Abstract
Ecological studies of thaumarchaeota often apply glycerol dibiphytanyl glycerol tetraether (GDGT)-based intact membrane lipids. However, these components have only been characterized for thaumarchaeota from aquatic environments. Thaumarchaeota have been shown to play an important role in the nitrogen cycle in soil as ammonium oxidizers, and GDGTs are common lipids encountered in soil. We report the core and intact polar lipid (IPL) GDGTs produced by three newly available thaumarchaeota isolated from grassland soil in Austria (“Nitrososphaera viennensis,” group I.1b) and enriched from agricultural soils in South Korea (“Candidatus Nitrosoarchaeum koreensis” MY1, group I.1a; and “Candidatus Nitrososphaera” strain JG1, group I.1b). The soil thaumarchaeota all synthesize crenarchaeol as their major core GDGT, in agreement with the fact that crenarchaeol has also been detected in thaumarchaeota from aquatic environments. The crenarchaeol regioisomer apparently is produced in significant quantities only by soil thaumarchaeota of the I.1b subgroup. In addition, GDGTs with 0 to 4 cyclopentane moieties and GDGTs containing an additional hydroxyl group were detected. The IPL head groups of their membrane lipids comprised mainly monohexose, dihexose, trihexose, phosphohexose, and hexose-phosphohexose moieties. The hexose-phosphohexose head group bound to crenarchaeol occurred in all soil thaumarchaeota, and this IPL is at present the only lipid that is detected in all thaumarchaeota analyzed so far. This specificity and its lability indicate that it is the most suitable biomarker lipid to trace living thaumarchaeota. This study, in combination with previous studies, also suggests that hydroxylated GDGTs occur in the I.1a, but not in the I.1b, subgroup of the thaumarchaeota.
INTRODUCTION
Recently a new phylum in the domain Archaea was proposed, the Thaumarchaeota (4, 42). Known members of the phylum perform ammonium oxidation, although there are indications that some thaumarchaeota have another physiology (21). Ammonia-oxidizing archaea (AOA) play an important role in the biogeochemical cycling of nitrogen since they perform the first step in nitrification (i.e., the aerobic oxidation of ammonium to nitrite) (33, 34). For more than a century, it was thought that this process was only mediated by bacteria (e.g., the genera Nitrosomonas and Nitrosococcus), but environmental studies (18, 24, 51) have indicated that AOA often outnumber ammonia-oxidizing bacteria (AOB) by orders of magnitude in ocean waters, sediments, and soil. The first AOA isolated, “Nitrosopumilus maritimus” SCM1, showed chemoautotrophic growth on ammonium (16), and this has been confirmed by enrichment and characterization of other AOA from aquatic environments representing three phylogenetic lineages within the group I AOA (6, 9, 23, 51).
The first recognition that archaea could also be involved in nitrification in soil in addition to the AOB was based on an environmental genomic study, in which a gene encoding a subunit of the key enzyme ammonia monooxygenase (amoA) originating from soil archaea was identified (45). Subsequent quantification of the amoA gene in a set of different soils revealed that the archaeal amoA gene was often much more abundant than that of AOB (18), suggesting that archaea could represent the most abundant ammonia-oxidizing organisms in soil ecosystems on Earth. Stable isotope probing studies with 13C-labeled CO2 in soil revealed ammonia-oxidizing activity for the chemoautotrophic thaumarchaeota belonging to both groups I.1a and I.1b (32, 52, 53), the two phylogenetic groups to which most of the soil thaumarchaeota belong (1, 7, 22). Recently, a pure isolate of group I.1b was obtained in a laboratory culture (44), and three more AOA belonging to both phylogenetic groups have been enriched from soil (11, 12, 17). While growth on ammonium with nitrite as the oxidation product was demonstrated for all cultures, the pure culture unexpectedly revealed growth dependence on organic substrate, although most of its cellular carbon was fixed from inorganic CO2 (44).
The membrane lipids of thaumarchaeota are composed of glycerol dibiphytanyl glycerol tetraethers (GDGTs) (6, 29, 30, 35, 41). These include a range of GDGTs with 0 to 3 cyclopentane moieties and crenarchaeol, the characteristic GDGT, including 4 cyclopentane moieties and a cyclohexane moiety (Fig. 1), which had not been encountered in any cultivated (hyper)thermophilic crenarchaeota or euryarchaeota until now. For environmental microbiological studies, intact polar lipid (IPL) counterparts of GDGTs (i.e., GDGTs bound to 1 or 2 polar head groups) can be an important tool since they are thought to derive from living microorganisms, as the covalently bound polar head groups are relatively rapidly lost upon cell senescence (8, 48). GDGT-based IPLs have been used previously as tracers of AOA in marine sediments and water columns (2, 27, 39) but not in soil. The additional structural information obtained from polar head group analysis of IPLs may also be potentially useful for inferring the presence of certain phylogenetic lineages.
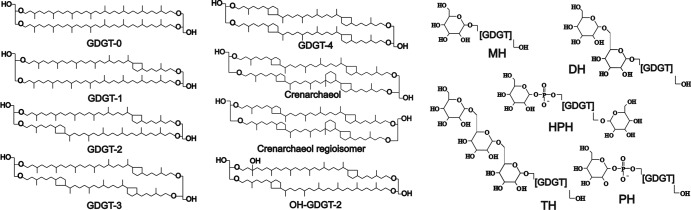
Fig 1.
Structures of diglycerol dialkyl tetraethers (GDGTs) core lipids synthesized by the studied soil thaumarchaeota. GDGT-0 to -4 are common archaeal GDGTs (15); crenarchaeol and its regioisomer were firstly identified in Cenarchaeum symbiosum (41). One example of a tentative structure of a hydroxylated GDGT is given (OH-GDGT-2). These types of GDGTs were recently identified by Liu et al. (20). At the right-hand side, general structures of IPLs detected in the studied soil thaumarchaeota are shown; polar head groups are shown in full structure: monohexose (MH), dihexose (DH), trihexose (TH), phosphohexose (PH), and hexose plus phosphohexose (HPH). It should be noted that the exact positions of the polar head groups are unknown.
A close relationship between crenarchaeol and AOA has been observed in diverse environments (5, 18, 26, 27), and this, coupled to its occurrence in cultivated representatives, suggests that it may in fact be specific to AOA or, more generally, to the phylum Thaumarchaeota. Analysis of archaeal membrane lipids in a wide variety of soils indeed revealed that crenarchaeol was present in almost every sample analyzed (47). Furthermore, a highly significant correlation between the amoA gene abundance of AOA and the concentration of crenarchaeol was reported (18). Together, these lipid biomarker data are consistent with the presumed important role of AOA in nitrification in soil. However, until recently no AOA were isolated from soil, and therefore, the relationship between the presence of AOA and crenarchaeol in soil remained somewhat ambiguous.
Here we compare the GDGT compositions (both as core lipids and as IPLs) of three of the four recently obtained soil cultures of group I.1a and I.1b thaumarchaeota for their membrane composition to test if thaumarchaeota can indeed be sources of GDGTs in soil.
MATERIALS AND METHODS
Growth of cultures.
