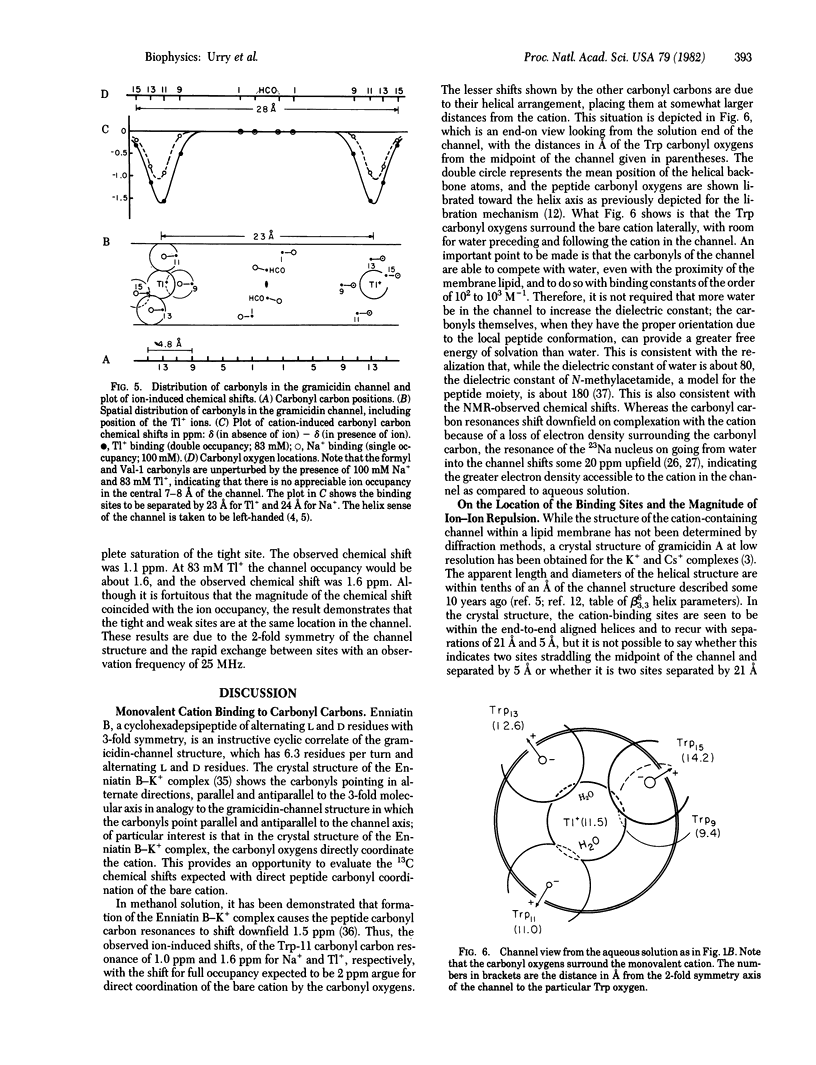
Abstract
Six syntheses of gramicidin A have been carried out, each with 90% 13 C enrichment of a single carbonyl carbon these being the formyl, Val-1, Trp-9, Trp-11, Trp-13, and Trp-15 carbonyl carbons. Each gramicidin A was incorporated as the channel state into phospholipid structures, and the chemical shift of the carbonyl carbon resonance was monitored by 13C NMR as a function of ion concentration. Plots of Na+- and Tl+-induced chemical shifts as a function of carbonyl location in the channel indicate two symmetrically related binding sites centered at the tryptophan carbonyls and separated by 23 A. The absence of ion-induced chemical shifts for the formyl and Val-1 carbonyl carbon resonances indicates that there is no binding site midway through the channel but rather a central free-energy barrier for ion transit through the channel. Ion induced chemical shifts of the tryptophan carbonyl carbon resonances at 100 mM Na+ verify that the tight binding constant (Kbt congruent to 70 M-1), observed with 23Na NMR, results from binding within the channel. This observation and the lateral, triangular distribution of the coordinating Trp-9, -11, and -13 carbonyls combine to provide an experimental demonstration that the carbonyls of the walls of the channel directly coordinate the ion, successfully competing with the polar solvent. With the binding sites verified and localized, it is possible to conclude that the transport mechanism for Na+ is well represented by the case of the two-site model [D. W. Urry, Venkatachalam, C. M., Spisni, A., Läuger, P. & Khaled, M. A. (1980) Proc. Natl. Acad. Sci. USA 77, 2028--2032].
