Abstract
We evaluated the performance of the DR. TBDR/NTM IVD kit, which was designed to detect Mycobacterium tuberculosis, rifampin-resistant M. tuberculosis, and nontuberculous mycobacteria, for detecting 110 positive and 50 negative cultures in Mycobacterium Growth Indicator Tubes. The accuracy rate of this kit for identification of Mycobacterium species was 95.5% (105/110).
TEXT
Mycobacterium species have long been divided into Mycobacterium tuberculosis complex (MTC) and nontuberculous mycobacteria (NTM). An increasing number of reports on infections due to NTM, including pulmonary, soft tissue, bone, bloodstream, and central nervous system infections, as well as intra-abdominal and genitourinary tract infections, have emerged over the past decade (5, 8, 12, 13, 18). Among NTM infections, pulmonary disease warrants special attention (5). Individualized treatment options tailored to each NTM species have been suggested to optimize treatment response (5).
Recently, the DR. Chip Corporation in Taiwan developed the DR. TBDR/NTM IVD kit. The kit is designed to target MTC, rifampin-resistant M. tuberculosis (likely multidrug-resistant M. tuberculosis [MDR-M. tuberculosis]), and 15 species of NTM, including Mycobacterium abscessus, Mycobacterium asciaticum, Mycobacterium avium, Mycobacterium intracellulare, Mycobacterium chelonae, Mycobacterium fortuitum, Mycobacterium gordonae, Mycobacterium kansasii, Mycobacterium lentiflavum, Mycobacterium malmoense, Mycobacterium marinum, Mycobacterium scrofulaceum, Mycobacterium shimodei, Mycobacterium szulgai, and Mycobacterium xenopi.
A total of 110 cultures that tested positive (≤42 days of incubation) and 50 cultures that tested negative (after 42 days of incubation) for Mycobacterium species in the Mycobacterium Growth Indicator Tube (MGIT) (Bactec MGIT 960 system; Becton, Dickinson Diagnostic Instrument Systems, Sparks, MD) from consecutive clinical respiratory specimens were collected at the National Taiwan University Hospital from January to October 2011. All respiratory specimens were processed and pretreated as previously described (15, 20). These processed respiratory specimens were also inoculated onto Lowenstein-Jensen (LJ) agar slants and cultured at 35°C in a 5% CO2 incubator (15).
The DR. TBDR/NTM IVD kit integrates nucleic acid amplification and specific probe hybridization methods for identification of species in the MTC, species resistant to rifampin, and identification of 15 NTM species. Multiplex PCR was used to amplify the 16S-23S rRNA gene internal transcribed spacer (ITS), the RNA polymerase B subunit (rpoB) gene, and PCR positive-control genes. The ITS ranges in size from approximately 270 to 360 bp and has been found to be a suitable probe for obtaining additional phylogenetic information (17). After amplification, species-specific and genotype-specific probes on the chip hybridize to target-amplified DNA sequences for identification.