“Nitrososphaera viennensis” EN76 was isolated from garden soil in Vienna (44). It can be requested from the authors and will soon be available in culture collections. This pure culture was grown as previously described (44). Briefly, the medium was inoculated and incubated aerobically without shaking at pH 7.5 and 37°C with 1 mM ammonium and 0.5 mM pyruvate. The medium also contained carbenicillin (100 μg ml−1). The freshwater medium (FWM) consisted of NaCl (1 g liter−1), MgCl2 · 6H2O (0.4 g liter−1), CaCl2 · 2H2O (0.1 g liter−1), KH2PO4 (0.2 g liter−1), and KCl (0.5 g liter−1). After autoclaving, 1 ml liter−1 nonchelated trace element mixture, 1 ml liter−1 vitamin mixture, 1 ml liter−1 NaFe EDTA solution (7.5 mM) and 2 ml liter−1 sodium bicarbonate (1 M) were added as previously described (16). The pH of the medium was adjusted to 7.5 with addition of 10 ml liter−1 of HEPES buffer (1 M HEPES, 0.6 M NaOH). Growth was assessed by measurements of ammonium consumption using a salicylic acid assay, and measurements of nitrite production were assessed by using the Griess reagent system (Promega), microscopy, and quantitative PCR. Cells were harvested in the late exponential phase at a cell density of ca. 5 × 107 cells ml−1 by centrifugation at 4°C at 9,000 rpm for 40 min. The cell pellet was washed with FWM and stored at −80°C until further analysis.
“Candidatus Nitrosoarchaeum koreensis” MY1, enriched from a soil sample from an agricultural station at Chungbuk National University of South Korea (127°27′E, 95 36°37′N), was grown as previously described (11). Briefly, the uniarchaeal enrichment culture (>90% archaea from the total cell number as determined by fluorescent in situ hybridization [FISH]) (11) was aerobically grown without shaking at 25°C in the dark and with 1 mM ammonium as the sole energy source using an artificial freshwater medium (AFM). The AFM contained the following components per liter of culture medium: 0.4 g MgCl2 · 6H2O, 0.5 g KCl, 0.2 g KH2PO4, 1.0 g NaCl, 0.42 g NaHCO3, and 0.1 g CaCl2 · 2H2O. After autoclaving, 1 ml nonchelated trace element solution (49), 1 ml NaFe EDTA solution (7.5 mM), and 3 ml NaHCO3 (1 M) were added as previously described (11). All other trace components were added as previously described (23). After oxidation of ammonium (typically after 3 weeks), 1% of the culture volume was transferred to fresh AFM. The pH of the medium remained almost constant (6.8 to 7.0) during the culture cycle.
Strain JG1 was also enriched from a soil sample from a Capsicum annuum cultivation field from the same agricultural station of the Chungbuk National University, as described in detail elsewhere (12). Since it is phylogenetically closely related to N. viennensis EN76 (12), we will refer to it as “Candidatus Nitrososphaera” strain JG1. The uniarchaeal enrichment culture (>90% archaea from the total cell number as determined by FISH) (12) was aerobically incubated in AFM, and the culture was grown in the dark without shaking at 37°C. The culture was supplemented with 1 mM ammonium as the sole energy source. The medium pH was kept constant at 6.5 by addition of 1 N NaOH or HCl.
Cells of “Ca. Nitrosoarchaeum koreensis” MY1 and “Ca. Nitrososphaera” strain JG1 were harvested before complete exhaustion of 1 mM ammonium chloride. One liter of culture with ca. 2 × 108 cells ml−1 was centrifuged at 6,700 × g and 4°C for 30 min, washed with cold phosphate (0.015 M; pH 6.0)-buffered saline solution, and stored at −70°C until extraction. This cell material is identical to that previously used for phylogenetic studies (11, 12).
Analysis of intact polar lipids.
Bligh and Dyer extracts (BDEs) containing IPLs of soil thaumarchaeota were obtained as described previously (29). An aliquot of the BDEs was analyzed with high-performance liquid chromatography-electrospray ionization-tandem mass spectrometry (HPLC-ESI-MS-MS) according to Peterse et al. (25) using an Agilent 1200 series LC instrument (Agilent, San Jose, CA), equipped with a thermostated autoinjector and column oven, coupled to a Thermo LTQ XL linear ion trap with an Ion Max source with an electrospray ionization (ESI) probe (Thermo Scientific, Waltham, MA). Briefly, BDE filtered through a 0.45-μm-pore regenerated cellulose (RC) filter (Alltech Associated, Inc., Deerfield, IL) was injected onto a Lichrosphere diol column (250 by 2.1 mm, 5-μm particles; Alltech Associates, Inc., Deerfield, IL) maintained at 30°C. The following elution program was used (flow rate, 0.2 ml min−1): 100% A (1 min), followed by a linear gradient to 66% A–34% B in 17 min (held for 12 min), followed by a linear gradient to 35% A–65% B in 15 min, where A = hexane–2-propanol–formic acid–14.8 M NH3 aqueous (aq) (79:20:0.12:0.04 [vol/vol/vol/vol]) and B = 2-propanol–water–formic acid–14.8 M NH3 aq (88:10:0.12:0.04 [vol/vol/vol/vol]). The total run time was 60 min, with a reequilibration period of 20 min between runs.
Each BDE was analyzed by way of a routine in which a positive ion scan (m/z 400 to 2,000 or 1,000 to 2,000) was followed by a data-dependent MS2 experiment where the base peak in the spectrum was fragmented (normalized collision energy [NCE], 25; isolation width, 5.0; activation Q, 0.175). The procedure was repeated on the 2nd to 4th most abundant ions in the initial spectrum.
Analysis of core GDGTs.
Acid hydrolysis was performed on aliquots of BDEs obtained from the cell material of soil thaumarchaeota, to cleave off polar head groups and release core GDGTs as described previously (29). Core GDGTs were analyzed by HPLC-atmospheric pressure chemical ionization-MS (HPLC-APCI-MS) using a modified procedure from Schouten et al. (37). GDGTs were quantified using selected ion monitoring (SIM) of GDGTs with m/z 1,302, 1,300, 1,298, 1,296, 1,294, 1,292, 1,290, and 1,288 as described previously (29).
Determination of the stable carbon isotopic composition.
The degree of 13C incorporation in archaeal core GDGTs when grown with labeled bicarbonate was measured as described previously (12); they GDGTs were analyzed using HPLC-APCI-MS with a modified SIM range of m/z 1,302 to 1,312 and m/z 1292 to 1,302 for selected time intervals. To determine the degree of labeling in core GDGTs and IPLs, isotope clusters of [M+H]+ (core GDGTs) and of [M+NH4]+/[M+Na]+ adducts (IPLs) were fitted with theoretical calculations on isotope abundance.
RESULTS
Distributions of core GDGTs.
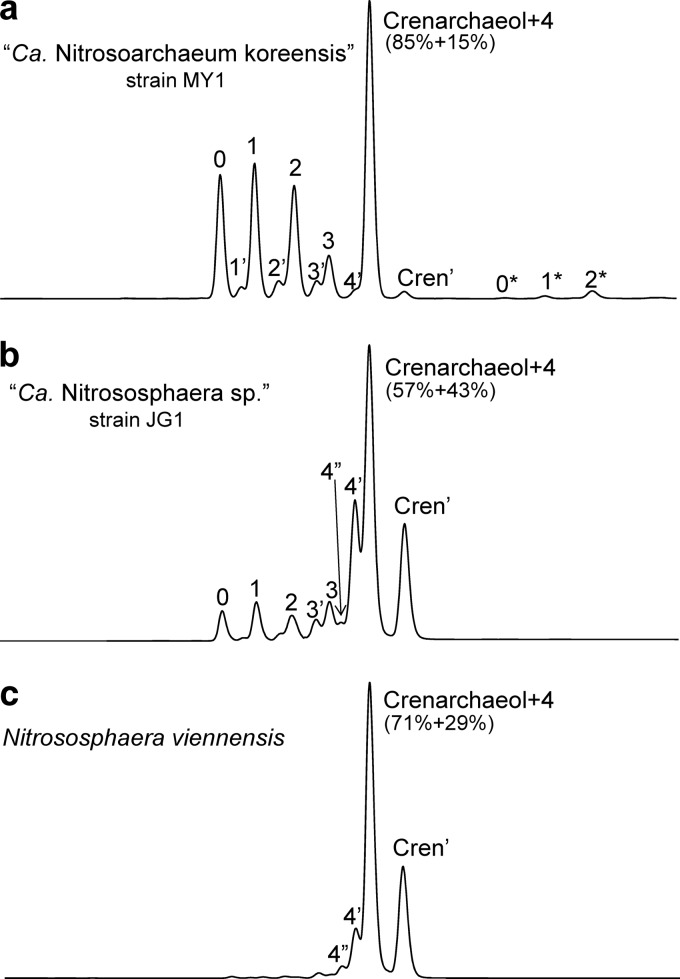
GDGT-0 to -4, crenarchaeol, and the regioisomer of crenarchaeol (Cren′) (see Fig. 1 for structures) were detected in all soil thaumarchaeotal (enrichment) cultures by HPLC-APCI-MS analysis after acid hydrolysis of the BDE (Fig. 2). Crenarchaeol was in all cases abundant, but the relative amounts of the other GDGTs were quite variable (Table 1).
