases | Rate of C. difficile cases per 10,000 patient days | No. of PIIs | Mean (min, max) no. of cases per PII | No. of PIIs confirmed as outbreaks by ribotyping | No. of PIIs confirmed as outbreaks by MLVA |
|---|---|---|---|---|---|---|---|
| A | Control | 32 | 1.7 | 3 | 3.0 (2, 4) | 1 | 1 |
| B | Control | 108 | 2.8 | 10 | 4.4 (2, 9) | 7 | 7 |
| C | Control | 48 | 2.1 | 5 | 2.6 (2, 3) | 1 | 1 |
| D | Control | 52 | 2.1 | 8 | 2.0 (2, 4) | 2 | 1 |
| E | Control | 246 | 3.5 | 58 | 3.2 (2, 10) | 11 | 5 |
| F | Control | 131 | 3.0 | 8 | 2.5 (2, 4) | 2 | 2 |
| G | Control | 156 | 3.1 | 8 | 5.8 (2, 10) | 5 | 4 |
| H | Control | 95 | 2.4 | 19 | 2.6 (2, 6) | 3 | 1 |
| I | Test | 63 | 2.7 | 3 | 3.3 (2, 4) | 1 | 1 |
| J | Test | 199 | 3.1 | 33 | 2.7 (2, 10) | 9 | 7 |
| K | Test | 46 | 4.3 | 3 | 3.3 (2, 5) | 1 | 1 |
| L | Test | 57 | 1.5 | 11 | 2.5 (2, 6) | 2 | 2 |
| M | Test | 74 | 1.8 | 15 | 2.9 (2, 8) | 5 | 5 |
| N | Test | 108 | 2.1 | 15 | 3.3 (2, 7) | 5 | 4 |
| O | Test | 167 | 3.5 | 46 | 3.7 (2, 15) | 20 | 17 |
| P | Test | 100 | 2.6 | 0 | 0 (NA, NA) | 0 | 0 |
Six PIIs contained two different outbreaks. NA, not available; min, minimum; max, maximum.
A total of 245 PIIs occurred, 119 in the control and 126 in the test arm, with an overall mean of 15 per hospital and a range of 0 to 58 per hospital (Table 1). The PIIs involved 785 (46.7%) patients of the 1,682 hospital-apportioned cases identified through mandatory reporting. The number of patients involved in a PII ranged from 2 to 15. The mean length of a PII in the control arm was 19.1 days and in the test arm was 19.9 days, with the longest PII observed being 117 days.
Of the 785 patients involved in PIIs, typing results were available for 669 (85.2%) samples, C. difficile was not isolated in 114 samples, and there were insufficient samples in 2 cases. One hospital in the test arm did not submit any samples for typing. The time taken from the identification of the PII, taken as the date the second sample in the PII was obtained, until receiving samples for typing in the laboratory was comparable in the two arms (14.0 and 14.4 days in the control and test arms, respectively). There was a significant difference in the mean turnaround time from sample receipt in the laboratory until reporting between the ribotyping (control) and MLVA (test) typing (13.6 days and 5.3 days, respectively [P < 0.001]).
Of the 245 PIIs, outbreaks were confirmed in 69 (28.2%) involving 218 cases by the typing method reported, MLVA in the test and ribotyping in the control. A total of 34 outbreaks were confirmed in the control arm by ribotyping in 32/119 PIIs (26.9%), with 2 outbreaks, caused by different ribotypes occurring in two of the PIIs. In the test arm, 40 outbreaks were confirmed by MLVA in 37/126 PIIs (29.4%), with three of the PIIs containing 2 outbreaks.