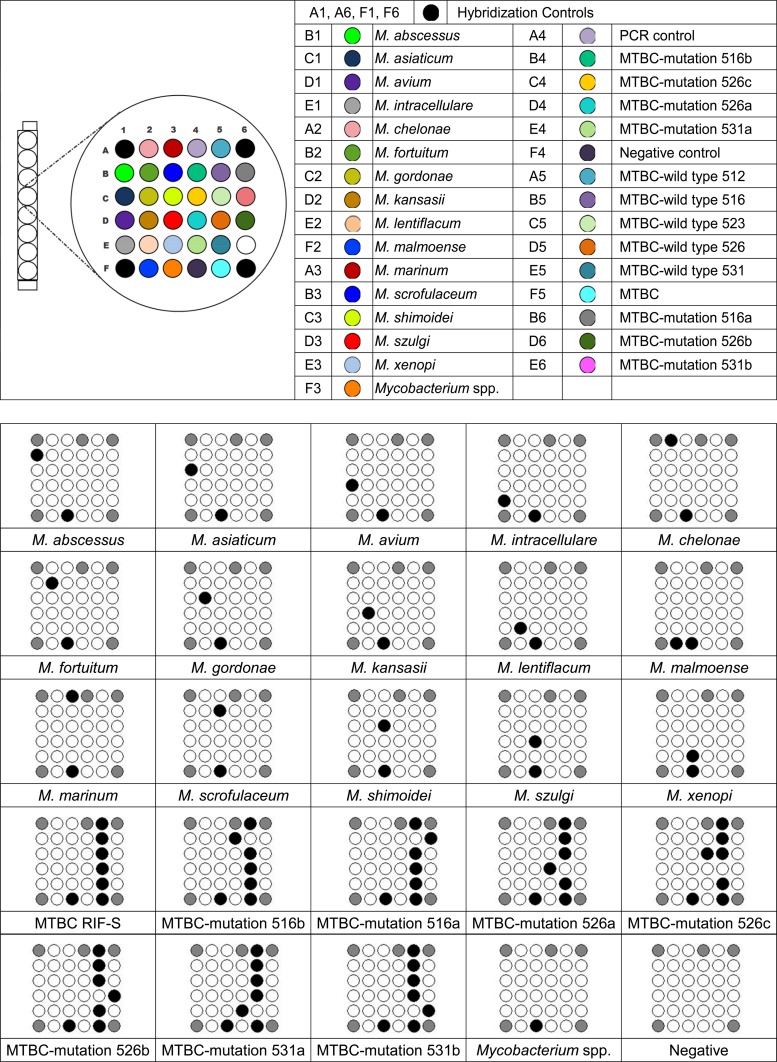
The protocol of the DR. TBDR/NTM IVD kit was as follows. First, 500 μl of each MGIT-positive culture was added to a 0.5-ml portion of E1 (phosphate buffer solution) buffer and centrifuged. With the supernatant removed, the pellet was then resuspended in 1 ml of E1 buffer, centrifuged, and again resuspended with 50 μl of E2 buffer (Tris-HCl solution with Triton X-100). After heating for 20 min and cooling on ice for 5 min followed by centrifugation, the supernatant containing extracted DNA was transferred to a new microcentrifuge vial. Then, 5 μl of extracted DNA was transferred to the PCR tube for amplification. The amplicons from specimen DNA were mixed with DR. Hyb buffer, denatured, and then transferred to a chiller rack at −20°C. A 100-μl aliquot of DR. Hyb buffer was then added to the DR. TBDR/NTM chip, and then 5 μl of PCR product (10 μl/well) was added to each well. The DR. AiM Reader (600 dots per in. [dpi]) was used to read the pattern that developed at the bottom of the well. The template “TBDR/NTM” was used to analyze data. Figure 1 demonstrates the patterns that developed at the bottom of the well of the DR. TBDR/NTM IVD kit.
Fig 1.
DR. TBDR/NTM IVD kit for identification of M. tuberculosis and NTM species. MTBC, M. tuberculosis complex.
The results of mycobacterial species identification by the DR. TBDR/NTM IVD kit and by the conventional biochemical identification method were initially evaluated and compared. When there was a difference in the species identification results obtained by the DR. TBDR/NTM IVD kit and by the conventional methods, 16S rRNA gene sequencing analysis was used for further species identification of the isolates that had produced different results (9). Sequencing analysis of the 16S rRNA gene (1,464 bp) was performed using two primers (8FPL and 1492) as previously described (9).
