Abstract
This work reports on the identification and molecular characterization of a two-component regulatory system (2CRS), encoded by serRK, which is believed to control the expression of the ser2003 locus in Bifidobacterium breve UCC2003. The ser2003 locus consists of two genes, Bbr_1319 (sagA) and Bbr_1320 (serU), which are predicted to encode a hypothetical membrane-associated protein and a serpin-like protein, respectively. The response regulator SerR was shown to bind to the promoter region of ser2003, and the probable recognition sequence of SerR was determined by a combinatorial approach of in vitro site-directed mutagenesis coupled to transcriptional fusion and electrophoretic mobility shift assays (EMSAs). The importance of the serRK 2CRS in the response of B. breve to protease-mediated induction was confirmed by generating a B. breve serR insertion mutant, which was shown to exhibit altered ser2003 transcriptional induction patterns compared to the parent strain, UCC2003. Interestingly, the analysis of a B. breve serU mutant revealed that the SerRK signaling pathway appears to include a SerU-dependent autoregulatory loop.
INTRODUCTION
Bifidobacteria are high-G+C-content, Gram-positive microorganisms which are considered to constitute an important bacterial group among the microbiota of the human gastrointestinal tract (GIT) (47, 51, 55). In recent years, they have become a focus of scientific attention due to the perceived beneficial or probiotic action that they generate in their host (4, 22, 30, 58). This has catalyzed their exploitation as active ingredients of various commercial functional foods (43). The health-promoting effects attributed to (certain strains of) bifidobacteria include prevention of infection by pathogenic bacteria (59, 60), immunostimulatory (10) and anticarcinogenic capabilities (23), protection against infectious diarrhea (38), lowering of serum cholesterol (61), anti-inflammatory activity (17), and alleviation of lactose maldigestion (14). Understanding the mechanisms of probiosis is essential for their rational inclusion as probiotics in functional foods (20); however, only a small number of genetic loci have been implicated in the interaction between bifidobacteria and their host (8, 9, 34, 54, 56). Among them, the serpin (serine protease inhibitor)-encoding genes have been proposed to be involved in host-bacterium cross talk. Serpins represent a large class of protease inhibitors that are involved in regulating a wide spectrum of protease-mediated processes (13, 42). They are widely distributed in higher eukaryotic organisms but are also found in some viruses, where they appear to modulate virus-host interactions and viral infectivity (13). In addition, serpins have recently been identified in bacteria and Archaea and are thus present in all major domains of life (16, 18). Among members of the genus Bifidobacterium, serpin-encoding homologs are not widely distributed but are restricted to a small number of species in this genus (50). The physiological function of serpins produced by bacteria is not fully understood, and it has been hypothesized that intestinal bacteria, such as bifidobacteria, produce serpins to protect themselves against host-derived proteases, providing an advantage for survival in a highly complex and competitive environment (19, 51). Inhibition of human proteases, such as α-antitrypsin and human neutrophil elastase, by bifidobacterial serpins suggests an intriguing possibility that their release at the sites of intestinal inflammation is beneficial because it reduces exaggerated serine protease activity, which in turn may cause pathological tissue damage (19, 44, 48).
Two-component regulatory systems (2CRSs) are employed extensively in nature by microorganisms to modify their cellular physiology in response to alterations in environmental conditions (for reviews, see references 5, 11, 15, 27, 31, and 45). A 2CRS typically consists of a membrane-associated sensor protein or histidine protein kinase (HPK), which monitors one or more environmental parameters, and a cytoplasmic effector protein or response regulator (RR), which induces a specific cellular adaptive response. Although 2CRSs have previously been identified in different bifidobacterial genomes (26, 41, 57), their physiological function remains largely unknown. Just one bifidobacterial 2CRS, the phoRP system from Bifidobacterium breve UCC2003, has been functionally characterized and shown to regulate the response to phosphate limitation (2).
The presence of serpin-encoding genes in several bifidobacterial species as well as their induced transcription in response to different proteases has previously been described; however, the molecular mechanism driving this regulated expression has remained elusive. In this work, we report that the specific protease-mediated, transcriptional induction of the serpin-encoding gene from B. breve UCC2003 is subject to autoregulatory control mediated by a 2CRS, here designated SerRK.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
Bacterial strains and plasmids used in this study are listed in Table 1. B. breve UCC2003 and its derivatives were grown at 37°C in de Man-Rogosa-Sharpe (MRS) medium or reinforced clostridial medium (RCM) (Oxoid, Hampshire, England) as standing cultures supplemented with 0.05% cysteine or on RCM agar plates containing 1.5% (wt/vol) agar under anaerobic conditions in a modular atmosphere-controlled system (Davidson & Hardy Ltd., Dublin, Ireland). Escherichia coli strains were grown in LB medium under aerobic conditions on a rotary shaker (150 rpm) at 37°C or plated on LB agar plates. Where appropriate, media were supplemented with ampicillin (Amp; 100 μg ml−1), erythromycin (Er; 100 μg ml−1), chloramphenicol (Cm; 20 μg ml−1), kanamycin (Kn; 50 μg ml−1), or tetracycline (Tet; 10 μg ml−1) for E. coli and Cm (2 μg ml−1) or Tet (10 μg ml−1) for B. breve.
Table 1.
Bacterial strains and plasmids used in this work
| Strain or plasmid | Genotype or phenotype | Reference or source |
|---|---|---|
| Strains | ||
| B. breve | ||
| UCC2003 | Isolate from nursling stool | 28 |
| UCC2003-serR | pORI19-tetW-serR insertion mutant of UCC2003 | This study |
| UCC2003-serU | pORI19-tetW-serU-containing gene insertion mutant of UCC2003 | This study |
| UCC2003-serR-pPKserRK | B. breve UCC2003-serR containing serRK region in pPKCm vector | This study |
| UCC2003-serU-pPKserU | B. breve UCC2003-serR containing fusion of serU gene p44 promoter and cloned in pPKCm vector | This study |
| UCC2003-lacZ | pORI19-tetW-lacZ insertion mutant of UCC2003 | M. O'Connell Motherway and D. van Sinderen (unpublished data) |
| E. coli | ||
| EC101 | E. coli JM101 with repA from pWV01 integrated in chromosome, Kmr | 21 |
| XL1-Blue | Δ(mcrA)183Δ(mcrCB-hsdSMR-mrr)173 endA1 supE44 thi-1 recA1 gyrA96 relA1 lac [F′ proAB lacIqZΔM15 Tn10 (Tetr)] | Stratagene |
| Plasmids | ||
| pNZ44 | Cmr, pNZ8048 containing constitutive p44 promoter from Lactococcus lactis chromosome | 29 |
| pNZ44-serR | Cmr; pNZ44 derivative containing translational fusion of serR-containing DNA fragment to p44 promoter | This study |
| pNZ44-serU | Cmr; pNZ44 derivative containing translational fusion of serU-containing DNA fragment to p44 promoter | This study |
| pQE30 | Apr, IPTG-inducible promoter expression plasmid for N-terminal His tag fusions | Qiagen |
| pQE30-serR | Apr, pQE30 carrying the serR gene of B. breve UCC2003 | This study |
| pQE30-serR-T | Apr, pQE30 carrying the truncated serR gene of B. breve UCC2003 | This study |
| pAM5 | pBC1-pUC19 Tetr [tetW] | 3 |
| pORI19 | Emr repA ori+; cloning vector | 21 |
| pORI19-serR | Internal 525-bp fragment of serR cloned in pORI19 | This study |
| pORI19-serU | Internal 696-bp fragment of serU gene cloned in pORI19 | This study |
| pORI19-tet-serR | Internal 525-bp fragment of serR and tetW cloned in pORI19 | This study |
| pORI19-tet-serU | Internal 696-bp fragment of serU gene and tetW cloned in pORI19 | This study |
| pUC18 | Apr lacZα; general cloning vector | 62 |
| pUC18:inter-serpin | Apr, pUC18 carrying the ser2003 promoter | This study |
| pUC18:inter-serpin-Mut1 | Apr, pUC18 carrying mutation 1 at the ser2003 promoter | This study |
| pUC18:inter-serpin-Mut2 | Apr, pUC18 carrying mutation 2 at the ser2003 promoter | This study |
| pUC18:inter-serpin-Mut3 | Apr, pUC18 carrying mutation 3 at the ser2003 promoter | This study |
| pUC18:inter-serpin-Mut4 | Apr, pUC18 carrying mutation 4 at the ser2003 promoter | This study |
| pNZ272 | Cmr, pSH71 derivative containing promoterless glucuronidase gene for promoter screening | 35 |
| pNZ272-inter-serpin | pNZ272 derivative carrying the ser2003 promoter | This study |
| pNZ272-inter-serpin Mut1 | pNZ272 derivative carrying the ser2003 promoter with mutation 1 | This study |
| pNZ272-inter-serpin-Mut2 | pNZ272 derivative carrying the ser2003 promoter with mutation 2 | This study |
| pNZ272-inter-serpin-Mut3 | pNZ272 derivative carrying the ser2003 promoter with mutation 3 | This study |
| pNZ272-inter-serpin-Mut4 | pNZ272 derivative carrying the ser2003 promoter with mutation 4 | This study |
| pPKCm | Cmr, E. coli-Bifidobacterium shuttle vector | 6 |
| pPK-serU | pPKCM derivative carrying the p44 promoter and the serU gene | This study |
| pPK-serRK | pPKCM derivative carrying the p44 promoter and the serRK genes | This study |
DNA techniques and transformation.