Following the completion of the study, all isolates in the test arm were typed using ribotyping and all isolates that were clustered using ribotyping in the control arm were typed with MLVA. This enabled exploration of any difference in the discriminatory ability of the two techniques to be highlighted. Of the 34 outbreaks confirmed by ribotyping in the control arm, MLVA typing confirmed 22, while ribotyping of the isolates in the test arm identified 51 outbreaks, as opposed to 40 with MLVA. There were no instances in the test arm where MLVA identified an outbreak that had not been identified by ribotyping. The ribotypes of the outbreaks that were not confirmed by MLVA differed. Combining the test and control arms, a total of 156 cases were considered part of an outbreak by MLVA, while with ribotype, 218 cases were considered part of an outbreak; thus, 62 cases were inappropriately considered to be part of an outbreak by ribotyping. On examining the number of outbreaks identified by MLVA, there were no statistical differences between the two intervention arms.
The appropriateness of defining an outbreak as isolates with an STRD of <4 was examined and demonstrated that, if an STRD of 1 had been used, only 45 of the 62 PIIs would have been classified as outbreaks and that, if an STRD of 10 had been adopted, only an additional 4 PIIs would have been classified as outbreaks. Three of the four PIIs that were not considered to be outbreaks by MLVA typing were the epidemic strains 106 and 027.
A total of 66 different ribotypes were identified in the control and test arms of the study, with 24 ribotypes only present in single samples. The most prevalent ribotype was 106 (81 samples), followed by 027 (68 samples), 002 (51 samples), 014 (50 samples), 015 (45 samples), 078 (44 samples), 005 (42 samples), 001 (41 samples), and 020 (35 samples). All of the remaining ribotypes had <16 samples. A total of 21 ribotypes were responsible for outbreaks, with 9 ribotypes being common to both the test and control arms and 11 ribotypes causing a single outbreak. The predominant ribotype causing outbreaks overall was 106 (19 outbreaks) in six different hospitals, three in the control and three in the test. However, the predominant ribotype causing outbreaks in the test arm was 027, causing outbreaks in five different hospitals (Fig. 1). Five outbreaks were due to ribotype 174 in the test arm, but this occurred only at one hospital.
Fig 1.
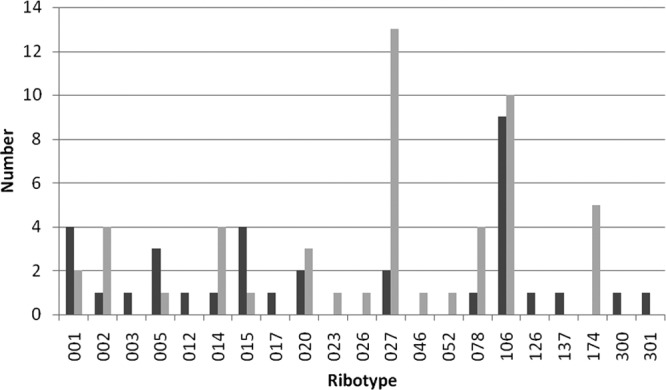
Number of outbreaks caused by each ribotype in the control and test arms of the study. Control, dark gray bars; test, light gray bars.
A total of 244 PII questionnaires, 120 from the control arm and 124 from the test, were completed. New measures were implemented following the reporting of the typing results on a total of 55 occasions, 13 in the control and 42 in the test. The most frequent interventions (55%) were the auditing of practices and the teaching of staff. Following the reporting of typing results, 55 measures in total were stopped, 41 in the control and 14 in the test. In the control arm, the majority of measures were the auditing of practices, while in the test, it was more varied. Physician feedback on the value of the typing result in the management of the clusters strongly favored MLVA rather than ribotyping. Thirteen (9.9%) of the respondents from the control arm strongly agreed that typing had aided their management compared to 54 (40.6%) in the test arm (Table 2).
Table 2.