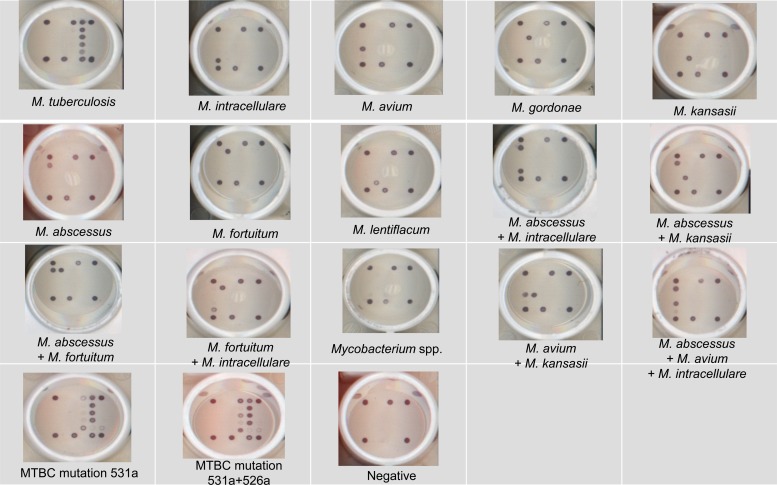
All 50 MGIT negative cultures had negative results by the DR. TBDR/NTM IVD kit. Growth on the LJ agar slants for the 50 specimens was also negative after incubation for 2 months. Table 1 shows the results of species identification by conventional identification methods and the DR. TBDR/NTM IVD kit and 16S rRNA gene sequencing analysis of 110 MGIT cultures. The results obtained by the DR. TBDR/NTM IVD kit are illustrated in Fig. 2.
Table 1.
Results obtained by conventional biochemical methods and the DR. TBDR/NTM IVD kit for positive cultures of Mycobacterium Growth Indicator Tubes (MGIT) and by 16S rRNA sequencing analysis of the isolates with discrepant results of species identification by conventional biochemical methods and the DR. TBDR/NTM IVD kita
| Mycobacterial species (no. of specimens) identified by conventional methods for positive MGIT cultures | Mycobacterial species (no. of specimens) identified by DR. TBDR/NTM IVD kit for positive MGIT cultures | Mycobacterial species (no. of specimens) identified by 16S rRNA sequencing analysis of isolates identified by conventional methods |
|---|---|---|
| M. tuberculosis, non-multidrug-resistant M. tuberculosis (MDR-M. tuberculosis) (16) | M. tuberculosis (14) | |
| M. abscessus (1) | M. tuberculosis | |
| Mycobacterium species (1) | M. tuberculosis | |
| MDR-M. tuberculosis (3) | MDR-M. tuberculosis (3) | |
| M. avium-M. intracellulare complex (28) | M. avium (3) | M. avium (3) |
| M. intracellulare (19) | M. intracellulare (19) | |
| M. avium/M. intracellulare (2) | M. avium | |
| M. lentiflavum (1) | M. lentiflavum | |
| M. abscessus/M. avium/M. intracllulare (2) | M. avium (2) | |
| Mycobacterium species (1) | M. intracellulare (1) | |
| M. abscessus (38) | M. abscessus (36) | |
| M. abscessus/M. kansasii (1) | M. abscessus (1) | |
| M. abscessus/M. avium/M. intracellulare (1) | M. abscessus (1) | |
| M. chelonae (2) | M. abscessus/M. intracellulare (2) | M. chelonae (2) |
| M. fortuitum (10) | M. fortuitum (7) | |
| M. fortuitum/M. intracellulare (1) | M. fortuitum (1) | |
| M. abscessus/M. fortuitum (1) | M. fortuitum (1) | |
| Mycobacterium species (1) | Mycobacterium species | |
| M. gordonae (6) | M. gordonae (6) | |
| M. kansasii (4) | M. kansasii (3) | |
| M. avium/M. kansasii (1) | M. kansasii (1) | |
| M. tuberculosis and M. avium (1) | M. avium/M. intracellulare (1) | M. avium (1) |
| M. abscessus and M. fortuitum (2) | M. abscessus/M. fortuitum (2) | M. abscessus/M. fortuitum (2) |
Isolates marked in bold indicate discrepant results obtained from the three identification methods. The discrepant results were defined as differences in mycobacterial species or complexes between the results from the DR. TBDR/NTM IVD Kit and 16S rRNA sequencing analysis.
Fig 2.
The performances of selected mycobacterial species for identification by the DR. TBDR/NTM IVD kit among 110 positive cultures in Mycobacterium Growth Indicator Tubes.
