Abstract
MIC and disk diffusion quality control (QC) ranges were established for two new pleuromutilin antimicrobials (BC-3205 and BC-3781) in an eight-laboratory study performed according to Clinical and Laboratory Standards Institute M23-A3 guidelines. Staphylococcus aureus ATCC 29213 and 25923, Streptococcus pneumoniae ATCC 49619, and Haemophilus influenzae ATCC 4927 strains were evaluated. The proposed QC ranges would aid clinical laboratories in testing these compounds following their development for treatment of respiratory and cutaneous infections.
TEXT
Two new pleuromutilin class agents (BC-3205 and BC-3781) have been developed by Nabriva Therapeutics AG (Vienna, Austria) for oral and intravenous treatment of acute bacterial skin and skin structure infections (ABSSSI) caused by Gram-positive organisms and community-acquired bacterial pneumonia (CABP) (6, 8, 9). BC-3781 and BC-3205 are semisynthetic pleuromutilin derivatives that interfere with bacterial protein synthesis by binding to the peptidyltransferase center of the ribosomal 50S subunit. Staphylococcus aureus, especially methicillin-resistant S. aureus (MRSA), Streptococcus pneumoniae, and Haemophilus influenzae represent important causative organisms of ABSSSI and CABP, and BC-3781 and BC-3205 have demonstrated potent activity against these pathogens (6, 8, 9). In this report, we describe the results of a multilaboratory quality control (QC) trial designed to propose ranges for both BC-3205 and BC-3781 for broth microdilution (MICs in micrograms per milliliter) and disk diffusion (zone diameters in millimeters) methods, following the guidelines published in the Clinical and Laboratory Standards Institute (CLSI) M23-A3 document (4).
Eight laboratories were used in these studies; however, only seven laboratories are needed to establish a QC range according to the CLSI M23-A3 document (4). These laboratories were experienced microbiology facilities, and each followed the CLSI procedures for disk diffusion (2) and broth microdilution (3) methods. The sites included Massachusetts General Hospital, Boston, MA (M. J. Ferraro; disk diffusion study only); Wheaton Franciscan Laboratory, Wauwatosa, WI (E. Munson; disk diffusion study only); JMI Laboratories, North Liberty, IA (R. N. Jones); TREK Diagnostics, Cleveland, OH (C. Knapp); University of Alberta, Edmonton, Alberta, Canada (R. Rennie); Duke University Medical Center, Durham, NC (L. B. Reller); University of Washington, Seattle, WA (S. Swanzy); Robert Wood Johnson Medical School, New Brunswick, NJ (M. Weinstein); University of Texas Medical Center, Houston, TX (A. Wanger; MIC study only); and the Cleveland Clinic Foundation, Cleveland, OH (G. Hall; MIC study only).
Reference frozen-form broth microdilution panels were prepared by TREK Diagnostics following good manufacturing practice (GMP) guidelines and shipped frozen to all participant sites. Panels contained three lots of cation-adjusted Mueller-Hinton broth (Oxoid, Hampshire, United Kingdom; BBL, Sparks, MD; and Difco, Detroit, MI). Also, panels containing three lots of Haemophilus Test Medium (HTM) and three lots of Mueller-Hinton broth supplemented with 2% to 5% lysed horse blood were provided by the same vendor. Azithromycin, retapamulin (7), and levofloxacin were utilized as control agents. For broth microdilution testing, each laboratory tested replicates of S. aureus ATCC 29213, H. influenzae ATCC 49247, and S. pneumoniae ATCC 49619. Participating laboratories were instructed to read endpoints in accordance with the CLSI M07-A8 document, which states that, for some antimicrobial agents with trailing endpoints, the MIC should be read at the first well that shows a prominent (≥80%) reduction in growth compared to the control, and tiny buttons of growth should be ignored (1, 3). Colony counts were performed on drug-free agar media and resulted in the following average counts: S. aureus ATCC 29213 = 4.0 × 105 CFU/ml, H. influenzae ATCC 49247 = 5.4 ×105 CFU/ml, and S. pneumoniae ATCC 49619 = 2.7 × 105 CFU/ml.