Fig 2.
Base peak chromatogram of core GDGT profiles of “Ca. Nitrosoarchaeum koreensis” MY1 (a), “Ca. Nitrososphaera” strain JG1 (adapted from reference 12) (b), and Nitrososphaera viennensis EN76 (c). Numbered peaks correspond to GDGT structures shown in Fig. 1. Numbers marked with a prime represent regioisomers with the same [M+H]+ as the corresponding GDGT. 4″ represents an unknown isomer of GDGT-4. The percentages listed below the peak “crenarchaeol + 4” indicate the respective contributions of each GDGT to the area of that peak, as corrected according to reference 10. Numbered peaks labeled with an asterisk are tentatively identified as by-products generated by acid hydrolysis of hydroxyl GDGTs with the number indicating the number of rings. They are characterized by [M+H]+ ions at 1,300, 1,298, and 1,296 Da for 0*, 1*, and 2*, respectively. These components have been detected previously (20, 29). Since they are not affected by hydrogenation (PtO2; ethyl acetate/acetic acid), they are probably H-shaped GDGTs formed as artifacts during removal of the polar head group and hydroxyl group by acid hydrolysis.
Table 1.
Fractional abundance of core GDGTs synthesized by the analyzed soil thaumarchaeota and TEX86 ratio and TEX86-derived temperature in comparison with the cultivation temperature
| Parametera | Result for: |
||
|---|---|---|---|
| “Ca. Nitrosoarchaeum koreensis” MY1 | “Ca. Nitrososphaera” strain JG1 | N. viennensis EN76 | |
| Fractional abundance (%) | |||
| GDGT-0 | 13.7 | 2.9 | 0.3 |
| GDGT-1 | 15.3 | 4.0 | 0.2 |
| GDGT-1′ | 1.1 | 0.3 | 0.1 |
| GDGT-2 | 14.3 | 3.4 | 0.2 |
| GDGT-2′ | 2.0 | 0.6 | 0.3 |
| GDGT-3 | 5.5 | 5.0 | 0.5 |
| GDGT-3′ | 2.1 | 2.2 | 1.0 |
| GDGT-4 | 6.3 | 21.8 | 9.2 |
| GDGT-4′ | 1.0 | 14.9 | 8.3 |
| GDGT-4″ | NDd | 2.1 | 2.0 |
| Cren | 35.8 | 29.2 | 55.1 |
| Cren′ | 1.1 | 13.7 | 22.9 |
| GDGT-0* | 0.1 | ND | ND |
| GDGT-1* | 0.4 | ND | ND |
| GDGT-2* | 1.3 | ND | ND |
| TEX86b | 0.58 | 0.85 | 0.99 |
| Temp (°C)c | 22 | 34 | 38 |
| Cultivation temp (°C) | 25 | 37 | 37 |
The numbers refer to the GDGTs shown in Fig. 1. GDGTs indicated with a prime are probably the regioisomers (antiparallel). GDGTs indicated with an asterisk represent by-products formed upon acid hydrolysis of IPLs containing a hydroxylated GDGT as the core lipid.
For a definition, see article by Schouten et al. (36).
Calculated by T = 68.4 × log (TEX86) + 38.6 (13).
ND, not determined.
The enrichment culture belonging to the I.1a group (i.e., “Ca. Nitrosoarchaeum koreensis”) showed the highest relative abundances of GDGT-0 to -2 (ca. 14% of total quantified GDGTs for each GDGT), while the contribution of GDGT-3 (6%) and GDGT-4 (6%; i.e., 15% of the peak area representing coeluting GDGT-4 and crenarchaeol) (Fig. 2a) was lower (Table 1). Remarkably, GDGT-1 to -4 were accompanied by earlier-eluting GDGT isomers. These have previously been identified as the corresponding regioisomers (41). Three later-eluting minor peaks labeled 0* to 2* (Fig. 2) were also detected, which were absent in the soil thaumarchaeota from the I.1b group. These unknown GDGTs have been identified previously in AOA enriched from marine sediments falling in group I.1a (29). Recently, they have been described as unknown products of hydroxylated GDGTs formed by acid hydrolysis (20).
The core GDGT distribution of N. viennensis (group I.1b) was dominated by crenarchaeol (55%), the regioisomer of crenarchaeol (23%), GDGT-4 (9%), and its presumed regioisomer (8%) (Fig. 2b and Table 1). The GDGT distribution of “Ca. Nitrososphaera sp.” JG1 (group I.1b) enrichment culture from South Korea was similar to that of N. viennensis, although it contained higher relative abundances of GDGT 0 to 3. However, GDGT-4 (22%), its regioisomer (15%), and the crenarchaeol regioisomer (14%) were in addition to crenarchaeol (29%) also abundant, just like in N. viennensis.
Distribution of IPL-GDGTs.