Responses to whether the typing result aided the management of clusters
| Study arm | Strongly agree | Agree | Disagree | Strongly disagree | No response |
|---|---|---|---|---|---|
| Control | 13 | 97 | 13 | 4 | 4 |
| Test | 54 | 61 | 12 | 1 | 5 |
DISCUSSION
Many studies have investigated the epidemiology of C. difficile using a variety of typing methodologies, but the majority of these have been retrospective, have not used the results to influence infection control practices in a timely manner, or have assessed the impact of the results on transmission (1, 17, 20). Although this study failed to demonstrate a difference in the number of hospital-apportioned C. difficile cases between the test and control arms, it did demonstrate that rapid results could be delivered and that the rapid typing assisted the physicians in the management of C. difficile PIIs. There are a number of possible reasons for the failure to observe a difference between the test and control arms. One of the most likely reasons is the wide range and potential combinations of C. difficile control measures. Following the large outbreaks and rise of C. difficile in the United Kingdom in the early 2000s, the awareness surrounding the control of C. difficile has been heightened and a wide range of control measures have been instituted. Guidance issued in the United Kingdom recommends the identification of PIIs, followed by the implementation of control measures, including investigating with ribotyping, increased cleaning, reviewing of antibiotics, auditing of infection control practices, and educating the ward staff (5). The rate of identification of PIIs was much higher in some hospitals than in others, which may be a true reflection of events, with some hospitals having a greater number of sporadic cases, or may be due to differences in protocols within hospitals for the identification of PIIs. This is highlighted by the hospital that, despite repeated encouragement, was not able to submit any samples for typing. If samples from PIIs were not sent for typing, this could have resulted in potential outbreaks being missed and potentially leading to an increase in cross transmission and a rise in cases. Another limitation of PIIs is that they will only identify clusters of ward-based transmission and not further hospital-wide transmission.
Due to the need to act quickly following the identification of a PII, most hospitals implement additional infection control measures prior to receiving the typing results, including increased cleaning and changing cleaning products. This may, in part, explain why no difference is observed between the test and control arms, with the measures being applied rapidly in both arms. However, the availability of rapid typing results did result in a larger number of new measures being introduced in the test arm compared to the control arm, probably due to the results being available in a time to undertake relevant interventions while positive patients were still in the ward. In the control arm, a greater number of measures were stopped after the results were received, with the availability of typing results potentially resulting in cost savings.
Currently, there is a lot of debate regarding the optimal test combinations for the diagnosis of C. difficile infection, with some previously commonly used enzyme-linked immunosorbent assay (ELISA) toxin detection tests being demonstrated to have poor sensitivity and specificity (3, 6). Combination testing is now advocated, but multiple algorithms are being employed both within laboratories in this study and throughout the United Kingdom (19). The problems with testing are highlighted in our study by the fact that C. difficile could not be grown from 14.8% samples; this could be due to the insensitivity of the culture technique or the lack of specificity of the diagnostic testing. The culture positivity rate is comparable to that observed by the CDRN in the United Kingdom, where recovery in 2010 and 2011 was 89.9%.
This study demonstrated that MLVA typing could be delivered in a timely manner with the ability to aid the management of C. difficile, with a difference of 8 days between the delivery of MLVA typing and ribotyping results. Providing timely results enables additional or more targeted control measures to be implemented in the case of an outbreak or the cessation of measures if the cluster is demonstrated not to be an outbreak.