For disk diffusion tests, three different lots of 20-μg disks were manufactured by two companies: MAST Group, Merseyside, United Kingdom (BC-3205 lot 257105 and lot 257106 and BC-3781 lot 257108 and lot 257109) and Bio-Rad, Hercules, CA (BC-3205 lot 9L0011 and BC-3781 lot 9L0011). Single lots of comparator disks from BD (Franklin Lakes, NJ) were used: azithromycin (lot 9118096) (15 μg), clindamycin (lot 9187750) (2 μg), and linezolid (lot 9225172) (30 μg). Three manufacturers (Remel, Lenexa, KS; Hardy Diagnostics, Santa Maria, CA; and BBL) were used to produce lots for Mueller-Hinton agar (lot 848387, lot 09352, and lot 9328321), Haemophilus Test Medium (lot 855369, lot 09355, and lot 9336525), and Mueller-Hinton agar with 5% sheep blood (lot 852672, lot 10006, and lot 9350462).
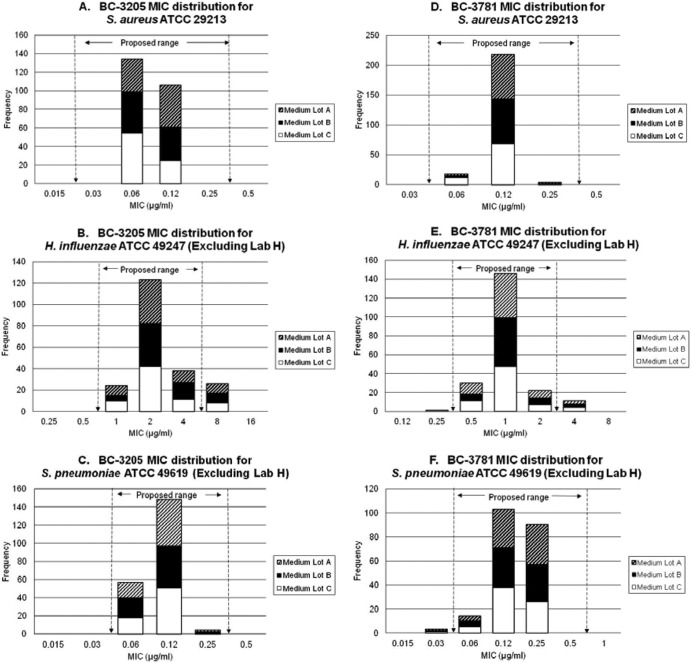
The BC-3205 and BC-3781 MIC results are summarized as proposed ranges in Table 1 and shown in Fig. 1 and 2. The S. aureus ATCC 29213 results for BC-3205 demonstrated a bimodal MIC distribution (0.06 and 0.12 μg/ml), with all results within the proposed 4-dilution-step range of 0.03 to 0.25 μg/ml. A slight medium variation occurred with two lots at a mode of 0.06 μg/ml and one lot at 0.12 μg/ml. BC-3781 tested against the same S. aureus strain (Fig. 1) resulted in a 3-dilution-step range of 0.06 to 0.25 μg/ml (100.0% of results within the proposed range) with no medium variations observed (Table 1).
Table 1.
Proposed quality control ranges of BC-3205 and BC-3781 disk diffusion and broth microdilution testsa
| QC organism | BC-3205 |
BC-3781 |
||
|---|---|---|---|---|
| Disk diffusion zone diam QC range (mm) (% in range) | Broth microdilution MIC QC range (μg/ml) (% in range) | Disk diffusion zone diam QC range (mm) (% in range) | Broth microdilution MIC QC range (μg/ml) (% in range) | |
| S. aureus ATCC 25923 | 26–32 (99.0) | 26–32 (97.4) | ||
| S. aureus ATCC 29213 | 0.03–0.25 (100.0) | 0.06–0.25 (100.0) | ||
| H. influenzae ATCC 49247 | 18–24 (95.6) | (87.6)b | 22–28 (96.0) | 0.5–2 (94.3)b |
| S. pneumoniae ATCC 49619 | 19–27 (98.6) | 0.06–0.25 (100.0)b | 19–27 (99.3) | 0.06–0.5 (98.6)b |
The Range Finder program calculated all ranges to be identical to the proposed range or 1 mm greater for the disk diffusion method. The CLSI calculated range was the proposed range found in this table.
Excluding laboratory H (outlier site); the proposed range for H. influenzae ATCC 49247 for BC-3205 contains only 87.6% of results, which does not meet the CLSI guidelines.
Fig 1.
MIC distributions of BC-3205 (A to C) and BC-3781 (D to F) for QC reference strains.