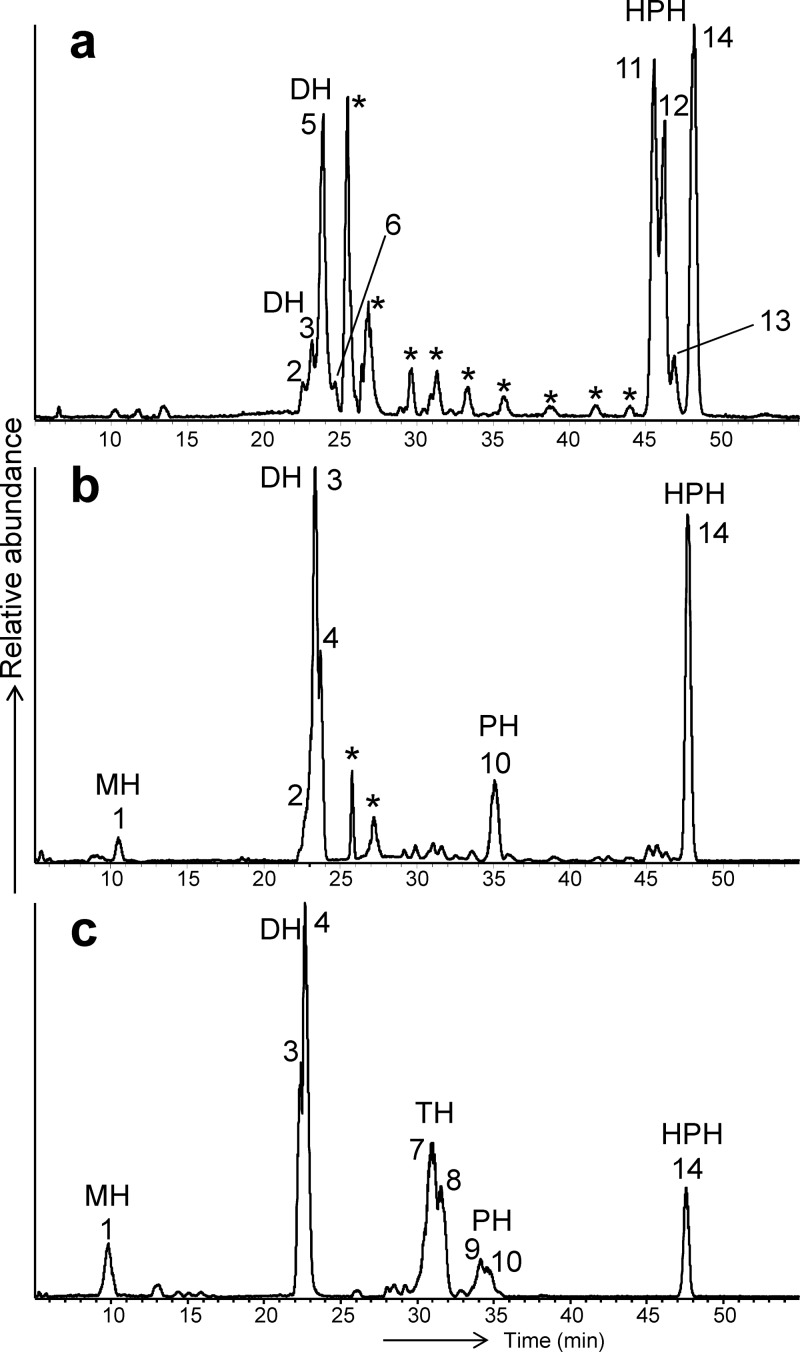
HPLC-ESI-MS-MS analysis of the three thaumarchaeotal enrichment cultures revealed the presence of two major clusters of peaks and a number of smaller peaks in the BDEs (Fig. 3), which were identified as GDGT-based IPLs. Our analyses revealed that all three soil thaumarchaeota synthesized GDGT-based IPLs containing both glyco- and phospho-head groups. The composition and relative abundances of these IPL classes differed substantially between different cultures (Fig. 3 and Table 2). Identification of the IPLs was performed by detection of characteristic ammoniated (NH4+) and/or sodiated (Na+) molecules ([M + 18]+ and [M + 23]+, respectively) in MS1, followed by detection of characteristic fragment ions in MS2 that led to identification of the core GDGTs associated with the different IPLs (Table 2).
Fig 3.
Base peak chromatogram of GDGT-based IPL profiles (m/z 1,000 to 2,000) of “Ca. Nitrosoarchaeum koreensis” MY1 (a), “Ca. Nitrososphaera” strain JG1 (b), and Nitrososphaera viennensis EN76 (c). Peak numbers correspond to the IPL structures described in Table 2. Stars indicate contaminants.
Table 2.
Main IPLs identified in the three soil thaumarchaeotal (enrichment) cultures with the characteristic ions, polar head groups, and major core GDGTs
| Peaka | Characteristic ion(s) (mass in Da) |
IPL head groupc | Main core GDGT(s) | |
|---|---|---|---|---|
| MS1b | Lost in MS2 | |||
| 1 | 1,471; [M+NH4]+ | 179 | MH | Crenarchaeol |
| 2 | 1,639, 1,637; [M+NH4]+ | 179, 341 | DH | GDGT-2, GDGT-3 |
| 3 | 1,635; [M+NH4]+ | 179, 341 | DH | GDGT-4 |
| 4 | 1,633; [M+NH4]+ | 179, 341 | DH | Crenarchaeol |
| 5 | 1,655; [M+NH4]+d | 179, 197, 341, 359 | DH | OH-GDGT-2 |
| 6 | 1,651; [M+NH4]+ | 179, 197, 341, 359 | DH | OH-GDGT-4 |
| 7 | 1,797; [M+NH4]+ | 179, 341, 503 | TH | GDGT-4 |
| 8 | 1,795; [M+NH4]+ | 179, 341, 503 | TH | Crenarchaeol |
| 9 | 1,558; [M+Na]+ | 180, 242, 260 | PH | GDGT-4 |
| 10 | 1,556; [M+Na]+ | 180, 242, 260 | PH | Crenarchaeol |
| 11 | 1,728; [M+Na]+ | 162, 180, 242, 260 | HPH | GDGT-0 |
| 12 | 1,726; [M+Na]+ | 162, 180, 242, 260 | HPH | GDGT-1 |
| 13 | 1,724; [M+Na]+ | 162, 180, 242, 260 | HPH | GDGT-2 |
| 14 | 1,718; [M+Na]+ | 162, 180, 242, 260 | HPH | Crenarchaeol |
The numbers refer to peaks indicated in Fig. 3.
Typically, [M+NH4]+, [M+Na]+, and [M+H]+ ions were observed; the ion reported was used for the MS2 experiment.
MH, monohexose; DH, dihexose; TH, trihexose; PH, phosphohexose; HPH, hexose-phosphohexose (Fig. 1).
Ions at 1,657 and 1,653 Da are also present in smaller relative amounts (comparable to that of peak 6) and are likely derived from DH IPLs with OH-GDGT-1 and OH-GDGT-3 as the core lipids.
All of the peaks in the first section of the base peak chromatogram (represented by peaks 1 to 8; Fig. 3, retention time window of 0 to 32 min) were identified as GDGT-based glycolipids. The first peak represents a monohexose (MH) with a crenarchaeol core lipid. Peaks 2 to 4 represent dihexose (DH) GDGTs, with each peak associated with a different major core lipid (Table 2). The mass spectral information of peak 5 and 6 was consistent with an IPL containing two sugar moieties and core GDGTs (dominated by the one with two cyclopentane moieties) with a molecular mass 16 Da higher than that of the regular core lipids. These IPLs were only observed in the BDE of “Ca. Nitrosoarchaeum koreensis” (Fig. 3). These IPLs, previously described as containing a 180-Da head group, have been identified in N. maritimus SCM1 (35) and other AOA (29) and have also been recovered from marine sediments (19). Recently, these IPLs were identified as GDGTs containing a tertiary hydroxyl group, analogous to the well-known hydroxyl archaeols (20). Peaks 7 and 8 were only detected in N. viennensis and reflect trihexose (TH) IPLs, characterized by the loss of three sugar moieties (Table 2).
The second section of the base peak chromatogram (represented by peaks 9 to 14) (Fig. 3; retention time window of 34 to 49 min) represented GDGT-based phospholipids. Peaks 9 and 10 represented IPLs with a phosphohexose (PH) head group coupled to GDGT-4 and crenarchaeol, respectively. Peaks 11 to 14, representing IPLs with a hexose head group in addition to a phosphohexose head group (HPH) coupled to GDGT-0, GDGT-1, GDGT-3, and crenarchaeol. They were abundant in the IPL profiles of all three thaumarchaeotal (enrichment) cultures (Fig. 3), but their distribution was strikingly different since in N. viennensis and “Ca. Nitrososphaera” only HPH IPLS with crenarchaeol as the core lipid were detected, whereas for “Ca. Nitrosoarchaeum koreensis” a more complex distribution was evident (Fig. 3).
DISCUSSION
Core GDGTs as phylogenetic markers.