The general procedures used for DNA manipulation were previously described (39) unless otherwise specified. Restriction enzymes and T4 DNA ligase were obtained from Roche (Roche Diagnostics, East Sussex, United Kingdom) and used according to the manufacturer's instructions. PCRs were performed using Taq PCR master mix (Qiagen GmbH, Hilden, Germany). Synthetic oligonucleotides were synthesized by MWG Biotech AG (Ebersberg, Germany) and are described in Table S1 in the supplemental material. PCR products were purified using the High-Pure PCR product purification kit (Roche). Plasmid DNA was introduced into E. coli and B. breve by electrotransformation as previously described (28). Plasmid DNA was obtained from B. breve and E. coli using the QIAprep spin plasmid miniprep kit (Qiagen GmbH, Hilden, Germany). An initial lysis step was performed using 30 mg ml−1 of lysozyme for 30 min at 37°C as part of the plasmid purification protocol for B. breve.
Bioinformatics.
Sequence data were obtained from the Artemis-mediated (37) genome annotations of B. breve UCC2003 (34). Database searches were performed using the nonredundant sequence database accessible at the National Center for Biotechnology Information website (http://www.ncbi.nlm.nih.gov) using TBLASTN, BLASTX, and BLASTP (1). Sequence analysis was performed using Clone Manager Professional Suite (Sci-Ed Central), and multiple local alignments were carried out with ClustalW software (46).
SerR overexpression in B. breve.
In order to achieve SerR overexpression in B. breve UCC2003, the complete coding region of the serR gene, including its presumed ribosomal binding site, was amplified by PCR from chromosomal DNA of B. breve UCC2003 using the primer combination SerR-for and SerR-rev (see Table S1 in the supplemental material). The resulting PCR product was restricted at the unique PstI and XbaI sites, which had been incorporated at the 5′ end of the forward and reverse primers, respectively; ligated into the similarly digested pNZ44 vector; and introduced into E. coli XL1-Blue by electroporation. The expected structure of the resulting plasmid, pNZ44-serR, was verified by sequence analysis prior to its transfer to B. breve UCC2003 by electroporation.
Transcriptome analysis.
DNA microarrays containing oligonucleotide primers representing each of the 1,864 annotated genes of the genome of B. breve UCC2003 (34) were obtained from Agilent Technologies (Palo Alto, CA). Overnight cultures of B. breve UCC2003 with pNZ44 or pNZ44-serR were used to inoculate 50 ml of MRS broth. Such cultures were then incubated at 37°C until an optical density at 600 nm (OD600) of 0.5 was reached, at which point cells were harvested by centrifugation at 8,000 × g for 1 min at room temperature and immediately frozen prior to RNA isolation. Methods for cell disruption, RNA isolation, RNA quality control, cDNA synthesis, and indirect labeling were used as described previously (63). Labeled cDNA was hybridized using the Agilent Gene Expression hybridization kit (catalog no. 5188-5242) as described in the Agilent Two-Color Microarray-Based Gene Expression Analysis v4.0 manual (publication number G4140-90050). Following hybridization, the microarrays were washed as described in the manual and scanned using Agilent's DNA G2565A microarray scanner. The scans were converted to data files with Agilent's Feature Extraction software (version 9.5).
DNA microarray data were processed as previously described (12, 52). Differential expression tests were performed with the Cyber-T implementation of a variant of the t test (25). A gene was considered differentially expressed between a test condition and a control when an expression ratio of >3 or <0.33 relative to the result for the control was obtained with a corresponding P value that was equal to or less than 0.001. Final data presented are the averages of 2 independent array experiments. The array data were deposited in the GEO database under accession number GSE37835.
EMSA.
In order to obtain the full serR gene or a 5′-truncated serR (here designated serR-T, lacking codons 1 through 114 of the full-length serR gene) from B. breve UCC2003, PCR amplification was performed using primer combination serR-p30F and serR-p30R or serR-CterF and serR-CterR, respectively (see Table S1 in the supplemental material). The DNA fragments encompassing serR or serR-T were restricted with BamHI and HindIII and ligated to pQE30 digested with the same enzyme combination. The ligation mixtures were introduced into E. coli XL1-Blue by electrotransformation, and transformants were selected based on Amp resistance. Ampr transformants were shown by restriction and sequence analysis to contain the expected plasmids, which were designated pQE30:serR and pQE30:serR-T. Crude cell extracts were prepared as follows: overnight cultures of E. coli XL1-Blue independently harboring pQE30:serR and pQE30:serR-T were diluted 1:50 into fresh LB medium supplemented with 100 μg/ml of Amp and grown at 37°C with vigorous shaking. At an OD600 of 0.6, expression of the relevant recombinant protein was induced by the addition of 1 mM isopropyl-β-d-thiogalactoside (IPTG). Growth was continued for 4 h, and cells from 200 ml of culture were collected by centrifugation (10 min, 8,000 × g, 4°C) in an Avanti J-20 XP centrifuge (Beckman Coulter, Mijdrecht, The Netherlands). The pellets were washed with 50 ml of buffer A (50 mM NaHPO4, 300 mM NaCl, 10 mM imidazole, 3.5% glycerol, 1 mM β-mercaptoethanol, pH 8.0) and stored at −80°C for future use. Cell extracts were prepared using 106-μm glass beads and a Mini-BeadBeater-8 cell disrupter (Biospec Products, Bartlesville, OK). After homogenization, the glass beads and cell debris were sedimented by centrifugation (30 min, 20,000 × g, 4°C) and the supernatant containing the cytoplasmic fraction was retained. DNA fragments representing different portions of the ser2003 promoter region were prepared by PCR using IRD800-labeled primer pairs (MWG Biotech; see Table S1 in the supplemental material). Electrophoretic mobility shift assays (EMSAs) were performed as described previously (53). Following electrophoresis, the presence and mobility position of the fluorescent PCR products in the gel were detected using an Odyssey infrared imaging system (Li-Cor Biosciences UK Ltd., Cambridge, United Kingdom) and captured using the supplied software Odyssey V3.0.
Site-directed mutagenesis.