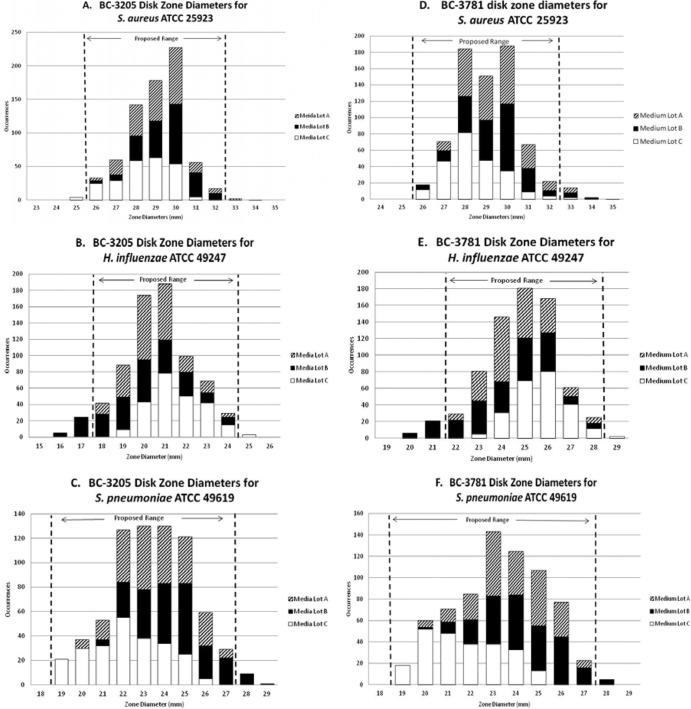
Fig 2.
Disk zone diameters of BC-3205 (A to C) and BC-3781 (D to F) disks for QC reference strains.
H. influenzae ATCC 49247, when tested with BC-3205, produced a 3-doubling-dilution range of 1 to 4 μg/ml (Table 1, Fig. 1). Using the results from all laboratories, only 81.8% of these values fell within the calculated range. By excluding the results from an outlier laboratory (laboratory H; BC-3205 modal value of 0.5 μg/ml) (10), the percentage of results included in the proposed range increased only to 87.6%; therefore, no range could be proposed. Laboratory H was also an outlier site for the BC-3781 results (10) and was excluded from analysis to provide a MIC range of 0.5 to 2 μg/ml for BC-3781, which included 94.3% of the results (slightly below the optimal target of ≥95% but still accepted by the CLSI) (1). For both drugs tested against H. influenzae, a trailing endpoint of 1 doubling dilution was encountered. Sites were instructed to ignore small, pinpoint buttons of growth when interpreting MIC panels (1).
Lastly, S. pneumoniae ATCC 49619 tested with BC-3205 had a proposed MIC QC range of 0.06 to 0.25 μg/ml (100.0% of results within the range; Table 1, Fig. 1), excluding laboratory H, which clearly had an outlier mode of 0.015 μg/ml (10). Data from laboratory H were also excluded from the analysis of the BC-3781 MIC range for S. pneumoniae, where the 0.06 to 0.5 μg/ml range included 98.6% of the reported MIC values. Results for the control agents (azithromycin, retapamulin, and levofloxacin) were within CLSI ranges (5), with the exception of only two values (2/630 [0.3%]), both contributed by laboratory H.
Almost 6,000 zone diameters were reported for the disk diffusion method, and BC-3205 and BC-3781 results are summarized in Table 1 and Fig. 2. Using CLSI M23-A3 criteria (4), a 7-mm range of 26 to 32 mm for S. aureus ATCC 25923 and BC-3205 was proposed that included 99.0% of all reported zone diameters. BC-3781 tested against S. aureus ATCC 25923 also produced a QC range of 26 to 32 mm, which included 97.4% of all results (Fig. 2). H. influenzae ATCC 49247 testing produced a QC range of 18 to 24 mm for BC-3205 and 22 to 28 mm for BC-3781. The Range Finder method (10) suggested a wider QC range for both agents. A 9-mm range was needed to include at least 95% of all reported values for S. pneumoniae ATCC 49619 and BC-3205 (19 to 27 mm). Using the CLSI M23-A3 guideline (4) for BC-3781 result analysis, a range of 20 to 26 mm included only 93.6% of results. The Range Finder program (10) calculated a range of 19 to 28 mm, which contained all reported results. A compromise range of 19 to 27 mm (99.3%) was proposed to include at least 95% of all results.
All clindamycin and linezolid comparator disk diffusion zones were within the CLSI published ranges, and nearly all (99.9%) of the azithromycin disk zones were within QC limits (5). There was very little median zone diameter variance between the lots of disks (BC-3205, ≤2 mm; BC-3781, ≤1 mm), and there were ≤2-mm differences between median zones for the medium lots across all organisms and investigational disks. A trend toward smaller zone diameters was noted with medium lot C against S. pneumoniae ATCC 49619. Future studies should be reviewed for any medium variations.