The discriminatory ability of a typing scheme is crucial in defining whether an isolate is part of the outbreak or sporadic. Multiple MLVA schemes have been described for C. difficile, containing different numbers of loci and having various degrees of discriminatory ability (13, 14, 18). The scheme adopted in this study has been demonstrated to have good discriminatory ability while also clustering isolates in concordance with PCR ribotyping (13). A further complication in the analysis of C. difficile MLVA is the definition of an outbreak. In MLVA schemes for other organisms, for example, Mycobacterium tuberculosis, an isolate is only considered part of the same outbreak if it has an identical MLVA profile (10). However, for C. difficile, this has been demonstrated to make the typing scheme too discriminatory with isolates that are epidemiologically part of the same outbreak differing slightly in the number of repeats and isolates from within the same feces having different MLVA profiles (16). Different approaches to overcome this have been proposed, with some studies using summed tandem-repeat differences (STRD) to define relatedness, with Marsh et al. stating that isolates with an STRD of ≤2 had a high degree of genetic relatedness and Fawley et al. stating that an STRD of ≤2 was indistinguishable and an STRD of <10 was highly related (7, 14). Broukhanski and colleagues used Manhattan distance-based clustering and then defined a cutoff of 90% to define similarity (2). This technique involves a rooted tree and is more suited to one of the studies rather than a routine service. Preliminary work investigating clusters with the loci used in this study demonstrated that an STRD of ≤4 correlated with the epidemiology, and this is further reinforced by study results where only four additional PIIs would have been called an outbreak if an STRD of <10 had been considered. Three of these PIIs were due to epidemic ribotypes, and therefore, it is highly likely that they were not linked and that they occurred by chance.
The difference in the discriminatory ability of the two techniques is highlighted by the number of outbreaks detected. Outbreaks defined by ribotyping could be further split by MLVA, thus reducing the number of true outbreaks. MLVA provided greater discrimination when applied to a wide range of ribotypes, highlighting its discriminatory ability in all strains and not just ribotype 027, as has been described previously. Interestingly, there were no outbreaks confirmed by MLVA for ribotype 005. It may be that transmission had not occurred between these patients or that this is a highly evolving strain in which the MLVA loci are relatively unstable and evolve rapidly.
A similar number of PIIs were observed in both the test and control arms of the study, but when the ribotyping of isolates is applied to both arms the test arm had a greater number of PIIs confirmed as outbreaks. This may indicate that more transmission was occurring in these hospitals in the test arm compared to the control arm.
There is quite a marked difference in the ribotypes causing outbreaks between the two arms, with the greatest number of outbreaks being caused by ribotype 027 in the test arm, but only two being due to ribotype 027 in the control. During the 2000s, ribotype 027 was the most dominant strain circulating in the United Kingdom, accounting for >50% of all isolates typed in the East and West Midlands in 2008 (www.hpa.org.uk). However, the picture is changing with a greater number of types being observed and this is reflected in this study.
Although this study does not demonstrate a reduction in C. difficile hospital-apportioned cases between the test and control arms, it does highlight and demonstrate the utility of rapid, discriminatory typing. There was a clear difference in starting interventions in the MLVA arm, resulting in a more efficient use of resources, which was supported by physician opinion. The availability of epidemiological typing at the local level is crucial in being able to provide a timely and tailored service to users. It enables the rapid sending and processing of samples and feedback of results, allowing targeted measures to be taken, which in turn should lead to reduced costs of interventions aimed at infection control.
ACKNOWLEDGMENTS
We thank all of the hospitals in the East and West Midlands, United Kingdom, that submitted fecal samples and completed questionnaires to make this study possible.
This is an independent report commissioned and funded by the Policy Research Programme at the Department of Health. The views expressed are not necessarily those of the Department of Health.