The ser2003 promoter region (170 bp; identified based on the identical and characterized ser210B promoter region of B. breve 210B described by Turroni et al. [50]) was PCR amplified using chromosomal DNA of B. breve UCC2003 as a template and primer combination pUC-interF and pUC-interR (see Table S1 in the supplemental material), primers which had restriction sites incorporated at their 5′ ends. The generated DNA fragment was cut with EcoRI and XbaI and ligated into the similarly digested vector pUC18 (Table 1). The ligation mixture was introduced by electroporation into E. coli XL1-Blue, and transformants were selected based on Amp resistance. The expected structure of the resulting plasmid, pUC18-interSer, was verified by restriction and sequence analysis prior to its use as a probe for the site-directed mutagenesis. In vitro site-directed mutagenesis of the putative SerR binding site was carried out using primer combinations Mut-1F/Mut-1R, Mut-2F/Mut-2R, Mut-3/Mut-3R, and Mut-4F/Mut-4R, respectively (Table S1) and the QuikChange II site-directed mutagenesis kit (Stratagene, La Jolla, CA) according to the manufacturer's instructions. Sequence analysis of the resulting plasmids was performed to confirm the presence of the specific mutations.
Transcriptional fusions and GusA assays.
The ser2003 promoter region, as well as the site-directed mutated versions of this region (see above), were amplified by PCR using primer combination pNZ272-InterF/pNZ272-InterR (see Table S1 in the supplemental material), which contained EcoRI and BglII restriction sites at their 5′ ends, respectively. Amplicons were digested with EcoRI and BglII and cloned upstream of the promoterless gusA gene present in the similarly restricted pNZ272 reporter vector (Table 1). Ligation mixtures were independently introduced by electroporation in E. coli XL1-Blue competent cells. The resulting plasmids, once verified by restriction and sequence analysis, were then transferred to B. breve UCC2003 by electroporation. GusA activity assays in B. breve UCC2003 were carried out in triplicate by independent assay as previously described by Cronin et al. (6) with the following modifications: cells were grown in MRS medium to an OD600 of approximately 0.4 to 0.5, at which point ser2003 transcription was induced by the addition of pancreatic elastase (final concentration of 1 mg/ml).
Construction of serR and serU insertion mutants.
The insertion mutants were constructed using a previously described method (33). An internal fragment of the serR gene (between codons 26 and 201) and one of the serU gene (from codon 164 to codon 336) were amplified by PCR using B. breve UCC2003 chromosomal DNA as a template and the oligonucleotide primers serRKOF and serRKOR and serpinKOF and serpinKOR, respectively (see Table S1 in the supplemental material). The two generated amplicons were restricted with PstI and XbaI (which had been incorporated in the PCR primers); individually cloned into similarly restricted pORI19, an Ori+ RepA− integration plasmid (21); and introduced into E. coli EC101 by electroporation. The expected structures of the recombinant plasmids, designated pORI19-serR and pORI19-serpin, were confirmed by restriction mapping and sequencing. The tetW gene, amplified by PCR using pAM5 plasmid DNA as the template (3) and primers tetWf and tetWr (Table S1), thereby incorporating a SalI site, was cloned into the SalI-cut pORI19-serR and pORI19-serpin plasmids (flanking the previously inserted fragment) to generate plasmids pORI19-tet-serR and pORI19-tet-serpin. The latter plasmids were introduced into E. coli EC101 harboring pNZ-M.BbrII-M.BbrIII to facilitate methylation (33), and the resulting methylated pORI19-tet-serR and pORI19-tet-serpin were then independently introduced into B. breve UCC2003 by electroporation. Mutants were selected on reinforced clostridial agar (RCA) plates supplemented with Tet, and their expected integrated position in either the serR or the serU gene was checked by PCR. A single colony of each of the B. breve UCC2003 derivatives containing either a disrupted serR or serU gene was selected and designated B. breve UCC2003-serR or B. breve UCC2003-serU, respectively.
Complementation experiments.
For the construction of the complementation plasmids pPK-serRK and pPK-serU, DNA fragments encompassing serRK, including its native promoter region, and the coding sequence of serU were generated by PCR amplification from chromosomal DNA of B. breve UCC2003 using KOD DNA polymerase and primer combination serRKF and serRKR or SerU-for and SerU-rev, respectively (see Table S1 in the supplemental material). The serRK-containing amplicon was digested with NotI and XbaI and ligated to similarly digested pPKCM1. The serU amplicon was digested with PstI and XbaI and ligated to similarly digested pNZ44. The ligations were introduced into E. coli XL1-Blue by electroporation, and the resulting plasmids were verified by sequence analysis and designated pPK-serRK and pNZ44-serU. Plasmid pNZ44-serU was used as a template for PCR using p44F and SerU-rev in order to amplify serU together with the p44 promoter. This amplicon was digested with NotI and ligated to similarly digested pPKCm1 before transformation into E. coli XL1-Blue. The integrity of the resulting plasmid, designated pPK-serU, was verified by restriction and sequence analysis. Finally, the plasmids pPK-serRK and pPK-serU were introduced by electroporation into B. breve UCC2003-serR and B. breve UCC2003-serU strains, to generate strains B. breve UCC2003-serR-pPKserRK and UCC2003-serU-pPKserU, respectively.
Measurement of protease-mediated transcriptional induction of the ser2003 genes by qRT-PCR.
Cultures of B. breve UCC2003, the mutant strains UCC2003-serR and UCC2003-serU, and their complemented derivatives UCC2003-serR-pPKserRK and UCC2003-serU-pPKserU were grown at 37°C in MRS medium to an OD600 of 0.5, after which enzyme treatment was initiated by the addition of plasmin (3 mg/ml), papain (0.5 mg/ml), kallikrein (0.55 mg/ml), or trypsin (0.25 mg/ml) (all enzymes were obtained from Sigma, Italy). Cultures were maintained at 37°C for 90 min, after which samples (30 ml) were briefly centrifuged to harvest cells, which were used for RNA isolation as described previously (57) and then treated with DNase (Roche). A set of quantitative reverse transcriptase PCR (qRT-PCR) primers was used to target sagA (the first gene of the ser2003 operon), and the reference genes atpD, tufA, rpoB, ldh, pdxS, gluC, and uvrD/Rep (49). Primer design criteria were based on a desired melting temperature (Tm) between 58 and 60°C and an amplicon size of approximately 100 bp. qRT-PCR was performed using the CFX96 system (Bio-Rad, CA). PCR products were detected with SYBR green fluorescent dye and amplified according to the following protocol: one cycle of 95°C for 3 min, followed by 39 cycles of 95°C for 5 s and 66°C for 20 s. The melting curve was 65°C to 95°C with increments of 0.5°C/s. Each PCR mix contained the following: 12.5 μl 2× SYBR SuperMix Green (Bio-Rad), 1 μl of cDNA dilution, and each of the forward and reverse primers at an 0.5 μM concentration; nuclease-free water was added to obtain a final volume of 20 μl. In each run, negative controls (no cDNA) for each primer set were included. The threshold cycle (2−ΔΔCT) method (24) was used to calculate relative changes in gene expression determined from real-time quantitative PCR experiments. Results were calculated from at least two independent RNA extractions. Statistical analysis of the obtained data was performed using the CFX Manager software (Bio-Rad).
Microarray data accession number.
The array data were deposited in the GEO database under accession number GSE37835.
RESULTS
Overexpression of the response regulator SerR in B. breve UCC2003 leads to increased transcription of the serpin-encoding gene serU.
The B. breve UCC2003 genome harbors a single serpin-like gene (34), here designated serU (Bbr_1320), whose deduced amino acid sequence shows a high level (>90%) of similarity with serpin proteins encoded by other bifidobacteria (e.g., B. breve 210B, Bifidobacterium longum subsp. longum NCC2705, B. longum subsp. longum DJO10A, and Bifidobacterium longum subsp. infantis ATCC 15697), although in the case of the serpin from Bifidobacterium dentium Bd1 and B. dentium ATCC 27679 the similarity level falls to 44% (50). The SerU sequence contains, in addition to the functional domains of the serpin superfamily (PFAM00079), domains identified in alpha-1 antitrypsin, antithrombin, and angiotensinogen.