Though concerns exist regarding the efficiency of the 16S rRNA sequencing method in identifying mycobacteria, it is still widely used as the gold standard in various studies assessing the performance of kits for identification of novel mycobacteria (11, 19, 21). The accuracy rate for identifying M. tuberculosis was 89.5% (17/19). Three rifampin-resistant M. tuberculosis isolates identified by the DR. TBDR/NTM IVD kit were resistant to both isoniazid (1 μg/ml) and rifampin (1 μg/ml) (MDR-M. tuberculosis) as determined using the conventional agar proportion method. In identifying NTM species, the DR. TBDR/NTM IVD kit correctly identified all isolates of M. abscessus, M. fortuitum, M. gordonae, and M. kansasii species. In contrast, it misidentified the two M. chelonae species. The DR. TBDR/NTM IVD kit identified 27 (96.4%) of 28 M. avium complex (MAC) isolates.
The strengths of the DR. TBDR/NTM IVD kit may lie in the correct species identification of NTM. The major flaw of the DR. TBDR/NTM IVD kit is its limited ability to correctly identify all M. tuberculosis isolates. This chip also failed to differentiate between M. abscessus (sensu stricto), M. massiliense, and M. bolletii (2, 4, 16). This may be clinically important, since M. abscessus (sensu stricto) and M. bolletii have induced macrolide resistance whereas M. massiliense does not (2, 6, 7). Furthermore, M. chelonae is a rare cause of NTM lung disease, so the lack of identification of M. chelonae is not of great importance from a practical standpoint.
Not all less-common Mycobacterium species that are known to cause various clinical infections are included in the identification list of the DR. TBDR/NTM IVD kit (1, 3, 9, 14). The clinical significance of less-common NTM species, however, is being criticized (5). Furthermore, it can be difficult to determine which NTM species are important to include in the identification list due to the rapidly emerging database as well as changing taxonomic status of NTM species (5, 10).
In summary, we have tested a novel commercially available kit designed to rapidly identify M. tuberculosis, rifampin-resistant M. tuberculosis, and 15 NTM species from positive MGIT cultures. Though the test specimen number may be relatively small, this kit was highly sensitive at identifying common NTM species in our study. This technology could serve as a rapid and effective method for identifying Mycobacterium species among positive MGIT cultures and as an important epidemiologic tool for diagnosis of NTM disease. Further corroboration of the utility of this technology in population-based studies will hopefully be forthcoming.
Footnotes
Published ahead of print 1 August 2012
REFERENCES
- 1. Adékambi T. 2009. Mycobacterium mucogenicum group infections: a review. Clin. Microbiol. Infect. 15:911–918 [DOI] [PubMed] [Google Scholar]
- 2. Bastian S, et al. 2011. Assessment of clarithromycin susceptibility in strains belonging to the Mycobacterium abscessus group by erm(41) and rrl sequencing. Antimicrob. Agents Chemother. 55:775–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen C, Lai HWCC, Tan CK. 2009. Arthritis caused by Mycobacterium terrae in a patient with rheumatoid arthritis. Int. J. Infect. Dis. 13:e145–e147 [DOI] [PubMed] [Google Scholar]
- 4. Duarte RS, et al. 2009. Epidemic of postsurgical infections caused by Mycobacterium massiliense. J. Clin. Microbiol. 47:2149–2155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Griffith DE, et al. 2007. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am. J. Respir. Crit. Care Med. 175:367–416 [DOI] [PubMed] [Google Scholar]
- 6. Kim HY, et al. 2010. Mycobacterium massiliense is differentiated from Mycobacterium abscessus and Mycobacterium bolletii by erythromycin ribosome methyltransferase gene (erm) and clarithromycin susceptibility patterns. Microbiol. Immunol. 54:347–353 [DOI] [PubMed] [Google Scholar]