Footnotes
Published ahead of print 25 July 2012
REFERENCES
- 1. Bauer MP, et al. 2011. Clostridium difficile infection in Europe: a hospital-based survey. Lancet 377:63–73 [DOI] [PubMed] [Google Scholar]
- 2. Broukhanski G, Simor A, Pillai DR. 2011. Defining criteria to interpret multilocus variable-number tandem repeat analysis to aid Clostridium difficile outbreak investigation. J. Med. Microbiol. 60:1095–1100 [DOI] [PubMed] [Google Scholar]
- 3. Carroll KC. 2011. Tests for the diagnosis of Clostridium difficile infection: the next generation. Anaerobe 17:170–174 [DOI] [PubMed] [Google Scholar]
- 4. Cohen SH, et al. 2010. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect. Control Hosp. Epidemiol. 31:431–455 [DOI] [PubMed] [Google Scholar]
- 5. Department of Health 2009. Clostridium difficile infection: how to deal with the problem. Department of Health, London, United Kingdom [Google Scholar]
- 6. Eastwood K, Else P, Charlett A, Wilcox M. 2009. Comparison of nine commercially available Clostridium difficile toxin detection assays, a real-time PCR assay for C. difficile tcdB, and a glutamate dehydrogenase detection assay to cytotoxin testing and cytotoxigenic culture methods. J. Clin. Microbiol. 47:3211–3217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fawley WN, Wilcox MH. 2011. An enhanced DNA fingerprinting service to investigate potential Clostridium difficile infection case clusters sharing the same PCR ribotype. J. Clin. Microbiol. 49:4333–4337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gerding DN. 2010. Global epidemiology of Clostridium difficile infection in 2010. Infect. Control Hosp. Epidemiol. 31(Suppl 1):S32–S34 [DOI] [PubMed] [Google Scholar]
- 9. Hardy KJ, Gossain S, Thomlinson D, Pillay DG, Hawkey PM. 2010. Reducing Clostridium difficile through early identification of clusters and the use of a standardised set of interventions. J. Hosp. Infect. 75:277–281 [DOI] [PubMed] [Google Scholar]
- 10. Hawkey PM, et al. 2003. Mycobacterial interspersed repetitive unit typing of Mycobacterium tuberculosis compared to IS6110-based restriction fragment length polymorphism analysis for investigation of apparently clustered cases of tuberculosis. J. Clin. Microbiol. 41:3514–3520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Killgore G, et al. 2008. Comparison of seven techniques for typing international epidemic strains of Clostridium difficile: restriction endonuclease analysis, pulsed-field gel electrophoresis, PCR-ribotyping, multilocus sequence typing, multilocus variable-number tandem-repeat analysis, amplified fragment length polymorphism, and surface layer protein A gene sequence typing. J. Clin. Microbiol. 46:431–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kuijper EJ, Coignard B, Tull P. 2006. Emergence of Clostridium difficile-associated disease in North America and Europe. Clin. Microbiol. Infect. 12(Suppl 6):2–18 [DOI] [PubMed] [Google Scholar]
- 13. Manzoor SE, et al. 2011. Extended multilocus variable-number tandem-repeat analysis of Clostridium difficile correlates exactly with ribotyping and enables identification of hospital transmission. J. Clin. Microbiol. 49:3523–3530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Marsh JW, et al. 2006. Multilocus variable-number tandem-repeat analysis for investigation of Clostridium difficile transmission in hospitals. J. Clin. Microbiol. 44:2558–2566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stubbs SL, Brazier JS, O'Neill GL, Duerden BI. 1999. PCR targeted to the 16S–23S rRNA gene intergenic spacer region of Clostridium difficile and construction of a library consisting of 116 different PCR ribotypes. J. Clin. Microbiol. 37:461–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tanner HE, Hardy KJ, Hawkey PM. 2010. Coexistence of multiple multilocus variable-number tandem-repeat analysis subtypes of Clostridium difficile PCR ribotype 027 strains within fecal specimens. J. Clin. Microbiol. 48:985–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tenover FC, et al. 2011. Comparison of strain typing results for Clostridium difficile isolates from North America. J. Clin. Microbiol. 49:1831–1837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. van den Berg RJ, Schaap I, Templeton KE, Klaassen CH, Kuijper EJ. 2007. Typing and subtyping of Clostridium difficile isolates by using multiple-locus variable-number tandem-repeat analysis. J. Clin. Microbiol. 45:1024–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wilcox MH, Planche T, Fang FC, Gilligan P. 2010. What is the current role of algorithmic approaches for diagnosis of Clostridium difficile infection? J. Clin. Microbiol. 48:4347–4353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wiuff C, et al. 2011. The epidemiology of Clostridium difficile in Scotland. J. Infect. 62:271–279 [DOI] [PubMed] [Google Scholar]