The serU gene is organized in a manner identical to that determined for B. breve 210B (50) (Fig. 1a): serU is preceded by a gene, here designated sagA (serpin-associated gene) (Bbr_1319), which encodes a membrane-associated protein and which together with serU forms a bicistronic operon, here designated ser2003. Furthermore, two genes, here designated serR (Bbr_1318) and serK (Bbr_1317), which constitute a response regulator and a histidine protein kinase, respectively, of a predicted two-component regulatory system (2CRS), were identified immediately upstream of the sagA gene of both B. breve UCC2003 and 210B. This genetic organization is only partly conserved among the various bifidobacterial genomes (50): for example, both genes of the 2CRS as well as sagA are partially or completely absent in the B. longum strains, while in the case of B. dentium the sagA gene is absent but the 2CRS-encoding genes are present. Although the expression of bifidobacterial serpin genes has previously been shown to be induced following treatment with specific proteases (50), the role, if any, of the serRK 2CRS in the regulation of this expression in B. breve has not been investigated. In order to determine the possible involvement of serRK in the regulation of ser2003 transcription, a B. breve UCC2003 SerR-overexpressing derivative was generated by cloning the serR gene into the high-copy-number pNZ44 vector (to generate pNZ44-serR), which carries a strong constitutive promoter (see Materials and Methods) (29). Microarray analysis showed that the level of serR transcription in B. breve UCC2003 (pNZ44-serR) was 203-fold higher than that in the B. breve control strain containing the empty pNZ44 vector. Furthermore, from a total of 1,864 identified open reading frames of B. breve UCC2003 (34), microarray analysis showed a significantly increased transcriptional activity for 19 genes (>3-fold; P ≤ 0.001), while 21 genes exhibited significantly decreased levels of transcription (<3-fold; P ≤ 0.001) (Table 2). Among the genes with the highest level of increased transcription was sagA (119-fold), which is located adjacent to the serRK locus. Although sagA and the adjacent serU gene are organized as an operon (50), the level of transcriptional increase of the serpin-encoding gene was just 4.8-fold (Table 2). However, when these microarray results were investigated by qRT-PCR (see Materials and Methods), an equal level of upregulation for these genes was observed (data not shown). The obtained microarray data provided a first view of global gene expression changes upon SerR overexpression. However, such changes may not necessarily be due to direct transcriptional effects of the two-component system, since they may also have been caused by secondary or indirect effects, for example, as a result of stress due to protein overexpression. In order to address this issue, further experiments were carried out.
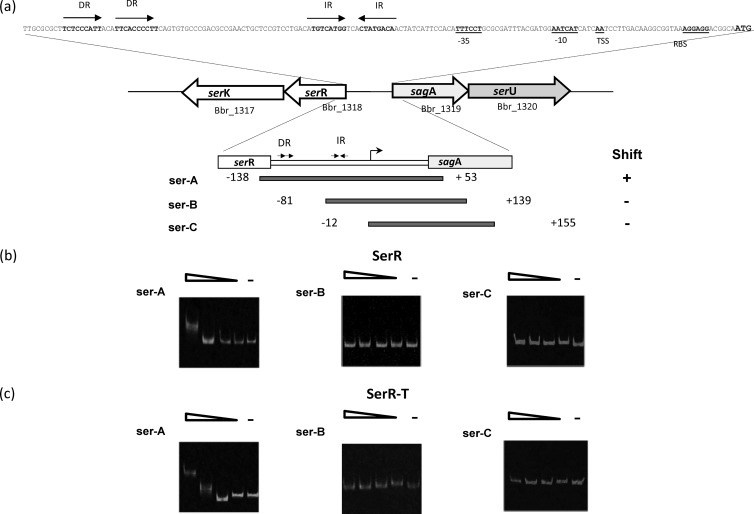
Fig 1.
(a) Schematic representation of the ser2003 gene locus, promoter region, and DNA fragments used in the gel mobility shift assays; the numbers indicate the ends of the fragments relative to the transcriptional start site. Direct repeats (DRs) and inverted repeats (IRs) are indicated; −10 and −35 hexamers, the transcriptional starting site (TSS), and the ribosomal starting site (RBS) are underlined. (b and c) Electrophoretic mobility shift assays carried out using ser2003 promoter fragments and cell crude extracts containing the full SerR protein (b) or its 5′-truncated fragment SerR-T (c). Gradients correspond to 2, 1, 0.5, and 0.25 μg of protein content. Cell extract from E. coli harboring the empty vector pQE30 was used as a negative control.
Table 2.
Summary of gene expression changes in B. breve UCC2003 upon SerR overexpressionb
| Expression category and locus tag | Putative function | SerR overexpressiona |
|---|---|---|
| Increased expression | ||
| Bbr_1318 | Bbr_1318; two-component response regulator, reverse | 203.05 |
| Bbr_1319 | Bbr_1319; conserved hypothetical membrane-spanning protein | 119.77 |
| Bbr_1656 | Bbr_1656; sugar ABC transporter, permease protein | 9.89 |
| Bbr_1657 | Bbr_1657; sugar-binding protein of ABC transporter system 2045106:2046023, reverse; MW, 33,668 | 8.01 |
| Bbr_1658 | Bbr_1658; sugar-binding protein of ABC transporter system 2046044:2047339, reverse; MW, 45,772 | 7.91 |
| Bbr_1655 | bgl3; beta-glucosidase | 6.55 |
| Bbr_0249 | Bbr_0249; ABC1 family protein kinase | 6.32 |
| Bbr_0107 | cebF; cellobiose/cellotriose transport system permease protein F 155651:156772, forward; MW, 41,448 | 5.76 |
| Bbr_1464 | Bbr_1464; conserved hypothetical membrane-spanning protein 1827409:1828293, reverse; MW, 31,617 | 5.45 |
| Bbr_0106 | cebE; cellobiose/cellotriose binding protein | 5.24 |
| Bbr_1320 | Bbr_1320; neuroserpin precursor | 4.82 |
| Bbr_0108 | cebG; cellobiose/cellotriose transport system permease protein 156780:157727, forward; MW, 33,983 | 4.16 |
| Bbr_1293 | Bbr_1293; myosin-cross-reactive antigen | 3.38 |
| Bbr_1434 | Bbr_1434; ATP-binding protein of ABC transporter system | 3.37 |
| Bbr_0109 | bgl1; beta-glucosidase | 3.35 |
| Bbr_1433 | Bbr_1433; ATP-binding protein of ABC transporter system | 3.27 |
| Bbr_1291 | Bbr_1291; short-chain dehydrogenase | 3.25 |
| Bbr_1506 | Bbr_1506; cyclopropane-fatty-acyl-phospholipid synthase | 3.16 |
| Bbr_1710 | rbsK5; ribokinase | 3.04 |
| Reduced expression | ||
| Bbr_1892 | Bbr_1892; PTS system, IIC component | 452.50 |
| Bbr_1893 | Bbr_1893; PTS system, IIB component | 194.45 |
| Bbr_1894 | Bbr_1894; PTS system, IIA component | 190.63 |
| Bbr_1898 | nrdF; ribonucleoside-diphosphate reductase beta chain | 13.02 |
| Bbr_1891 | Bbr_1891; transcriptional regulator, GntR family | 12.95 |
| Bbr_1899 | nrdE; ribonucleoside-diphosphate reductase alpha chain | 9.45 |
| Bbr_0273 | Bbr_0273; ATP-binding protein of ABC transporter system | 5.91 |
| Bbr_0274 | Bbr_0274; permease protein of ABC transporter system | 5.19 |
| Bbr_0925 | Bbr_0925; permease MFS superfamily | 4.95 |
| Bbr_1501 | Bbr_1501; ATP-binding protein of ABC transporter | 4.76 |
| Bbr_0195 | Bbr_0195; conserved hypothetical membrane-spanning protein | 3.92 |
| Bbr_1500 | Bbr_1500; conserved hypothetical membrane-spanning protein | 3.84 |
| Bbr_0939 | Bbr_0939; solute binding protein of ABC transporter | 3.82 |
| Bbr_0285 | lacZ2; beta-galactosidase | 3.54 |
| Bbr_1446 | nrdG; anaerobic ribonucleoside-triphosphate reductase activating protein | 3.53 |
| Bbr_0940 | Bbr_0940; narrowly conserved hypothetical protein | 3.41 |
| Bbr_1558 | Bbr_1558; permease protein of ABC transporter system | 3.39 |
| Bbr_0734 | Bbr_0734; sensory transduction protein kinase | 3.34 |
| Bbr_0116 | malQ1; 4-alpha-glucanotransferase | 3.30 |
| Bbr_0039 | trxB1; thioredoxin reductase/thioredoxin/glutaredoxin family | 3.25 |
| Bbr_1843 | Bbr_1843; narrowly conserved hypothetical membrane-spanning protein | 3.08 |
Expression ratios presented have a Bayesian P value of <0.001 according to the Cyber-T test (25).