The results from this multilaboratory QC study were intended to provide the initial ranges for routine susceptibility testing using disk diffusion and broth microdilution methods (2, 3, 5) as these two novel pleuromutilins progress through phase 2 and 3 human clinical trials. All QC ranges for BC-3781 (Table 1) were presented to the CLSI Subcommittee on Antimicrobial Susceptibility Testing (June 2010) and were approved for future publication in the M100 document (1, 5).
ACKNOWLEDGMENTS
This study was funded by an educational/research grant to JMI Laboratories (North Liberty, IA) provided by Nabriva Therapeutics AG (Vienna, Austria).
JMI Laboratories, Inc., has received research and educational grants in 2009 to 2011 from Achaogen, Aires, American Proficiency Institute (API), Anacor, Astellas, AstraZeneca, Bayer, bioMérieux, Cempra, Cerexa, Cosmo Technologies, Contrafect, Cubist, Daiichi, Dipexium, Enanta, Furiex, GlaxoSmithKline, Johnson & Johnson (Ortho McNeil), LegoChem Biosciences Inc., Meiji Seika Kaisha, Merck, Nabriva, Novartis, Paratek, Pfizer (Wyeth), PPD Therapeutics, Premier Research Group, Rempex, Rib-X Pharmaceuticals, Seachaid, Shionogi, Shionogi USA, The Medicines Co., Theravance, ThermoFisher, TREK Diagnostics, Vertex Pharmaceuticals, and some other corporations. Some JMI employees are advisors/consultants for Astellas, Cubist, Pfizer, Cempra, Cerexa-Forest, J&J, and Theravance. S.P. and Z.I.-S. are employees and owners of stock options of Nabriva Therapeutics AG.
Footnotes
Published ahead of print 18 July 2012
REFERENCES
- 1. Clinical and Laboratory Standards Institute 1 February 2012, accession date CLSI Subcommittee on Antimicrobial Susceptibility Testing meeting minutes, 2010. CLSI, Wayne, PA: http://www.clsi.org/Content/NavigationMenu/Committees/Microbiology/AST/ArchiveofPreviousEvents/Summary_Minutes_Jan_2010.pdf [Google Scholar]
- 2. Clinical and Laboratory Standards Institute 2009. M02–A10. Performance standards for antimicrobial disk susceptibility tests; approved standard: 10th ed. CLSI, Wayne, PA [Google Scholar]
- 3. Clinical and Laboratory Standards Institute 2009. M07–A8. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard: 8th ed. CLSI, Wayne, PA [Google Scholar]
- 4. Clinical and Laboratory Standards Institute 2008. M23–A3. Development of in vitro susceptibility testing criteria and quality control parameters: 3rd edition. CLSI, Wayne, PA [Google Scholar]
- 5. Clinical and Laboratory Standards Institute 2012. M100–S22. Performance standards for antimicrobial susceptibility testing: 22nd informational supplement. CLSI, Wayne, PA [Google Scholar]
- 6. Novak R, Shlaes DM. 2010. The pleuromutilin antibiotics: a new class for human use. Curr. Opin. Investig. Drugs 11:182–191 [PubMed] [Google Scholar]
- 7. Ross JE, Jones RN. 2005. Quality control guidelines for susceptibility testing of retapamulin (SB-275833) by reference and standardized methods. J. Clin. Microbiol. 43:6212–6213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sader HS, Biedenbach DJ, Paukner S, Ivezic-Schoenfeld Z, Jones RN. 2012. Antimicrobial activity of the investigational pleuromutilin compound BC-3781 tested against gram-positive organisms commonly associated with acute bacterial skin and skin structure infections. Antimicrob. Agents Chemother. 56:1619–1623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sader HS, et al. 2012. Antimicrobial activity of the novel pleuromutilin antibiotic BC-3781 against organisms responsible for community-acquired respiratory tract infections (CARTIs). J. Antimicrob. Chemother. 67:1170–1175 [DOI] [PubMed] [Google Scholar]
- 10. Turnidge J, Bordash G. 2007. Statistical methods for establishing quality control ranges for antibacterial agents in Clinical and Laboratory Standards Institute susceptibility testing. Antimicrob. Agents Chemother. 51:2483–2488 [DOI] [PMC free article] [PubMed] [Google Scholar]