Abstract
A Planctomyces limnophilus mutant generated using the EZ-Tn5 transposome was found to possess an insertion within pckA, encoding phosphoenolpyruvate carboxykinase. Disruption of pckA expression and elimination of enzymatic activity resulted in poor growth in glucose-free medium, demonstrating a gluconeogenic role for pckA in P. limnophilus.
INTRODUCTION
Bacteria belonging to the phylum Planctomycetes possess several distinctive phenotypic and molecular characteristics, including a proteinaceous cell wall that is devoid of peptidoglycan (17), reproduction by yeast-like division, and in many cases, internal cell compartmentalization resembling the eukaryotic nucleus (2, 19). They are found in aquatic and soil environments (3), as well as among microbial communities associated with the intestinal tracts of many organisms, including mammals (11) and fish (15). The Planctomycetes are significant participants in the global carbon and nitrogen cycles and contain a unique group capable of carrying out anaerobic ammonium oxidation (anammox) (5, 8). While the availability of genome sequences for several Planctomycetes (cited in reference 6), as well as biochemical, physiological, and ecological studies, have provided some insight into this fascinating group of bacteria, the lack of molecular genetic tools has made it difficult to examine them in greater detail.
Recently, Jogler et al. (6) described a genetic approach for studying Planctomyces limnophilus and suggested that this bacterium be used as a model species for the Planctomycetes. P. limnophilus is a chemoheterotroph that has a distinct cell cycle, with sessile cells that form stalks; its ability to form multicellular rosettes, its low salt tolerance, pinkish red pigmentation, high growth rate compared to the growth of other Planctomycetes (4), intracytoplasmic membrane structure, and the availability of a genome sequence (10) make it an ideal subject for molecular genetic studies. We have been examining the use of the Epicentre (Madison, WI) R6Kγori/Kan-2 transposon kit (EZ-Tn5) (9) for creating P. limnophilus mutants by a procedure similar to that described by Jogler et al. (6). Here, we report the isolation of mutants obtained using this approach and describe the characterization of one mutant that contains an insertion within the gene encoding phosphoenolpyruvate carboxykinase, pckA.
Transposon mutagenesis and isolation of mutants.
Electrocompetent cells of P. limnophilus (DSM 3776), a gift from Naomi Ward, University of Wyoming, were prepared from a 5-day-old culture grown in 500 ml of modified 621 medium (DSMZ) (peptone and yeast extract concentrations were doubled to 0.05% each) at 28°C and 190 rpm that was washed with 10% glycerol and suspended in 1.5 ml 10% glycerol. Cells prepared in this manner were electrocompetent for several months when stored at −80°C. Electroporation was carried out by mixing 1 μl of EZ-Tn5 transposome solution and 1 μl of TypeOne restriction inhibitor (Epicentre) with 70-μl cells in a microcentrifuge tube, incubating on ice for 5 min, and then transferring to a cold electroporation cuvette with a 0.1-cm gap and pulsing using the Ec3 program of a Bio-Rad MicroPulser electroporator (∼3 kV and 600 Ω for 3.5 s). Cells were then transferred to 5 ml of 621 medium and incubated at 28°C with 190 rpm shaking for 2 h, after which they were plated onto 621 agar plates containing kanamycin (50 μg/ml) and incubated at 28°C for 6 to 7 days. Cells that were not treated with the EZ-Tn5 solution yielded no transformants. By this approach, we were able to obtain between 1 × 103 and 3 × 103 transformants per microgram of DNA using 108 cells, which is similar to the efficiency reported by Jogler et al. (6) using the EZ-Tn5 transposome solution. The transformation efficiency increased approximately 10-fold when wild-type cells were transformed with chromosomal DNA isolated from a Kanr transformant, which was probably due to homologous recombination. We note that elimination of the TypeOne restriction inhibitor resulted in a 10-fold decrease in transformation efficiency, which may reflect activity of a restriction modification system identified in the P. limnophilus genome (Plim_1220).
Identification of insertion sites by rescue cloning.