A decade ago, crenarchaeol was postulated to be a specific membrane lipid for marine Thaumarchaeota based on its ubiquity in the marine environment and its presence in the only available uniarchaeal enrichment culture at that time (41). This hypothesis has been confirmed by the identification of crenarchaeol synthesis by N. maritimus (35), an AOA isolated from tropical aquarium gravel (16) and in three marine AOA enrichment cultures, including “Candidatus Nitrosoarchaeum limnia” (29). Molecular ecological studies have indicated, however, that thaumarchaeota are not restricted to the marine environment and also thrive in freshwater, hot springs, and soil environments. In agreement with this, crenarchaeol has indeed been detected in such settings (3, 26, 47) and enrichment of AOA from hot springs (6, 9) and subsequent studies of their membrane lipids (6, 30) confirmed the production of crenarchaeol by nonmarine AOA. On the basis of these culture studies, it has been postulated that crenarchaeol is an appropriate biomarker for AOA (30). Various environmental microbiological studies indeed report a significant correlation between the archaeal amoA gene abundance and expression and crenarchaeol concentration (18, 26, 27). Furthermore, labeling studies of North Sea water using 13C-labeled bicarbonate over the annual cycle revealed incorporation of inorganic carbon into crenarchaeol at the time of maximum archaeal amoA abundance, directly showing chemoautotrophic production of Thaumarchaeota biomass (28). However, recently thaumarchaeota, falling in the I.1b cluster, have been identified in a wastewater treatment plant that produced crenarchaeol, contained amoA genes, but did not seem to perform chemoautotrophic ammonium oxidation (21), suggesting that crenarchaeol is a biomarker for Thaumarchaeota but not necessarily in all environments for active ammonia oxidizers.
The three (enrichment) cultures of thaumarchaeota from soil falling in both the I.1a (“Ca. Nitrosoarchaeum koreensis”) and I.1b (N. viennensis and “Ca. Nitrososphaera”) phylogenetic clusters all produce crenarchaeol (Fig. 2 and Table 1). This is in agreement with the idea that crenarchaeol is specific for Thaumarchaeota and confirms that crenarchaeol present in soil is derived from thaumarchaeota probably involved in ammonium oxidation. It also confirms the presence of crenarchaeol in group I.1b Thaumarchaeota that was so far only evident from the study of the moderately thermophile “Ca. Nitrososphaera gargensis,” isolated from a hot spring (30). In all three soil thaumarchaeota, crenarchaeol is the most abundant GDGT (Table 1). However, there is a marked difference in the fractional abundance of the crenarchaeol regioisomer; it is quite abundant in the two soil thaumarchaeota falling in group I.1b, whereas it is only present in small relative amounts in the group I.1a thaumarchaeote “Ca. Nitrosoarchaeum koreensis” (Table 1; Fig. 2). This is fully consistent with other studies: the group I.1a N. maritimus (35) and marine surface sediment enrichment cultures (29) contain no or low abundances of the crenarchaeol regioisomer (<5% of summed crenarchaeol) (Table 3), whereas it is far more dominant in “Ca. Nitrososphaera gargensis” (24%) (Table 3), which belongs to the group I.1b cluster. “Candidatus Nitrosocaldus yellowstonii,” falling into the so-called thermophilic phylogenetic cluster, does also not contain substantial amounts of the crenarchaeol regioisomer (6), but its relative abundance was not reported. This indicates that relatively high abundances of the crenarchaeol regioisomer (>10 to 20%) (Table 3) may be indicative for group I.1b thaumarchaeota. This is consistent with environmental GDGT data (Table 3) since soils, which host in addition to group I.1a thaumarchaeota group I.1b thaumarchaeota, have in general higher abundances of the crenarchaeol regioisomer relative to crenarchaeol than marine and lacustrine samples, where group I.1b thaumarchaeota are far less common than group I.1a thaumarchaeota.
Table 3.
Crenarcheaol regioisomer fractional abundance in pure and enrichment cultures of thaamarchaeota and environmental samples
| Culture or sample type | Crenarchaeol regioisomer fractional abundancea | Reference |
|---|---|---|
| Thaumarchaeotal cultures | ||
| Nitrosopumilus maritimus | 0.001 | 35 |
| “Ca. Nitrosoarchaeum limnia” | 0.01 | 29 |
| Enrichment SJ′ | 0.05 | 29 |
| Enrichment AR′ | 0.02 | 29 |
| “Ca. Nitrosoarchaeum koreensis” MY1 | 0.03 | This study |
| Nitrososphaera viennensis EN76 | 0.42 | This study |
| “Ca. Nitrososphaera” strain JG1 | 0.47 | This study |
| “Ca. Nitrososphaera gargensis” (n = 4) | 0.24 ± 0.01 | 30 |
| Environmental samples | ||
| Soils (n = 118) | 0.09 ± 0.05 | 47 |
| Kilimanjaro soils (n = 16) | 0.15 ± 0.07 | 40 |
| Marine sediments (n = 426) | 0.04 ± 0.04 | 14 |
| Lake sediments (n = 28) | 0.01 ± 0.01 | 3 |
Calculated as [crenarchaeol regioisomer]/([crenarchaeol regioisomer] + [crenarchaeol]). Values with “±” are averages based on the number of samples (n) specified ± the standard deviation of the average.
Other GDGTs produced by thaumarchaeota, apart from crenarchaeol and its regioisomer, are GDGTs with 0 to 4 cyclopentane moieties. GDGT-0 is produced in similar relative abundances to crenarchaeol, except in the case of the group 1.Ib archaeon “Ca. Nitrososphaera gargensis” (30), while GDGT-1 to -4 are typically produced in lower relative amounts than GDGT-0 and crenarchaeol, although their fractional abundances vary substantially between cultures (6, 29, 30, 35). The GDGT distribution of N. viennensis is rather different from those of most other thaumarchaeotal cultures; apart from crenarchaeol, it only contains GDGT-4 in high relative abundance (Fig. 3) with only low (<1%) (Table 1) fractional abundances of GDGT-1 to -3. In this respect, it is somewhat comparable to that of the moderately thermophilic “Ca. Nitrososphaera gargensis” (30). This may be a consequence of the relatively high temperature (37°C) at which it was cultured (discussed below). GDGT distributions of the two other soil thaumarchaeota studied here do not show the high abundance of GDGT-0 as observed in other marine thaumarchaeota (29, 35); it only accounts for 3 to 14% of total GDGTs. The high abundance of GDGT-4 (6 to 22%) (Table 1) in all three soil thaumarchaeota is remarkable, as it is typically not observed in such high abundances in other thaumarchaeota. However, the thaumarchaeotal enrichment culture SJ obtained from marine sediment also contained GDGT-4 with a high fractional abundance of 12% (29). GDGT-4 is often the most abundant GDGT in (hyper)thermophilic archaea and is thought to promote dense packing of the membrane, which helps to maintain its integrity at high temperatures (46).
Lipids with the same molecular weight as GDGT-1 to -4 but with slightly earlier elution times have been identified in environmental samples (26) and in sedimentary thaumarchaeotal enrichments (29). GDGT-4′, isolated from the thermophilic archaeon Sulfolobus solfataricus, was identified by nuclear magnetic resonance (NMR) spectroscopy as the regioisomer of GDGT-4 (41) and, by inference, GDGT-1′ to -3′ are probably the regioisomers of GDGT-1 to -3. Their presence in the soil thaumarchaeota confirms that these lipids in soil are likely also derived from thaumarchaeota. In “Ca. N. koreensis,” GDGTs apparently produced from hydroxylated GDGTs were detected in relatively small amounts (Fig. 2a) (GDGT-0*, -1*, and -2*). These GDGTs have been detected previously in other group I.1a AOA enriched from marine sediments (29) but are absent in all studied group I.1b thaumarchaeota, indicating that they may be characteristic of this lineage of thaumarchaeota. This is evidently related to the absence of IPLs with a hydroxylated GDGT core in group I.1b thaumarchaeota (see below).