Abbreviations: MW, molecular weight; PTS, phosphotransferase; MFS, major facilitator superfamily.
SerR specifically binds the ser2003 promoter.
In order to obtain evidence for the possible regulatory role of the serRK 2CRS with regard to ser2003 transcription, the ability of SerR and an N-terminally truncated version, which includes the predicted SerR DNA binding domain (residues 171 to 217), to bind to the ser2003 locus promoter region was analyzed by electrophoretic mobility shift assay (EMSA). A DNA region containing the promoter (from position −343 to +50, relative to the presumed transcriptional start site [50] [Fig. 1a]) was prepared by PCR using IRD800-labeled primers. EMSA was carried out using crude cell extracts obtained from E. coli strains harboring plasmid pQE30-serR or pQE30-serR-T, while a cell extract from E. coli harboring the empty vector pQE30 was used as a negative control. The obtained EMSA results revealed that in contrast to the negative control, crude cell extracts obtained from E. coli expressing either the full-length SerR protein or its truncated version SerR-T were capable of a specific interaction with the ser2003 promoter region (Fig. 1b and c, ser-A panels). Additional control EMSAs, in order to probe the specificity of the DNA-protein interaction, were performed using the pstS (Bbr_1682) promoter (2), and as expected, this promoter region was not bound by either SerR or SerR-T (results not shown). These binding results, which demonstrate a physical interaction between the SerR protein and the ser2003 promoter region, support the notion of a direct regulatory role of SerR in ser2003 transcription, as suggested by our microarray data.
In order to identify which sequences within the promoter region are required for SerR binding, three IRD800-labeled amplicons, namely, ser-A, ser-B, and ser-C, which encompass different sections of the promoter region, were generated (Fig. 1a). The results obtained from the corresponding EMSAs showed that the SerR binding site is localized within a fragment between positions −138 and −81 (relative to the transcriptional start site of ser2003), which contains an imperfect direct repeat (DR) sequence (DRser; TTCTCCCATT-3N-TTCACCCCTT), suggesting that this DR plays a role in SerR binding. Interestingly, this fragment does not contain the inverted repeat (IR) that had previously been suggested to play a regulatory role in ser2003 transcription (50).
Directed mutagenesis of DRser affects SerR binding activity.
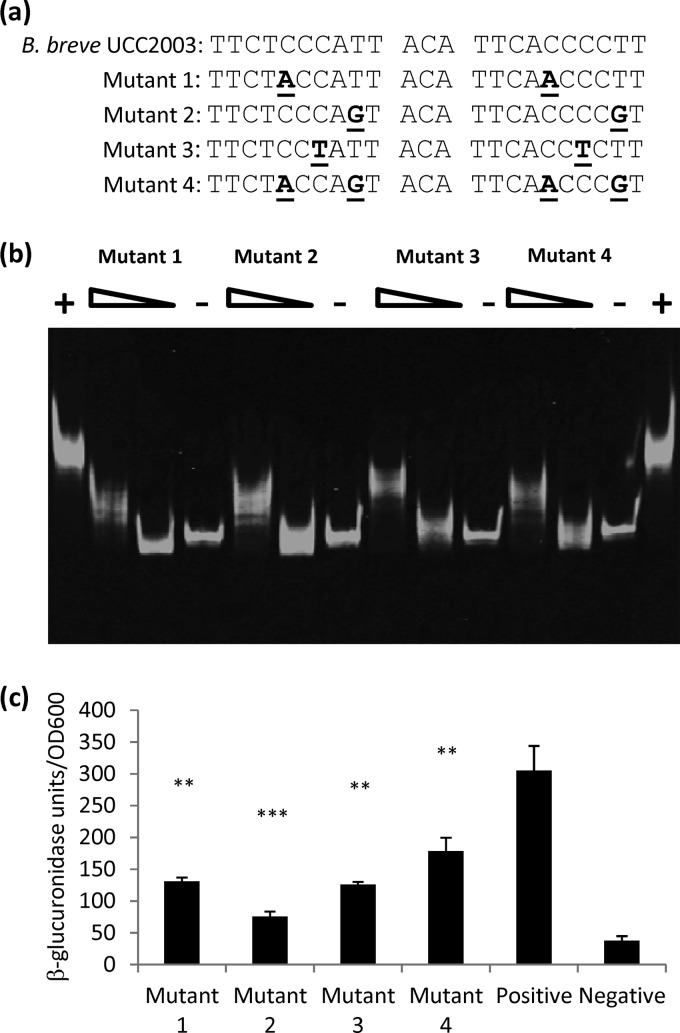
In order to verify the presumed role of DRser as a recognition sequence for SerR binding to the ser2003 locus promoter region, mutated versions of this region in which the sequence of the DR was changed were generated and then individually tested for their ability to be bound by SerR. In vitro site-directed mutagenesis was performed (see Materials and Methods), generating four different plasmids, pUC18-BSMut1, pUC18-BSMut2, pUC18-BSMut3, and pUC18-BSMut4, which each harbor a differently mutated version of the putative SerR binding site (Fig. 2). These plasmids were used as the templates to amplify the mutated DR-containing fragments, which were then used for EMSAs. The results reveal that SerR retains the capacity to interact with the mutated binding site, although apparently with decreased efficacy compared to that obtained with the nonmutated binding site, as these mutated fragments were much less affected in their mobility by the presence of SerR than was the DNA fragment containing the unmutated DRser (Fig. 2).
Fig 2.
(a) Schematic representation of the different mutations performed in the ser2003 locus promoter. (b) Gel mobility shift assays carried out with crude cell extracts containing SerR and various mutated versions of the ser2003 promoter region (see panel a); as a positive control, the wild-type ser2003 promoter region was used. (c) GusA assays testing protease-mediated induction of the ser2003 promoter of B. breve UCC2003 harboring plasmid pNZ272-BSMut1, pNZ272-BSMut2, pNZ272-BSMut3, or pNZ272-BSMut4; B. breve UCC2003 harboring pNZ272 with the wild-type ser2003 promoter or without any insert was used as a positive or a negative control, respectively. Data correspond to mean values of three independent experiments, and they were compared by an unpaired t test analysis. Error bars correspond to the standard deviations. **, P ≤ 0.01; ***, P ≤ 0.001.
In order to relate these observed binding differences to SerR-directed transcriptional activation of the ser2003 promoter, transcriptional fusions were constructed by cloning the wild-type ser2003 promoter and the four mutated derivatives upstream of the promoterless gusA reporter gene in pNZ272 (see Materials and Methods). B. breve UCC2003 strains harboring these gusA fusion plasmids, designated pNZ272-ser, pNZ-ser-Mut1, pNZ-ser-Mut2, pNZ-ser-Mut3, and pNZ-ser-Mut4, were used for GusA assays following protease-mediated induction. Pancreatic elastase treatment clearly induced the wild-type ser2003 locus promoter activity. In contrast, the mutated promoters did in all cases exhibit significantly lower β-glucuronidase (GUS) activity than did the wild-type promoter (Fig. 2). Although the number of point mutations present in DRser does not show an inverse correlation with the obtained GUS activity level, since mutant 4 (harboring 4 mutations) exhibited a higher level of activity than did the other 3 mutants (harboring just 2 mutations), these results confirm the regulatory role of DRser in protease-mediated transcriptional induction of the ser2003 promoter and concur with the presumed role of SerR in directing this induction.
Disruption and complementation of the serR gene in B. breve UCC2003.