From two independent transformations, we grew a total of 18 colonies in 621 medium with kanamycin (50 μg/ml) and isolated DNA using the Promega genomic DNA purification kit. After digestion with EcoRI (which resides outside the EZ-Tn5 cassette within adjacent chromosomal DNA) and ligation under dilute conditions (14), samples were transformed into Escherichia coli strain DH5α λpir (laboratory stock) and plated onto Luria-Bertani agar (16) containing kanamycin (50 μg/ml). The presence of the EZ-Tn5 segment in Kanr transformants was confirmed by PCR, and the sequence of adjacent P. limnophilus DNA was determined using Epicentre primers (R6Kan-2 RP-1 and Kan-2 FP-1). Of the 18 clones examined, 10 contained insertions that occurred at random positions throughout the ∼5.4-Mb chromosome (Table 1). Consistent with our small sample size, we did not find insertions within the large, 37-Kb plasmid. Eight mutants contained inserts within Plim_2259, suggesting that this is a likely hot spot for integration, a phenomenon reported for this transposon in other bacteria (12). Jogler et al. (6) also reported regional selectivity, although insertion at the Plim_2259 locus was not noted.
Table 1.
Locations and predicted functions of P. limnophilus genes disrupted by EZ-Tn5 transposome insertionsa
| Mutant | Gene | Putative function |
|---|---|---|
| HJS56 | Plim_0275 | Unknown hypothetical protein |
| HJS61 | Plim_0470 | ABC transporter-related protein |
| HJS24 | Plim_0685 | 60-kDa outer membrane protein |
| HJS13 | Plim_1151 | Unknown hypothetical protein |
| HJS63 | Plim_1384 | ATP-dependent HrpA-like helicase |
| WD9 | Plim_2259 | Phosphoenolpyruvate carboxykinase |
| WD13 | Plim_2263 | Unknown hypothetical protein |
| HJS52 | Plim_2514 | Carboxyl-terminal protease |
| HJS59 | Plim_3012 | Unknown hypothetical protein |
| HJS62 | Plim_3972 | Unknown hypothetical protein |
Gene designations and putative functions as noted in the Kegg database (www.genome.jp/kegg/kegg2.html).
Growth characteristics of strain WD9.
The Plim_2259 open reading frame contains a PEPCK_ATP motif characteristic of the enzyme phosphoenolpyruvate carboxykinase (ATP) (PEPCK; EC 4.1.1.49) (GenBank accession number ADG68085). Encoded by pckA, PEPCK catalyzes the formation of phosphoenolpyruvate (PEP) and carbon dioxide from oxaloacetate and ATP in a reversible manner and is the first committed step for gluconeogenesis from C3 and C4 compounds in virtually all organisms. The creation of a P. limnophilus mutant having an insertion within a putative pckA gene provided us with the opportunity to examine the role of a key metabolic enzyme in this organism, and we characterized this isolate further. Sequence analysis of the E. coli clone derived from the WD9 chromosome revealed that the insertion occurred at position 1107 (codon 368 out of 529) within the 1,587-bp Plim_2259 open reading frame. The presence of the 2001-bp EZ-Tn5 transposome within Plim_2259 sequences was confirmed by PCR amplification using primers B and C (5′-CATTGCTCAGCCACTGAAGA and 5′-CCGTGTAGCCACTGACAAA, respectively) that spanned the insertion site (Fig. 1a) and increased the expected 475-bp wild-type fragment by approximately 2 kb (Fig. 1b).
Fig 1.
Analysis of pckA gene structure and mRNA of wild type and strain WD9. (a) Diagram of the pckA open reading frame (arrow) and position of primer sets AD and BC relative to the Tn5 insertion, with their expected amplification sizes. (b) Results of PCR amplification of wild-type and WD9 chromosomal DNA using primer sets AD and BC. (c) RT-PCR analysis of mRNA extracted from cultures grown in 621 medium with 1.5 mM glucose using primer sets AD and BC, as shown; controls include reactions with (+) and without (−) DNase and RT. Numbers to the left of the gels represent sizes in base pairs.
