Abstract
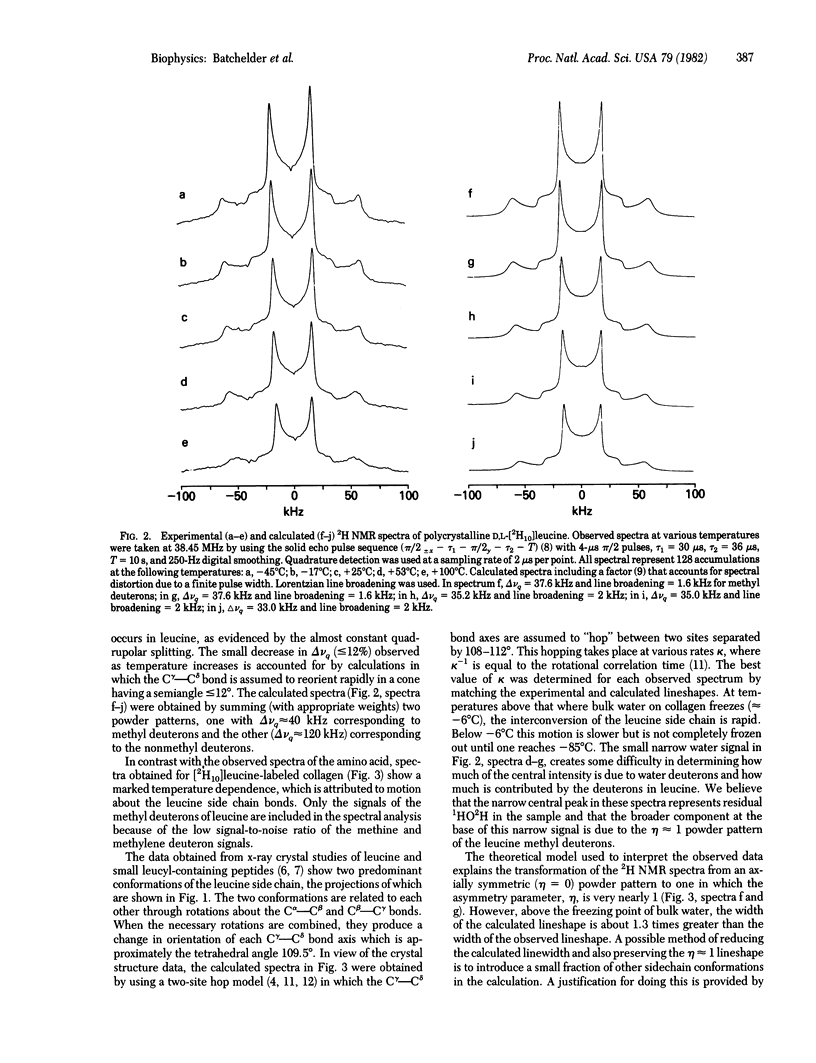
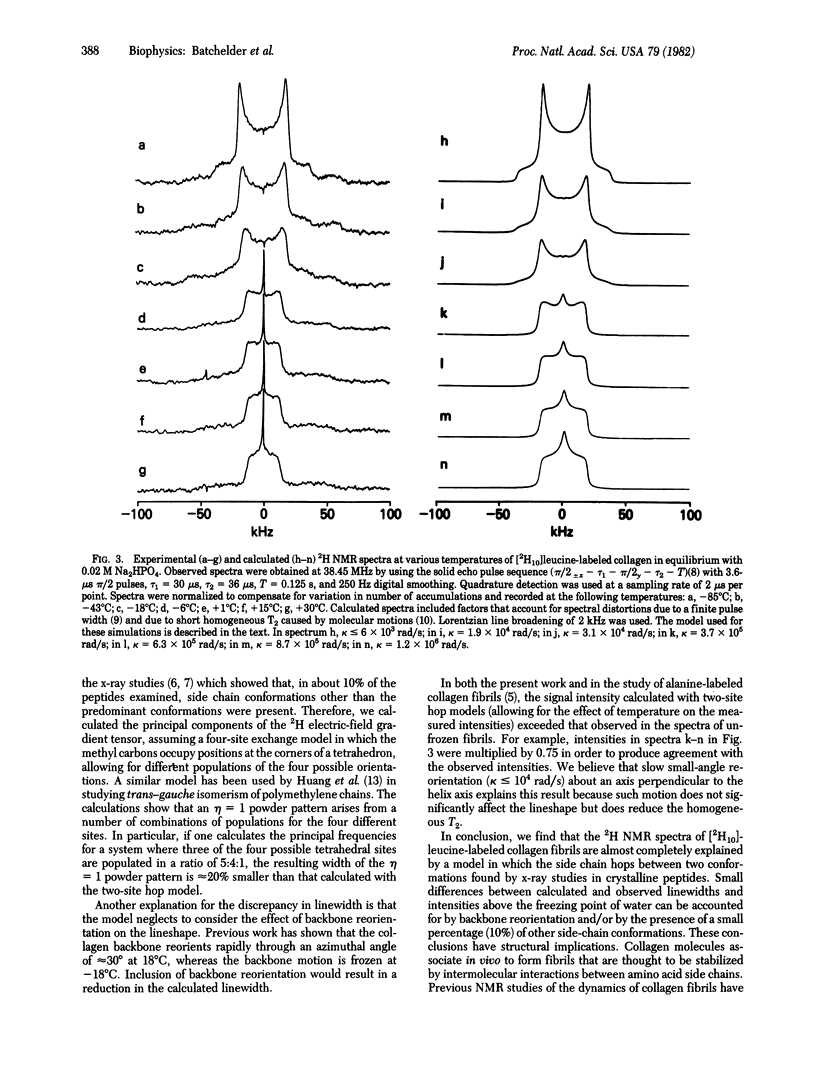
We have used 2H quadrupole-echo NMR spectroscopy to study the molecular dynamics of the leucine side chain in collagen fibrils labeled with [2H10]leucine. X-ray crystallographic studies of leucine and small leucyl-containing peptides and proteins [Benedetti, C. (1977) in Proceedings of the Fifth American Peptides Symposium, eds, Goodman, M. & Meienhofer, J. (Wiley, New York), pp. 257--274; Janin, J., Wodak, S., Levitt, M. & Maigret, B. (1978) J. Mol. Biol. 125, 357--386] show that the amino acid side chain exists predominantly in only two of the nine possible conformations. 2H NMR spectra of polycrystalline D,L [2H10]leucine obtained from -45 degrees C to +100 degrees C showed that interconversion of the two conformations did not take place on the 2H NMR timescale in this temperature range. In contrast, experimental lineshapes observed for [2H10]leucine-labeled collagen fibrils from -85 degrees C to +30 degrees C were simulated by using a model in which the side chain hops at various rates between the two predominant conformations found by the x-ray studies. A small difference between calculated and observed linewidths above the freezing point of water can be accounted for by backbone reorientation or by the presence of a small percentage of other side-chain conformations. Thus, these results provide strong evidence that the two predominant x-ray conformations not only exist in the fibrils as the preferred orientations but interconvert at rates that are proportional to temperature over the range - 85 degrees C to +30 degrees C. These observations concur with previous NNR studies of collagen fibrils that demonstrated a mobile contact region between collagen molecules.