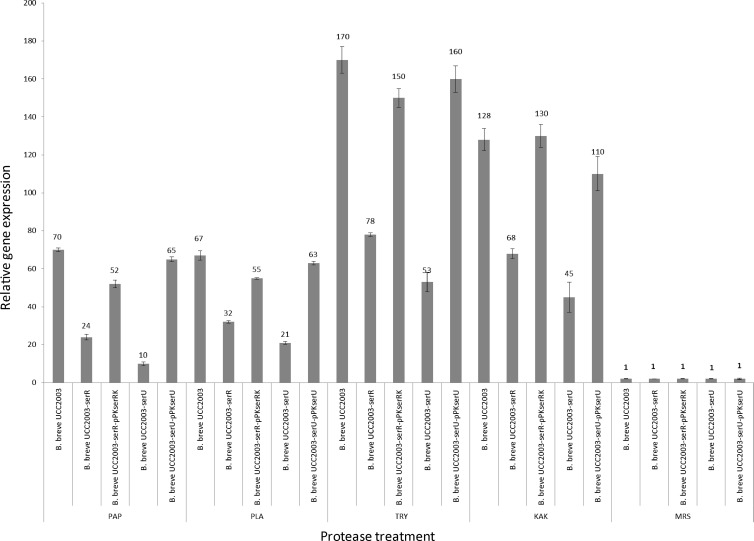
In order to verify and further understand the regulatory connection between the serRK-encoded 2CRS and protease-mediated induction of the ser2003 operon, an insertion mutant in the serR gene was constructed (designated UCC2003-serR). In addition, this mutant strain was complemented by introducing a plasmid containing the complete 2CRS serRK, yielding B. breve UCC2003-serR-pPKserRK (see Materials and Methods). Wild-type, mutant, and complemented strains were analyzed for their ability to induce the ser2003 promoter by different proteases. qRT-PCR experiments were carried out in triplicate (Fig. 3). For the wild-type strain B. breve UCC2003, different proteases were shown to induce various levels of transcription of the ser2003 locus (as measured by qRT-PCR targeting the sagA gene), varying from 70-fold induction with papain to 170-fold induction following exposure to trypsin, thus confirming a protease-specific transcriptional induction. In contrast, transcriptional induction of ser2003 was significantly reduced in B. breve UCC2003-serR, thereby providing further evidence that SerR regulates expression of ser2003. Protease-mediated induction of ser2003 transcription in the B. breve UCC2003-serR mutant strain was restored to levels observed for the wild-type strain B. breve UCC2003 when plasmid pPKserRK was introduced into UCC2003-serR, thus demonstrating complementation of the phenotype of the latter mutant (Fig. 3). Attempts to achieve complementation of UCC2003-serR using a plasmid that contained just serR resulted in a partial restoration of protease-mediated ser2003 transcription (results not shown). This partial complementation is likely due to a polar effect of the serR insertion mutation on serK expression, thus implicating the latter gene in ser2003 transcriptional regulation.
Fig 3.
The relative transcription levels of ser2003 in B. breve UCC2003 and various mutant and complemented strains upon serine protease treatment versus growth in MRS-based medium as analyzed by quantitative reverse transcriptase PCR assays. The histograms indicate the relative amounts of ser2003 mRNAs for the specific samples. The cDNAs were generated from RNA collected following exposure of the bifidobacterial cultures, for 90 min, to kallikrein (KAK), papain (PAP), plasmin (PLA), or trypsin (TRY) or left untreated (MRS; taken as a value of 1).
The SerRK signaling pathway involves serU and suggests protease-mediated ser2003 autoregulation.
In order to investigate the role, if any, of the serU gene in protease-mediated induction of the ser2003 operon, a strain that carried a disrupted serU gene was created (designated UCC2003-serU; see Materials and Methods). Interestingly, protease-mediated transcriptional induction of ser2003 was significantly reduced in strain UCC2003-serU, while it was restored to wild-type levels in UCC2003-serU, in which the serU gene was present on plasmid pPKserU (Fig. 3). These results clearly indicate that SerU regulates ser2003 transcription and thus its own expression through an autoregulatory mechanism.
DISCUSSION
Members of the serpin superfamily are widely distributed throughout all kingdoms of life, including the Eukarya, Prokarya, Archaea, and certain viruses (18, 42). Nevertheless, their presence in the genus Bifidobacterium is not ubiquitous and, in fact, appears to be restricted to strains belonging to the species B. breve, B. longum subsp. longum, B. longum subsp. infantis, Bifidobacterium longum subsp. suis, Bifidobacterium cuniculi, Bifidobacterium scardovia, and B. dentium (50). Very little is known about the physiological function of serpins in bacteria beyond their protease-inhibiting activity, although previous work has shown that serpin genes of different bifidobacterial species and subspecies (B. breve, B. longum subsp. infantis, and B. longum subsp. longum) are highly induced when bacterial cultures are exposed to various proteases (19, 36, 50). Some of these proteases can be found in the human gut; therefore, this serpin activity can be employed by bifidobacteria to protect themselves against host-derived proteases and to provide an advantage for survival in a highly complex and competitive environment. In contrast, other gut-associated bifidobacterial species, such as Bifidobacterium adolescentis, Bifidobacterium catenulatum, and Bifidobacterium bifidum, do not contain a serpin-encoding gene in their genomes, suggesting that these species may exert an alternative way of protection or may not need protection. The activation of the bifidobacterial serpin-encoding genes seems to be very specific: in strains of the subspecies B. longum subsp. infantis and B. longum subsp. longum, the highest level of induction is reached in response to human elastase and, although to a lower degree, to papain and pancreatic elastase, respectively (19). In contrast, in the B. breve 210B strain the highest induction was shown to occur following exposure to papain (50). The reason for the differences in the patterns of serpin gene induction between these two closely related bifidobacterial species may be linked to the different ecological niches of these bifidobacterial species, a notion that is consistent with the finding that there is no significant induction of the serpin gene of B. dentium Bd1 in response to any of the serine proteases examined (50).
Our data clearly implicate the 2CRS serRK of B. breve UCC2003 in the transcriptional regulation of the ser2003 locus. The transcriptome results combined with qRT-PCR analyses demonstrate increased transcription of the ser2003 locus when the response regulator SerR is overexpressed. In order to further substantiate SerRK involvement, we generated two independent insertion mutant strains, B. breve UCC2003-serU and B. breve UCC2003-serR, and demonstrated that ser2003 induction was significantly reduced in both mutants, although this effect was more pronounced in the former mutant, where the serU gene is interrupted, compared to the latter strain, which carried a disrupted serR gene. These data clearly suggest an autoregulatory role for SerU and perhaps the existence of an additional SerRK-independent but SerU-dependent regulatory mechanism that controls protease-mediated transcription of the ser2003 locus, although further research will have to substantiate this notion. The identification of the specific signal(s) that is sensed by the SerRK system will be the key to uncovering how this signal is generated by the protease exposure. Such signals may be specific peptides generated following protease-mediated degradation of cell envelope proteins or cell wall-anchored structures or may even be degradation products of serU itself, thus providing an explanation for the observed positive autoregulatory loop, which ensures further production of SerU when the encountered proteolytic activity is higher than the provided serpin-mediated protease inhibition.