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apell H. J., Bamberg E., Alpes H., Läuger P. Formation of ion channels by a negatively charged analog of gramicidin A. J Membr Biol. 1977 Feb 24;31(1-2):171–188. doi: 10.1007/BF01869403. [DOI] [PubMed] [Google Scholar]
- Bamberg E., Apell H. J., Alpes H. Structure of the gramicidin A channel: discrimination between the piL,D and the beta helix by electrical measurements with lipid bilayer membranes. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2402–2406. doi: 10.1073/pnas.74.6.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamberg E., Läuger P. Temperature-dependent properties of gramicidin A channels. Biochim Biophys Acta. 1974 Oct 29;367(2):127–133. doi: 10.1016/0005-2736(74)90037-6. [DOI] [PubMed] [Google Scholar]
- Dobler M., Dunitz J. D., Krajewski J. Structure of the K+ complex with enniatin B, a macrocyclic antibiotic with K+ transport properties. J Mol Biol. 1969 Jun 28;42(3):603–606. doi: 10.1016/0022-2836(69)90249-6. [DOI] [PubMed] [Google Scholar]
- Eisenman G., Sandblom J., Neher E. Interactions in cation permeation through the gramicidin channel. Cs, Rb, K, Na, Li, Tl, H, and effects of anion binding. Biophys J. 1978 May;22(2):307–340. doi: 10.1016/S0006-3495(78)85491-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henze R., Neher E., Trapane T. L., Urry D. W. Dielectric relaxation studies of ionic processes in lysolecithin-packaged gramicidin channels. J Membr Biol. 1982;64(3):233–239. doi: 10.1007/BF01870890. [DOI] [PubMed] [Google Scholar]
- Hägglund J., Enos B., Eisenman G. Multi-site, multi-barrier, multi-occupancy models for the electrical behavior of single filing channels like those of gramicidin. Brain Res Bull. 1979 Jan-Feb;4(1):154–158. doi: 10.1016/0361-9230(79)90077-7. [DOI] [PubMed] [Google Scholar]
- Koeppe R. E., 2nd, Berg J. M., Hodgson K. O., Stryer L. Gramicidin A crystals contain two cation binding sites per channel. Nature. 1979 Jun 21;279(5715):723–725. doi: 10.1038/279723a0. [DOI] [PubMed] [Google Scholar]
- Masotti L., Spisni A., Urry D. W. Conformational studies on the gramicidin A transmembrane channel in lipid micelles and liposomes. Cell Biophys. 1980 Sep;2(3):241–251. doi: 10.1007/BF02790452. [DOI] [PubMed] [Google Scholar]
- Myers V. B., Haydon D. A. Ion transfer across lipid membranes in the presence of gramicidin A. II. The ion selectivity. Biochim Biophys Acta. 1972 Aug 9;274(2):313–322. doi: 10.1016/0005-2736(72)90179-4. [DOI] [PubMed] [Google Scholar]
- Ramachnandran G. N., Chandrasekaran R. Conformation of peptide chains containing both L- & D-residues. I. Helical structures with alternating L- & D-residues with special reference to the LD-ribbon & the LD-helices. Indian J Biochem Biophys. 1972 Mar;9(1):1–11. [PubMed] [Google Scholar]
- Sandblom J., Eisenman G., Neher E. Ionic selectivity, saturation and block in gramicidin A channels: I. Theory for the electrical properties of ion selective channels having two pairs of binding sites and multiple conductance states. J Membr Biol. 1977 Mar 23;31(4):383–347. doi: 10.1007/BF01869414. [DOI] [PubMed] [Google Scholar]
- Urban B. W., Hladky S. B., Haydon D. A. The kinetics of ion movements in the gramicidin channel. Fed Proc. 1978 Oct;37(12):2628–2632. [PubMed] [Google Scholar]
- Urry D. W. A molecular theory of ion-conductng channels: a field-dependent transition between conducting and nonconducting conformations. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1610–1614. doi: 10.1073/pnas.69.6.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry D. W. Basic aspects of calcium chemistry and membrane interaction: on the messenger role of calcium. Ann N Y Acad Sci. 1978 Apr 28;307:3–27. doi: 10.1111/j.1749-6632.1978.tb41933.x. [DOI] [PubMed] [Google Scholar]
- Urry D. W. Basic aspects of calcium chemistry and membrane interaction: on the messenger role of calcium. Ann N Y Acad Sci. 1978 Apr 28;307:3–27. doi: 10.1111/j.1749-6632.1978.tb41933.x. [DOI] [PubMed] [Google Scholar]
- Urry D. W., Goodall M. C., Glickson J. D., Mayers D. F. The gramicidin A transmembrane channel: characteristics of head-to-head dimerized (L,D) helices. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1907–1911. doi: 10.1073/pnas.68.8.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry D. W., Long M. M., Jacobs M., Harris R. D. Conformation and molecular mechanisms of carriers and channels. Ann N Y Acad Sci. 1975 Dec 30;264:203–220. doi: 10.1111/j.1749-6632.1975.tb31484.x. [DOI] [PubMed] [Google Scholar]
- Urry D. W. Molecular perspectives of monovalent cation selective transmembrane channels. Int Rev Neurobiol. 1979;21:311–334. doi: 10.1016/s0074-7742(08)60642-x. [DOI] [PubMed] [Google Scholar]
- Urry D. W., Spisni A., Khaled A. Characterization of micellar-packaged gramicidin A channels. Biochem Biophys Res Commun. 1979 Jun 13;88(3):940–949. doi: 10.1016/0006-291x(79)91499-2. [DOI] [PubMed] [Google Scholar]
- Urry D. W. The gramicidin A transmembrane channel: a proposed pi(L,D) helix. Proc Natl Acad Sci U S A. 1971 Mar;68(3):672–676. doi: 10.1073/pnas.68.3.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry D. W., Venkatachalam C. M., Spisni A., Bradley R. J., Trapane T. L., Prasad K. U. The malonyl gramicidin channel: NMR-derived rate constants and comparison of calculated and experimental single-channel currents. J Membr Biol. 1980 Jun 30;55(1):29–51. doi: 10.1007/BF01926368. [DOI] [PubMed] [Google Scholar]
- Urry D. W., Venkatachalam C. M., Spisni A., Läuger P., Khaled M. A. Rate theory calculation of gramicidin single-channel currents using NMR-derived rate constants. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2028–2032. doi: 10.1073/pnas.77.4.2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein S., Wallace B. A., Blout E. R., Morrow J. S., Veatch W. Conformation of gramicidin A channel in phospholipid vesicles: a 13C and 19F nuclear magnetic resonance study. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4230–4234. doi: 10.1073/pnas.76.9.4230. [DOI] [PMC free article] [PubMed] [Google Scholar]