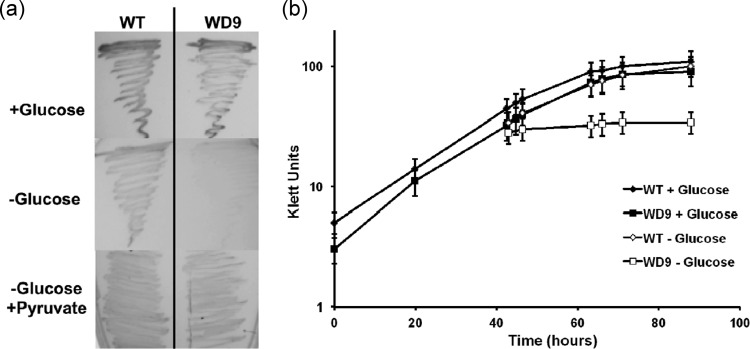
In many bacteria, the utilization of C3 and C4 compounds as sole carbon sources requires the PEP-producing activity of PEPCK for the production of hexoses via gluconeogenesis (e.g., Rhizobium [13] and Staphylococcus [18]). Therefore, a mutation in pckA should result in growth inhibition in a medium that is not supplemented with glucose. As predicted, while the wild type was capable of growing on solid 621 medium with and without glucose (Fig. 2a), the growth of strain WD9 was inhibited in the absence of glucose (Fig. 2a). In liquid medium with glucose, WD9 grew with a doubling time of approximately 14 h, which was similar to the growth of wild-type cultures under the same conditions (Fig. 2b). The growth of a mid-exponential-phase wild-type culture that was washed and resuspended in glucose-free 621 medium was comparable to that of the glucose-containing culture (Fig. 2b). In contrast, resuspension of a WD9 culture grown in glucose-deficient medium resulted in a significant reduction in growth (Fig. 2b). Poor growth of WD9 was also obtained when glucose was replaced by Kreb's cycle intermediates (succinate and malate) or precursors (glutamate and aspartate) (each at 5 mM) (not shown), which is consistent with a defect in the pckA gene and is indicative of a gluconeogenic role for PEPCK in P. limnophilus.
Fig 2.
Growth of the wild type (WT) and strain WD9 (pckA) in the presence and absence of glucose. (a) Cultures on solid agar plates containing 621 medium with (+) and without (−) added glucose (1.5 M) or pyruvate (5 mM) were grown for 7 days at 28°C. (b) Growth curves of cultures grown in 621 medium. Results are representative of three independent experiments. Culture turbidity was measured with a Klett-Summerson colorimeter (filter no. 66).
Interestingly, the growth of WD9 could be restored to wild-type levels in the absence of glucose by the addition of 5 mM pyruvate to 621 medium (Fig. 2a). The ability of a pckA mutant to utilize pyruvate may be due to the activity of PEP synthase (EC 2.7.9.1), which would provide PEP from pyruvate and has been shown to be necessary for growth on pyruvate in E. coli (1). A PEP synthase homolog can be found in the P. limnophilus genome (Plim_2420), and a role for this gene in gluconeogenesis remains to be determined. The capability of P. limnophilus to synthesize PEP from pyruvate may explain why WD9 did not cease growth in the absence of glucose but continued to grow with an apparent doubling time of >200 h, compared to approximately 14 h for the wild type (Fig. 2b), eventually achieving nearly the same growth yields as the wild type under similar conditions after several additional days of incubation (not shown). The reduced growth rate could be explained by a low rate of pyruvate production from amino acids in 621 medium, which is then used by PEP synthase to make PEP in the absence of PEPCK. Several pyruvate-generating activities have been identified in the P. limnophilus genome, including homologs of alanine dehydrogenase (EC 1.4.1.1; Plim_3804) and threonine dehydratase (EC 4.3.1.9; Plim_1722), as well as the malic enzyme (EC 1.1.1.38; Plim_1786), which catalyzes the formation of pyruvate from malate.
Expression of the Plim_2259 gene.