Recently, Turroni and coworkers (50) performed a comparison of the promoter regions of ser genes from different bifidobacteria, including B. breve 210B, B. breve UCC2003, B. longum subsp. longum NCC2705, B. longum subsp. longum DJO10A, B. longum subsp. infantis CCUG52486, and B. longum subsp. infantis ATCC 55813, finding a number of DNA motifs, including the putative −10 and −35 hexamers and a nearly perfect inverted repeat (IR) (TGTCATGG-3N-CTATGACA). These elements are also conserved in the ser2003 promoter sequence, but in addition a direct repeat (DR) (TTCTCCCATT-3N-TTCACCCCTT), located upstream of the IR, was found, and it may also have a regulatory role in serpin expression. The presence of this DR was investigated in the promoter regions of genes previously showing an increased transcriptional activity by microarray analysis, but it was not found in any case. In order to clarify the role of the IR and DR, EMSAs between SerR and fragments encompassing different elements of the promoter region were carried out. Our results show that the presence of the DR, but not of the IR, is necessary for SerR binding to the promoter. Both repeats are absent from the B. dentium Bd1 and B. dentium ATCC 27679 genomes, suggesting a different regulatory mechanism for the serpin gene in this bifidobacterial species. Other response regulator proteins, such as PhoR in Corynebacterium glutamicum, have also been shown to bind a DR (40); for instance, the DNA binding targets of response regulators belonging to the largest subfamily (i.e., the OmpR family) are typically direct repeats, whereas response regulator proteins belonging to the second largest family (the NarL family) typically bind to inverted repeats. This difference in the types of DNA sequences that are targeted by these two response regulator families reflects a fundamentally different organization of the bound RR dimers (7). SerR belongs to the LuxR family, a very heterogeneous family, in which the DNA binding sequences are not fully characterized but in many cases have been identified as IRs (32). The role of DR2003 in SerR binding capacity was further investigated by mutagenesis experiments combined with EMSAs. The results demonstrated that SerR was able to bind the mutated binding site, though with decreased binding efficiency, which is then thought to diminish SerR's activity as a transcriptional activator, an assumption that was corroborated by GUS assays performed under protease induction conditions. These results confirm the role of DR2003 in the transcriptional regulation of the ser2003 operon and further advance our understanding of serpin gene expression in B. breve.
The results described in this work allow us to conclude that SerRK is a 2CRS that controls the transcriptional induction of the ser2003 locus from B. breve UCC2003 following specific protease treatment. Data derived from the analysis of a B. breve-serU mutant indicate that the SerRK signaling pathway includes a SerU-dependent autoregulatory feedback. However, the molecular mechanism driving this regulated expression, as well as the specific signals sensed by the SerRK 2CRS, remains elusive. Further investigation, with particular emphasis on the in vivo biological role of the serpin in the gut, is ongoing in order to elucidate these questions.
Supplementary Material
ACKNOWLEDGMENTS
The Alimentary Pharmabiotic Centre is a research center funded by Science Foundation Ireland (SFI), through the Irish Government's National Development Plan. We and our work were supported by SFI (grants 02/CE/B124 and 07/CE/B1368), a postdoctoral fellowship from the Spanish Ministry of Science and Innovation (MICINN) to P.A.-M., and an HRB postdoctoral fellowship (grant PDTM/20011/9) awarded to M.O.M. This work was also financially supported by a FEMS Advanced Fellowship 2011 and an IRCSET Embark postdoctoral fellowship to F.T.
Footnotes
Published ahead of print 27 July 2012
P.A.-M. and M.O.M. contributed equally to this work.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1. Altschul SF, et al. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alvarez-Martin P, et al. 2012. A conserved two-component signal transduction system controls the response to phosphate starvation in Bifidobacterium breve UCC2003. Appl. Environ. Microbiol. 78:5258–5269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alvarez-Martin P, O'Connell-Motherway M, van Sinderen D, Mayo B. 2007. Functional analysis of the pBC1 replicon from Bifidobacterium catenulatum L48. Appl. Microbiol. Biotechnol. 76:1395–1402 [DOI] [PubMed] [Google Scholar]
- 4. Bisanz JE, Reid G. 2011. Unraveling how probiotic yogurt works. Sci. Transl. Med. 3:106ps41 doi:10.1126/scitranslmed.3003291 [DOI] [PubMed] [Google Scholar]
- 5. Crépin S, et al. 2011. The Pho regulon and the pathogenesis of Escherichia coli. Vet. Microbiol. 153:82–88 [DOI] [PubMed] [Google Scholar]
- 6. Cronin M, Knobel M, O'Connell-Motherway M, Fitzgerald GF, van Sinderen D. 2007. Molecular dissection of a bifidobacterial replicon. Appl. Environ. Microbiol. 73:7858–7866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. de Been M, Bart MJ, Abee T, Siezen RJ, Francke C. 2008. The identification of response regulator-specific binding sites reveals new roles of two-component systems in Bacillus cereus and closely related low-GC Gram-positives. Environ. Microbiol. 10:2796–2809 [DOI] [PubMed] [Google Scholar]
- 8. Fanning S, et al. 2012. Bifidobacterial surface-exopolysaccharide facilitates commensal-host interaction through immune modulation and pathogen protection. Proc. Natl. Acad. Sci. U. S. A. 109:2108–2113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fukuda S, et al. 2011. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 469:543–547 [DOI] [PubMed] [Google Scholar]
- 10. Furrie E, et al. 2005. Synbiotic therapy (Bifidobacterium longum/Synergy 1) initiates resolution of inflammation in patients with active ulcerative colitis: a randomised controlled pilot trial. Gut 54:242–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gao R, Mack TR, Stock AM. 2007. Bacterial response regulators: versatile regulatory strategies from common domains. Trends Biochem. Sci. 32:225–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. García de la Nava GJ, van Hijum SAFT, Trelles O. 2003. PreP: gene expression data pre-processing. Bioinformatics 19:2328–2329 [DOI] [PubMed] [Google Scholar]
- 13. Gettins PG. 2002. Serpin structure, mechanism, and function. Chem. Rev. 102:4751–4804 [DOI] [PubMed] [Google Scholar]
- 14. He T, et al. 2008. Effects of yogurt and bifidobacteria supplementation on the colonic microbiota in lactose-intolerant subjects. J. Appl. Microbiol. 104:595–604 [DOI] [PubMed] [Google Scholar]
- 15. Hoch JA. 2000. Two-component and phosphorelay signal transduction. Curr. Opin. Microbiol. 3:165–170 [DOI] [PubMed] [Google Scholar]
- 16. Huntington JA. 2011. Serpin structure, function and dysfunction. J. Thromb. Haemost. 9:26–34 [DOI] [PubMed] [Google Scholar]
- 17. Imaoka TS, et al. 2008. Anti-inflammatory activity of probiotic Bifidobacterium: enhancement of IL-10 production in peripheral blood mononuclear cells from ulcerative colitis patients and inhibition of IL-8 secretion in HT-29 cells. World J. Gastroenterol. 14:2511–2516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Irving JA, et al. 2002. Serpins in prokaryotes. Mol. Biol. Evol. 19:1881–1890 [DOI] [PubMed] [Google Scholar]
- 19. Ivanov D, et al. 2006. A serpin from the gut bacterium Bifidobacterium longum inhibits eukaryotic elastase-like serine proteases. J. Biol. Chem. 281:17246–17252 [DOI] [PubMed] [Google Scholar]
- 20. Kullen MJ, Klaenhammer TR. 2000. Genetic modification of intestinal lactobacilli and bifidobacteria. Curr. Issues Mol. Biol. 2:41–50 [PubMed] [Google Scholar]
- 21. Law J, et al. 1995. A system to generate chromosomal mutations in Lactococcus lactis which allows fast analysis of targeted genes. J. Bacteriol. 177:7011–7018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Leahy SC, Higgins DG, Fitzgerald GF, van Sinderen D. 2005. Getting better with bifidobacteria. J. Appl. Microbiol. 98:1303–1315 [DOI] [PubMed] [Google Scholar]
- 23. Le Leu RK, Hu Y, Brown IL, Woodman RJ, Young GP. 2010. Synbiotic intervention of Bifidobacterium lactis and resistant starch protects against colorectal cancer development in rats. Carcinogenesis 31:246–251 [DOI] [PubMed] [Google Scholar]