- 7. Koh WJ, et al. 2011. Clinical significance of differentiation of Mycobacterium massiliense from Mycobacterium abscessus. Am. J. Respir. Crit. Care Med. 183:405–410 [DOI] [PubMed] [Google Scholar]
- 8. Lai CC, Wang HC. 2011. Clinical significance of Mycobacterium abscessus isolates at a medical center in Northern Taiwan. J. Microbiol. Immunol. Infect. 44:488–489 [DOI] [PubMed] [Google Scholar]
- 9. Lai CC, et al. 2008. Fatal meningitis caused by Mycobacterium nonchromogenicum in a patient with nasopharyngeal carcinoma. Clin. Infect. Dis. 46:325–326 [DOI] [PubMed] [Google Scholar]
- 10. Leao SC, Tortoli E, Euzeby JP, Garcia MJ. 2011. Proposal that Mycobacterium massiliense and Mycobacterium bolletii be united and reclassified as Mycobacterium abscessus subsp. bolletii comb. nov., designation of Mycobacterium abscessus subsp. abscessus subsp. nov. and emended description of Mycobacterium abscessus. Int. J. Syst. Evol. Microbiol. 61(Pt 9):2311–2313 [DOI] [PubMed] [Google Scholar]
- 11. Lee AS, Jelfs P, Sintchenko V, Gilbert GL. 2009. Identification of non-tuberculous mycobacteria: utility of the GenoType Mycobacterium CM/AS assay compared with HPLC and 16S rRNA gene sequencing. J. Med. Microbiol. 58:900–904 [DOI] [PubMed] [Google Scholar]
- 12. Lee MR, et al. 2012. CNS infections caused by Mycobacterium abscessus complex: clinical features and antimicrobial susceptibilities of isolates. J. Antimicrob. Chemother. 67:222–225 [DOI] [PubMed] [Google Scholar]
- 13. Lee MR, et al. 2012. Risk factors for Mycobacterium chelonae-abscessus pulmonary disease persistence and deterioration. J. Infect. 64:228–230 [DOI] [PubMed] [Google Scholar]
- 14. Lindeboom JA, Bruijnesteijn van Coppenraet LE, van Soolingen D, Prins JM, Kuijper EJ. 2011. Clinical manifestations, diagnosis, and treatment of Mycobacterium haemophilum infections. Clin. Microbiol. Rev. 24:701–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu YC, et al. 2011. Differential diagnosis of tuberculous and malignant pleurisy using pleural fluid adenosine deaminase and interferon gamma in Taiwan. J. Microbiol. Immunol. Infect. 44:88–94 [DOI] [PubMed] [Google Scholar]
- 16. Nash KA, Brown-Elliott BA, Wallace RJ., Jr 2009. A novel gene, erm(41), confers inducible macrolide resistance to clinical isolates of Mycobacterium abscessus but is absent from Mycobacterium chelonae. Antimicrob. Agents Chemother. 53:1367–1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Roth A, et al. 1998. Differentiation of phylogenetically related slowly growing mycobacteria based on 16S–23S rRNA gene internal transcribed spacer sequences. J. Clin. Microbiol. 36:139–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shu CC, Wang JT, Wang JY, Yu CJ, Lee LN. 2012. Mycobacterial peritonitis: difference between non-tuberculous mycobacteria and Mycobacterium tuberculosis. Clin. Microbiol. Infect. 18:246–258 [DOI] [PubMed] [Google Scholar]
- 19. Turenne CY, Tschetter L, Wolfe J, Kabani A. 2001. Necessity of quality-controlled 16S rRNA gene sequence databases: identifying nontuberculous Mycobacterium species. J. Clin. Microbiol. 39:3637–3648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang JY, et al. 2004. Performance assessment of a nested-PCR assay (the RAPID BAP-MTB) and the BD ProbeTec ET system for detection of Mycobacterium tuberculosis in clinical specimens. J. Clin. Microbiol. 42:4599–4603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wu TL, et al. 2008. Rapid identification of mycobacteria from smear-positive sputum samples by nested PCR-restriction fragment length polymorphism analysis. J. Clin. Microbiol. 46:3591–3594 [DOI] [PMC free article] [PubMed] [Google Scholar]