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Janin J., Wodak S. Conformation of amino acid side-chains in proteins. J Mol Biol. 1978 Nov 5;125(3):357–386. doi: 10.1016/0022-2836(78)90408-4. [DOI] [PubMed] [Google Scholar]
- Jelinski L. W., Sullivan C. E., Batchelder L. S., Torchia D. A. Deuterium nuclear magnetic resonance of specifically labeled native collagen. Investigation of protein molecular dynamics using the quadrupolar echo technique. Biophys J. 1980 Oct;32(1):515–529. doi: 10.1016/S0006-3495(80)84987-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelinski L. W., Sullivan C. E., Torchia D. A. 2H NMR study of molecular motion in collagen fibrils. Nature. 1980 Apr 10;284(5756):531–534. doi: 10.1038/284531a0. [DOI] [PubMed] [Google Scholar]
- Jelinski L. W., Torchia D. A. 13C/1H high power double magnetic resonance investigation of collagen backbone motion in fibrils and in solution. J Mol Biol. 1979 Sep 5;133(1):45–65. doi: 10.1016/0022-2836(79)90250-x. [DOI] [PubMed] [Google Scholar]
- Jelinski L. W., Torchia D. A. Investigation of labeled amino acid side-chain motion in collagen using 13C nuclear magnetic resonance. J Mol Biol. 1980 Apr;138(2):255–272. doi: 10.1016/0022-2836(80)90286-7. [DOI] [PubMed] [Google Scholar]
- Torchia D. A., VanderHart D. L. 13C Magnetic resonance evidence for anisotropic molecular motion in collagen fibrils. J Mol Biol. 1976 Jun 14;104(1):315–321. doi: 10.1016/0022-2836(76)90018-8. [DOI] [PubMed] [Google Scholar]