- 24. Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- 25. Long AD, et al. 2001. Improved statistical inference from DNA microarray data using analysis of variance and a Bayesian statistical framework. Analysis of global gene expression in Escherichia coli K12. J. Biol. Chem. 276:19937–19944 [DOI] [PubMed] [Google Scholar]
- 26. MacConaill LE, Butler D, O'Connell-Motherway M, Fitzgerald GF, van Sinderen D. 2003. Identification of two-component regulatory systems in Bifidobacterium infantis by functional complementation and degenerate PCR approaches. Appl. Environ. Microbiol. 69:4219–4226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mascher T, Helmann JD, Unden G. 2006. Stimulus perception in bacterial signal-transducing histidine kinases. Microbiol. Mol. Biol. Rev. 70:910–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mazé A, O'Connell-Motherway M, Fitzgerald GF, Deutscher J, van Sinderen D. 2007. Identification and characterization of a fructose phosphotransferase system in Bifidobacterium breve UCC2003. Appl. Environ. Microbiol. 73:545–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McGrath S, Fitzgerald GF, van Sinderen D. 2001. Improvement and optimization of two engineered phage resistance mechanisms in Lactococcus lactis. Appl. Environ. Microbiol. 67:608–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McNulty NP, et al. 2011. The impact of a consortium of fermented milk strains on the gut microbiome of gnotobiotic mice and monozygotic twins. Sci. Transl. Med. 3:106ra106 doi:10.1126/scitranslmed.3002701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mitrophanov AY, Groisman EA. 2008. Signal integration in bacterial two-component regulatory systems. Genes Dev. 22:2601–2611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nasser W, Reverchon S. 2007. New insights into the regulatory mechanisms of the LuxR family of quorum sensing regulators. Anal. Bioanal. Chem. 387:381–390 [DOI] [PubMed] [Google Scholar]
- 33. O'Connell Motherway M, O'Driscoll J, Fitzgerald GF, van Sinderen D. 2009. Overcoming the restriction barrier to plasmid transformation and targeted mutagenesis in Bifidobacterium breve UCC2003. Microb. Biotechnol. 2:321–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. O'Connell Motherway M, et al. 2011. Functional genome analysis of Bifidobacterium breve UCC2003 reveals type IVb tight adherence (Tad) pili as an essential and conserved host-colonization factor. Proc. Natl. Acad. Sci. U. S. A. 108:11217–11222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Platteeuw C, Simons G, de Vos WM. 1994. Use of the Escherichia coli beta-glucuronidase (gusA) gene as a reporter gene for analyzing promoters in lactic acid bacteria. Appl. Environ. Microbiol. 60:587–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Roberts TH, Hejgaard J, Saunders NF, Cavicchioli R, Curmi PM. 2004. Serpins in unicellular Eukarya, Archaea, and Bacteria: sequence analysis and evolution. J. Mol. Evol. 59:437–447 [DOI] [PubMed] [Google Scholar]
- 37. Rutherford KJ, et al. 2000. Artemis: sequence visualization and annotation. Bioinformatics 10:944–945 [DOI] [PubMed] [Google Scholar]
- 38. Saavedra JM, Bauman NA, Perman JA, Yolken RH, Oung I. 1994. Feeding of Bifidobacterium bifidum and Streptococcus thermophilus to infants in hospital for prevention of diarrhoea and shedding of rotavirus. Lancet 344:1046–1049 [DOI] [PubMed] [Google Scholar]
- 39. Sambrook J, Russell DW. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 40. Schaaf S, Bott M. 2007. Target genes and DNA-binding sites of the response regulator PhoR from Corynebacterium glutamicum. J. Bacteriol. 189:5002–5011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schell M, et al. 2002. The genome sequence of Bifidobacterium longum reflects its adaptation to the human gastrointestinal tract. Proc. Natl. Acad. Sci. U. S. A. 99:14422–14427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Silverman GA, et al. 2001. The serpins are an expanding superfamily of structurally similar but functionally diverse proteins. Evolution, mechanism of inhibition, novel functions, and a revised nomenclature. J. Biol. Chem. 276:33293–33296 [DOI] [PubMed] [Google Scholar]
- 43. Stanton C, Ross RP, Fitzgerald GF, van Sinderen D. 2005. Fermented functional foods based on probiotics and their biogenic metabolites. Curr. Opin. Biotechnol. 16:198–203 [DOI] [PubMed] [Google Scholar]
- 44. Steck N, et al. 2011. Enterococcus faecalis metalloprotease compromises epithelial barrier and contributes to intestinal inflammation. Gastroenterology 141:959–971 [DOI] [PubMed] [Google Scholar]
- 45. Stock AM, Robinson VL, Goudreau PN. 2000. Two-component signal transduction. Annu. Rev. Biochem. 69:183–215 [DOI] [PubMed] [Google Scholar]
- 46. Thompson JD, Higgins DG, Gibson TJ. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids. Res. 22:4673–4680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Turroni F, Ribbera A, Foroni E, van Sinderen D, Ventura M. 2008. Human gut microbiota and bifidobacteria: from composition to functionality. Antonie Van Leeuwenhoek 94:35–50 [DOI] [PubMed] [Google Scholar]
- 48. Turroni F, et al. 2009a. Exploring the diversity of the bifidobacterial population in the human intestinal tract. Appl. Environ. Microbiol. 75:1534–1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Turroni F, et al. 2011. Global genome transcription profiling of Bifidobacterium bifidum PRL2010 under in vitro conditions and identification of reference genes for quantitative real-time PCR. Appl. Environ. Microbiol. 77:8578–8587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Turroni F, et al. 2010. Characterization of the serpin encoding gene of Bifidobacterium breve 210B. Appl. Environ. Microbiol. 76:3206–3219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Turroni F, et al. 2009b. Microbiomic analysis of the bifidobacterial population in the human distal gut. ISME J. 3:745–751 [DOI] [PubMed] [Google Scholar]
- 52. van Hijum SAFT, et al. 2005. A generally applicable validation scheme for the assessment of factors involved in reproducibility and quality of DNA-microarray data. BMC Genomics 6:77 doi:10.1186/1471-2164-6-77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Van Sinderen D, et al. 1995. comK encodes the competence transcription factor, the key regulatory protein for competence development in Bacillus subtilis. Mol. Microbiol. 15:455–462 [DOI] [PubMed] [Google Scholar]
- 54. Ventura M, et al. 2007. Genomics of actinobacteria: tracing the evolutionary history of an ancient phylum. Mol. Biol. Rev. 71:495–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ventura M, Canchaya C, Fitzgerald GF, Gupta RS, van Sinderen D. 2007. Genomics as a means to understand bacterial phylogeny and ecological adaptation: the case of bifidobacteria. Antonie Van Leeuwenhoek 91:351–372 [DOI] [PubMed] [Google Scholar]
- 56. Ventura M, et al. 2009. The Bifidobacterium dentium Bd1 genome sequence reflects its genetic adaptation to the human oral cavity. PLoS Genet. 5:e1000785 doi:10.1371/journal.pgen.1000785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ventura M, Foley S, Bruttin A, Canchaya C, Brüssow H. 2002. Transcription mapping as a tool in phage genomics: the case of the temperate Streptococcus thermophilus phage Sfi21. Virology 296:62–76 [DOI] [PubMed] [Google Scholar]
- 58. Ventura M, et al. 2009. Genome-scale analyses of health-promoting bacteria: probiogenomics. Nat. Rev. Microbiol. 7:61–71 [DOI] [PubMed] [Google Scholar]
- 59. Wang KY, et al. 2004. Effects of ingesting Lactobacillus- and Bifidobacterium-containing yogurt in subjects with colonized Helicobacter pylori. Am. J. Clin. Nutr. 80:737–741 [DOI] [PubMed] [Google Scholar]
- 60. Wang Y, Jones PJH, Ausman LM, Lichtenstein AH. 2004. Soy protein reduces triglyceride levels and triglyceride fatty acid fractional synthesis rate in hypercholesterolemic subjects. Atherosclerosis 173:269–275 [DOI] [PubMed] [Google Scholar]
- 61. Xiao JZ, et al. 2003. Effects of milk products fermented by Bifidobacterium longum on blood lipids in rats and healthy adult male volunteers. J. Dairy Sci. 86:2452–2461 [DOI] [PubMed] [Google Scholar]
- 62. Yanisch-Perron C, Vieira J, Messing J. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103–119 [DOI] [PubMed] [Google Scholar]
- 63. Zomer A, et al. 2009. An interactive regulatory network controls stress response in Bifidobacterium breve UCC2003. J. Bacteriol. 191:7039–7049 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.