It is still not precisely known why the GDGT distributions of thaumarchaeotal cultures differ from each other. This may have to do with physical conditions in addition to a genetic control. The GDGT composition of archaea is substantially affected by temperature, as has been shown for both (hyper)thermophilic crenarchaeota and euryarchaeota (46) and mesophilic thaumarchaeota (50). Based on this observation, the TEX86 temperature proxy has been developed (13, 31, 36). It uses an empirical correlation between the GDGT distribution (GDGT-1 to -3 and the crenarchaeol regioisomer) and the temperature of oceans and lakes to estimate temperatures in the past based on fossil GDGT assemblages. The rationale of this proxy is that planktonic marine and lacustrine thaumarchaeota adapt their GDGT distribution according to temperature and that this signal is recorded in sediments upon fossilization of the GDGTs. The widely varying abundance of the crenarchaeol regioisomer in group I.1a and I.1b thaumarchaeota observed here is worrying in this respect because it suggests a strong genetic control on the GDGT distribution in addition to a physiological effect. However, when the GDGT distributions of the three studied soil thaumarchaeota are used to calculate temperatures using the TEX86, it becomes apparent that for all of them the TEX86-derived temperature is in reasonable agreement with the actual growth temperatures (25 and 37°C) (Table 1), even for the group I.1b thaumarchaeota that are characterized by a high abundance of the crenarchaeol regioisomer relative to that of crenarcheaol.
Distribution of IPL-GDGTs in thaumarchaeota.
Five thaumarchaeotal (enrichment) cultures have been previously profiled for their GDGT-IPL composition; N. maritimus (35), “Ca. Nitrososphaera gargensis” (a group I.1b thaumarchaeote enriched from a hot spring) (30), and three thaumarchaeotal cultures enriched from sediments (29). The four thaumarchaeota falling in the I.1a group all have similar IPL profiles (Table 4). The group I.1a thaumarchaeote enriched from soil, “Ca. Nitrosoarchaeum koreensis,” analyzed here has a similar profile to the other four group I.1a thaumarchaeota (Table 4): HPH IPLs are the most important peaks in the profile (Fig. 3a), and the minor IPLs are similar to those encountered in the other group I.1a thaumarchaeota (Table 4). The DH IPL with the hydroxyl-GDGT core lipids seems to be characteristic of this group since these IPLs were not detected in the group I.1b thaumarchaeota (Table 4). The two soil thaumarchaeota from group I.1b analyzed here are both characterized by DH IPLs as the major peak in the IPL profile (Fig. 3; Table 4). These IPLs are present in much lower abundance in the group I.1a thaumarchaeota (Table 4). It is also not present in large amounts in the group I.1b thaumarchaeote “Ca. Nitrososphaera gargensis,” which has an IPL profile distinct from the other thaumarchaeotal (enrichment) cultures because of the abundance of unknown MH + 176 and DH + 176 IPLs (30) (Table 4). The TH IPLs were unique for N. viennensis (Table 4). IPLs with >2 sugar groups appear to be characteristic for (hyper)thermophilic archaea (15), so the presence of this IPL may be the consequence of the higher cultivation temperature at which it was grown (i.e., 37°C), although they were not observed for “Ca. Nitrososphaera” grown at the same temperature. It seems that both genetic and physiological factors play a role in the IPL composition.
Table 4.
IPL profiles of group I.1a and I.1b thaumarchaeota
| Thaumarchaeote or culture condition | IPL profilea |
Reference | |||||||
|---|---|---|---|---|---|---|---|---|---|
| MH | MH + 176b | DH | DHc | DH + 176b | TH | PH | HPH | ||
| Group I.1a | |||||||||
| Nitrosopumilus maritimus | + | + | ++ | tr | +++ | 35 | |||
| “Ca. Nitrosoarchaeum limnia” | + | + | tr | +++ | 29 | ||||
| Enrichment SJ′ | + | ++ | +++ | 29 | |||||
| Enrichment AR′ | + | + | +++ | 29 | |||||
| “Ca. Nitrosoarchaeum koreensis” MY1 | + | ++ | +++ | This study | |||||
| Group I.1b | |||||||||
| Nitrososphaera viennensis EN76 | + | +++ | ++ | + | + | This study | |||
| “Ca. Nitrososphaera” strain JG1 | tr | +++ | + | ++ | This study | ||||
| “Ca. Nitrososphaera gargensis” | tr | + | + | ++ | + | +++ | 30 | ||
+++, most abundant in base peak chromatogram (BPC); ++, 50 to 100%; +, 10 to 50%; tr, trace. Note that the ionization efficiency under the LC/MS conditions of different IPLs may vary substantially, which indicates that peak heights in BPCs are not necessarily a reflection of their abundance.
Possibly a glucuronic acid head group.
This IPL contains a hydroxy-GDGT core instead of a more common GDGT core.
Our analyses reveal that GDGTs are associated with particular head groups: in case of the group I.1b soil thaumarchaeote the HPH and MH IPLs only contain crenarchaeol but not GDGT-4 as a core GDGT (Table 2), whereas GDGT-4 is only associated with DH, TH, and PH IPLs. In “Ca. Nitrososphaera gargensis,” crenarchaeol is also the only core GDGT encountered in the HPH IPL (30). For “Ca. Nitrosoarchaeum koreensis” this is quite different since in addition to crenarchaeol, GDGT-0, -1, and, to a lesser extent, -2 (the major GDGTs) (Fig. 2a) form the core of the HPH IPLs, while the minor GDGTs, GDGT-2 and -3, are predominantly associated with DH (Table 2). This situation is quite comparable to those of N. maritimus and the sedimentary thaumarchaeotal enrichment cultures (all belonging to group I.1a) (29, 35). Hydroxylated GDGTs with 1 to 4 cyclopentane moieties only possess a DH head group, in good agreement what has been reported for marine sediments (20), although these DH IPLs have the hydroxylated GDGT-0, and not GDGT-2, as their predominant core lipid. These findings indicate that there is a strong biological control of the head group composition of the different core GDGTs, probably to optimize the physical properties of the membrane composition of the thaumarchaeota. Future studies will have to address if this “GDGT speciation” is a function of temperature (and pH), since this has important implications for our understanding of the fate of fossil GDGT (38). The similarities in “GDGT speciation” between thaumarchaeota of group I.1a on the one hand and group I.1b on the other hand suggest that there is also a genetic control. All thaumarchaeota examined so far produce IPL GDGTs with the HPH head group in often relatively large amounts and with crenarchaeol as one of the core GDGTs (Table 4), indicating that HPH crenarchaeol is probably the most useful biomarker for detecting living AOA and other living, closely related thaumarchaeota (21) in a wide variety of environments.
Recently it has been proposed that GDGT-containing archaea in deep sea sediments biosynthesize the glycerol units of GDGTs de novo but use the isoprenoid alkyl chains from relic archaeal membranes and detritus (43). This was evident from a labeling study with 13C-labeled glucose; the label was incorporated into the glycerol backbone of archaeal membranes, but the isoprenoid alkyl chain of the GDGT remained unlabeled. It was suggested that this was a strategy to cope with the energy-limited conditions in the deep sea environment. We previously determined incorporation of labeled bicarbonate into the core GDGTs (11, 12). The experiments performed here also allowed us to look for isotopic heterogeneity within the IPLs produced by soil thaumarchaeota. However, the degree of 13C labeling of the IPLs produced during chemoautotrophic growth was similar to that of the core lipid (Table 5), indicating that the polar head groups were labeled to the same extent as the GDGT core lipid.
Table 5.
Fractional abundance of 13C in IPL GDGT and the corresponding core GDGT in labeling experiments with 5% 13C-labeled bicarbonate during chemoautotrophic growth of “Ca. Nitrosoarchaeum koreensis” and “Ca. Nitrososphaera”
| Thaumarchaeote and GDGT type |
13C fractional abundance ofa: |
|
|---|---|---|
| IPL GDGT | Core GDGT | |
| “Ca. Nitrosoarchaeum koreensis” MY1 | ||
| Crenarchaeol, HPH | 0.051 | 0.053 |
| GDGT-0, HPH | 0.048 | 0.051 |
| “Ca. Nitrososphaera” strain JG1 | ||
| Crenarchaeol, HPH | 0.054 | 0.054b |
| Crenarcheaol, PH | 0.054 | 0.054b |
| GDGT-4, DH | 0.056 | 0.055c |
Determined by fitting isotope clusters of [M+H]+ (core GDGTs) and of [M+NH4]+/[M+Na]+ adducts (IPLs) with theoretical calculations of isotope abundance.
Measured on the crenarchaeol regioisomer due to coelution of crenarcheaol with GDGT-4.
Measured on GDGT-4′ due to coelution of GDGT-4 with crenarchaeol.
Conclusions.
We report the core and IPL GDGTs synthesized by three thaumarchaeota that originate from garden and agricultural soils, respectively, derived from Austria and South Korea. They all produce crenarchaeol, confirming earlier studies that crenarchaeol is a biomarker for AOA. However, there are indications (21) that crenarchaeol may also be produced by thaumarchaeota that are not performing ammonium oxidation and may thus be considered a more general biomarker for the phylum Thaumarchaeota. The investigation of more representatives of this phylum will be required in order to decide if crenarchaeol would be more accurately renamed thaumarchaeol in the future. Marked differences were apparent in the abundance of the crenarchaeol regioisomer, with a much higher abundance in group I.1b than in group I.1a thaumarchaeota. This fits with GDGT distributions observed in environmental samples. Major GDGT-based IPLs synthesized by the three soil thaumarchaeota contained HPH head groups attached to crenarchaeol in all three cases, and GDGT-0, -1, and -2 and hydroxylated GDGTs in the case of the group I.1a soil thaumarchaeota. Comparison with other thaumarchaeota indicates that there is strong genetic control of the IPL signature. IPLs with hydroxylated GDGTs as core lipids have so far only been detected in group I.1a thaumarchaeota.
ACKNOWLEDGMENTS
The research leading to these results has received funding from the European Research Council under the European Union's Seventh Framework Programme (FP7/2007-2013)/ERC grant agreement no. 226600. The work of M.S. and C.S. was supported by the Austrian Science Fund (grant P23000 to C.S.).
Footnotes
Published ahead of print 20 July 2012
REFERENCES
- 1. Auguet JC, Barberan A, Casamayor EO. 2010. Global ecological patterns in uncultured Archaea. ISME J. 4:182–190 [DOI] [PubMed] [Google Scholar]
- 2. Biddle JF, et al. 2006. Heterotrophic Archaea dominate sedimentary subsurface ecosystems off Peru. Proc. Natl. Acad. Sci. U. S. A. 103:3846–3851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Blaga CI, Reichart G-J, Heiri O, Sjuijs A, Sinninghe Damsté JS. 2009. Tetraether membrane lipid distributions in water-column particulate matter and sediments: a study of 47 European lakes along a north-south transect. J. Paleolimnol. 41:523–540 [Google Scholar]
- 4. Brochier-Armanet C, Boussau B, Gribaldo S, Forterre P. 2008. Mesophilic crenarchaeota: proposal for a third archaeal phylum, the Thaumarchaeota. Nat. Rev. Microbiol. 6:245–252 [DOI] [PubMed] [Google Scholar]
- 5. Coolen MJL, et al. 2007. Putative ammonia-oxidizing Crenarchaeota in suboxic waters of the Black Sea: a basin-wide ecological study using 16S ribosomal and functional genes and membrane lipids. Environ. Microbiol. 9:1001–1016 [DOI] [PubMed] [Google Scholar]
- 6. de la Torre JR, Walker CB, Ingalls AE, Konneke M, Stahl DA. 2008. Cultivation of a thermophilic ammonia oxidizing archaeon synthesizing crenarchaeol. Environ. Microbiol. 10:810–818 [DOI] [PubMed] [Google Scholar]
- 7. Hansel CM, Fendorf S, Jardine PM, Francis CA. 2008. Changes in bacterial and archaeal community structure and functional diversity along a geochemically variable soil profile. Appl. Environ. Microbiol. 74:1620–1633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Harvey HR, Fallon RD, Patton JS. 1986. The effect of organic matter and oxygen on the degradation of bacterial membrane lipids in marine sediments. Geochim. Cosmochim. Acta 50:795–804 [Google Scholar]
- 9. Hatzenpichler R, et al. 2008. A moderately thermophilic ammonia-oxidizing crenarchaeote from a hot spring. Proc. Natl. Acad. Sci. U. S. A. 105:2134–2139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hopmans EC, Schouten S, Pancost RD, van der Meer MTJ, Sinninghe Damsté JS. 2000. Analysis of intact tetraether lipids in archaeal cell material and sediments by high performance liquid chromatography/atmospheric pressure chemical ionization mass spectrometry. Rapid Commun. Mass Spectrom. 14:585–589 [DOI] [PubMed] [Google Scholar]
- 11. Jung MY, et al. 2011. Enrichment and characterization of an autotrophic ammonia-oxidizing archaeon of mesophilic crenarchaeal group I.1a from an agricultural soil. Appl. Environ. Microbiol. 77:8635–8647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kim JG, et al. 2012. Cultivation of an ammonia-oxidizing archaeon of thaumarchaeotal group I.1b from an agricultural soil. Environ. Microbiol. 14:1528–1543 [DOI] [PubMed] [Google Scholar]
- 13. Kim JH, et al. 2010. New indices and calibrations derived from the distribution of crenarchaeal isoprenoid tetraether lipids: implications for past sea surface temperature reconstructions. Geochim. Cosmochim. Acta 74:4639–4654 [Google Scholar]
- 14. Kim J-H, Schouten S, Hopmans EC, Donner B, Sinninghe Damsté JS. 2008. Global sediment core-top calibration of the TEX86 paleothermometer in the ocean. Geochim. Cosmochim. Acta 72:1154–1173 [Google Scholar]
- 15. Koga Y, Nishihara M, Morii H, Akagawa-Matsushita M. 1993. Ether polar lipids of methanogenic bacteria: structures, comparative aspects, and biosyntheses. Microbiol. Rev. 57:164–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Könneke ME, de la Torre JR, Walker CB, Waterbury JB, Stahl DA. 2005. Isolation of an autotrophic ammonia-oxidizing marine archaeon. Nature 437:543–546 [DOI] [PubMed] [Google Scholar]
- 17. Lehtovirta-Morley LE, Stoecker K, Vilcinskas A, Prosser JI, Nicol GW. 2011. Cultivation of an obligate acidophilic ammonia oxidizer from a nitrifying acid soil. Proc. Natl. Acad. Sci. U. S. A. 108:15892–15897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Leininger S, et al. 2006. Archaea predominate among ammonia-oxidizing prokaryotes in soils. Nature 442:806–809 [DOI] [PubMed] [Google Scholar]
- 19. Lipp JS, Hinrichs KU. 2009. Structural diversity and fate of intact polar lipids in marine sediments. Geochim. Cosmochim. Acta 73:6816–6833 [Google Scholar]
- 20. Liu X-L, Lipp JS, Simpson JH, Lin Y-S, Summons RE, Hinrichs K-U. 2012. Mono- and dihydroxyl glycerol dibiphytanyl glycerol tetraethers in marine sediments: identification of both core and intact polar lipid forms. Geochim. Cosmochim. Acta 89:102–115 [Google Scholar]
- 21. Mussmann M, et al. 2011. Thaumarchaeotes abundant in refinery nitrifying sludges express amoA but are not obligate autotrophic ammonia oxidizers. Proc. Natl. Acad. Sci. U. S. A. 108:16771–16776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ochsenreiter T, Selezi D, Quaiser A, Bonch-Osmolovskaya L, Schleper C. 2003. Diversity and abundance of Crenarchaeota in terrestrial habitats studied by 16S RNA surveys and real time PCR. Environ. Microbiol. 5:787–797 [DOI] [PubMed] [Google Scholar]
- 23. Park B-J, et al. 2010. Cultivation of autotrophic ammonia-oxidizing archaea from marine sediments in coculture with sulfur-oxidizing bacteria. Appl. Environ. Microbiol. 76:7575–7587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Park S-J, Park B-J, Rhee S-K. 2008. Comparative analysis of archaeal 16S rRNA and amoA genes to estimate the abundance and diversity of ammonia-oxidizing archaea in marine sediments. Extremophiles 12:605–615 [DOI] [PubMed] [Google Scholar]
- 25. Peterse F, et al. 2011. Identification and distribution of intact polar branched tetraether lipids in peat and soil. Org. Geochem. 42:1007–1015 [Google Scholar]
- 26. Pitcher A, Schouten S, Sinninghe Damsté JS. 2009. In situ production of crenarchaeol in two California hot springs. Appl. Environ. Microbiol. 75:4443–4451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pitcher A, et al. 2011. Niche segregation of ammonia-oxidizing Archaea and anammox bacteria in the Arabian Sea oxygen minimum zone as determined by a combined intact polar lipid and gene-based approach. ISME J. 12:1896–1904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pitcher A, Wuchter C, Siedenberrg K, Schouten S, Sinninghe Damsté JS. 2011. Crenarchaeol tracks winter blooms of ammonia-oxidizing Thaumarchaeota in the coastal North Sea. Limnol. Oceanogr. 56:2308–2318 [Google Scholar]
- 29. Pitcher A, et al. 2011. Core and intact polar glycerol dibiphytanyl glycerol tetraether lipids of ammonia-oxidizing archaea enriched from marine and estuarine sediments. Appl. Environ. Microbiol. 77:3468–3477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pitcher A, et al. 2010. Crenarchaeol dominates the membrane lipids of Candidatus Nitrososphaera gargensis, a thermophilic group I.1b archaeon. ISME J. 4:542–552 [DOI] [PubMed] [Google Scholar]
- 31. Powers L, et al. 2010. Applicability and calibration of the TEX86 paleothermometer in lakes. Org. Geochem. 41:404–413 [Google Scholar]
- 32. Pratscher J, Dumont MG, Conrad R. 2011. Ammonia oxidation coupled to CO2 fixation by archaea and bacteria in an agricultural soil. Proc. Natl. Acad. Sci. U. S. A. 108:4170–4175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Prosser JI, Nicol GW. 2008. Relative contributions of archaea and bacteria to aerobic ammonia oxidation in the environment. Environ. Microbiol. 10:2931–2941 [DOI] [PubMed] [Google Scholar]
- 34. Schleper C, Nicol GW. 2010. Ammonia-oxidising archaea—physiology, ecology and evolution. Adv. Microb. Physiol. 57:1–41 [DOI] [PubMed] [Google Scholar]
- 35. Schouten S, et al. 2008. Intact membrane lipids of “Candidatus Nitrosopumilus maritimus,” a cultivated representative of the cosmopolitan mesophilic group I crenarchaeota. Appl. Environ. Microbiol. 74:2433–2440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schouten S, Hopmans EC, Schefuß E, Sinninghe Damsté JS. 2002. Distributional variations in marine crenarchaeotal membrane lipids: a new tool for reconstructing ancient sea water temperatures? Earth Planet. Sci. Lett. 204:265–274 [Google Scholar]
- 37. Schouten S, Huguet C, Hopmans EC, Kienhuis MVM, Sinninghe Damsté JS. 2007. Analytical methodology for TEX86 paleothermometry by high-performance liquid chromatography/atmospheric pressure chemical ionization-mass spectrometry. Anal. Chem. 79:2940–2944 [DOI] [PubMed] [Google Scholar]
- 38. Schouten S, Middelburg JJ, Hopmans EC, Sinninghe Damsté JS. 2010. Fossilization and degradation of intact polar lipids in deep subsurface sediments: a theoretical approach. Geochim. Cosmochim. Acta 74:3806–3814 [Google Scholar]
- 39. Schubotz F, Wakeham SG, Lipp JS, Fredricks HF, Hinrichs KU. 2009. Detection of microbial biomass by intact polar membrane lipid analysis in the water column and surface sediments of the Black Sea. Environ. Microbiol. 11:2720–2734 [DOI] [PubMed] [Google Scholar]
- 40. Sinninghe Damsté JS, Ossebaar J, Schouten S, Verschuren D. 2008. Altitudinal shifts in the branched tetraether lipid distribution in soil from Mt. Kilimanjaro (Tanzania): implications for the MBT/CGT continental palaeothermometer. Org. Geochem. 39:1072–1076 [Google Scholar]
- 41. Sinninghe Damsté JS, Schouten S, Hopmans EC, van Duin ACT, Geenevasen JAJ. 2002. Crenarchaeol: the characteristic core glycerol dibiphytanyl glycerol tetraether membrane lipid of cosmopolitan pelagic crenarchaeota. J. Lipid Res. 43:1641–1651 [DOI] [PubMed] [Google Scholar]
- 42. Spang A, et al. 2010. Distinct gene set in two different lineages of ammonia-oxidizing archaea supports the phylum Thaumarchaeota. Trends Microbiol. 18:331–340 [DOI] [PubMed] [Google Scholar]
- 43. Takano Y, et al. 2010. Sedimentary membrane lipids recycled by deep-sea benthic archaea. Nat. Geosci. 3:858–861 [Google Scholar]
- 44. Tourna M, et al. 2011. Nitrososphaera viennensis, an ammonia oxidizing archaeon from soil. Proc. Natl. Acad. Sci. U. S. A. 108:8420–8425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Treusch AH, et al. 2005. Novel genes for nitrite reductase and Amo-related proteins indicate a role of uncultivated mesophilic crenarchaeota in nitrogen cycling. Environ. Microbiol. 7:1985–1995 [DOI] [PubMed] [Google Scholar]
- 46. Uda I, Sugai A, Itoh YH, Itoh T. 2001. Variation in molecular species of polar lipids from Thermoplasma acidophilum depends on growth temperature. Lipids 36:103–105 [DOI] [PubMed] [Google Scholar]
- 47. Weijers JWH, Schouten S, Spaargaren OC, Sinninghe Damsté JS. 2006. Occurrence and distribution of tetraether membrane lipids in soils: implications for the use of the TEX86 proxy and the BIT index. Org. Geochem. 37:1680–1693 [Google Scholar]
- 48. White DC, Davis WM, Nickels JS, King JD, Bobbie RJ. 1979. Determination of the sedimentary microbial biomass by extractable lipid phosphate. Oecologia 40:51–62 [DOI] [PubMed] [Google Scholar]
- 49. Widdel F, Bak F. 1992. Gram-negative mesophilic sulfate-reducing bacteria, p 3352–3378 In Balows A, Dworkin M, Schlegel HG, Truper H. (ed), The prokaryotes, 2nd ed, vol 4 Springer, New York, NY [Google Scholar]
- 50. Wuchter C, Schouten S, Coolen MJL, Sinninghe Damsté JS. 2004. Temperature-dependent variation in the distribution of tetraether membrane lipids of marine Crenarchaeota: implications for TEX86 paleothermometry. Paleoceanography 19:PA4028 doi:10.1029/2004PA001041 [Google Scholar]
- 51. Wuchter C, et al. 2006. Archaeal nitrification in the ocean. Proc. Natl. Acad. Sci. U. S. A. 103:12317–12322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Xia W, et al. 2011. Autotrophic growth of nitrifying community in an agricultural soil. ISME J. 5:1226–1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhang LM, et al. 2010. Autotrophic ammonia oxidation by soil thaumarchaea. Proc. Natl. Acad. Sci. U. S. A. 107:17240–17245 [DOI] [PMC free article] [PubMed] [Google Scholar]