To determine whether Plim_2259 is expressed in P. limnophilus, we isolated total RNA, using TRIzol reagent (Life Technologies) according to the manufacturer's instructions, from 250 ml of glucose-grown wild-type and WD9 cultures and examined pckA mRNA production by reverse transcriptase (RT)-PCR. After DNase treatment (Fermentas) in the presence of RNasin (Promega), RT-PCRs were carried out using the SuperScript III system (Invitrogen) according to the manufacturer's directions, with a cDNA synthesis step of 50°C for 30 min, followed by 2 min at 94°C, and PCR amplification of 40 cycles with denaturation at 94°C for 15 s, annealing at 55°C for 30 s, and extension at 68°C for 1 min. As shown in Fig. 1a, a 396-bp RT-dependent product was obtained using primers A and D (5′-AACACCCAGTCGAGGCTTTA and 5′-GGAACTCCCCGTCTTTTTGT, respectively), which targeted sequences upstream of the Tn5 insertion in WD9, thereby confirming Plim_2259 expression in both the wild type and mutant. As expected, primers B and C (5′-CATTGCTCAGCCACTGAAGA and 5′-CCGTGTAGCCACTGACAAAA, respectively), which targeted sequences straddling the insertion site (Fig. 1a), generated a 475-bp product from wild-type RNA but did not yield any product from WD9 RNA (Fig. 1c) due to the EZ-Tn5 insertion.
PEPCK assays.
To confirm that Plim_2259 encodes PEPCK, 500 ml of mid-exponential-phase wild-type and WD9 cultures grown in 621 medium (with glucose) were centrifuged at 5,000 × g for 5 min at 4°C, washed twice with buffer A (50 mM imidazole, pH 7.0, 5 mM MnCl2, and 1 mM β-mercaptoethanol) and suspended in 1 ml buffer A. Cell extracts were prepared by two passes through a French pressure cell at 20,000 lb/in2 and centrifuging at 15,000 × g for 5 min at 4°C. The protein concentrations in the clarified supernatants were determined by the method of Kalb and Bernlohr (7), and PEPCK activity was measured spectrophotometrically by monitoring oxaloacetate production via a coupled malate dehydrogenase system at 25°C as described previously (18), subtracting background NADH oxidase activity. The PEPCK activity in wild-type cultures was 20.3 ± 2.1 nmol/min/mg protein (mean ± standard deviation). On the other hand, the PEPCK activity in WD9 cultures was undetectable (<0.2 nmol/min/mg protein). Thus, the absence of detectable PEPCK activity is consistent with the growth characteristics of WD9 and confirms that the Plim_2259 locus is pckA.
Conclusions.
The ability to create mutants of P. limnophilus by transposon mutagenesis has provided a powerful tool for examining the molecular biology, physiology, and genetics of this interesting group of bacteria. Showing that the growth characteristics of a P. limnophilus pckA mutant generated by this method are due to loss of PEPCK activity validates this approach for identifying gene function in this bacterium. To our knowledge, this is the first report confirming the function of a P. limnophilus gene predicted from bioinformatics, and it provides the basis for future studies that focus on the unique features of this group of bacteria. The ability to target specific genes by electroporation using adaptations of the EZ-Tn5 cassette will enable analyses of gene function and activity. Recently, we have applied this approach to introduce both gus- and gfp-containing constructs into the P. limnophilus chromosome at the pckA locus and are evaluating their efficacy to dissect mechanisms involved in pckA-directed expression (A. R. Parniani, T. Harvey, J. B. Wolf, and H. J. Schreier, unpublished). We are now in a position to continue the development of molecular genetic tools for P. limnophilus that will allow the dissection of many of the unusual characteristics of this bacterium and other members of the Planctomycetes.
ACKNOWLEDGMENTS
We thank Naomi Ward and Haifeng Geng for helpful discussions and Sabeena Nazar, Bio Analytical Services Laboratory, University of Maryland Center for Environmental Science, for sequencing.
This work was supported, in part, by a grant from the United States-Israel Binational Agricultural Research and Development Fund (MB-8720-08).
Footnotes
Published ahead of print 13 July 2012
REFERENCES
- 1. Fraenkel DG. 1987. Glycolysis, p 189–198. In Neidhardt FC, Ingraham JL, Low KB, Magasanik B, Schaechter M, Umbarger HE. (ed), Escherichia coli and Salmonella typhimurium: cellular and molecular biology. American Society for Microbiology, Washington DC [Google Scholar]
- 2. Fuerst JA. 2005. Intracellular compartmentation in planctomycetes. Annu. Rev. Microbiol. 59:299–328 [DOI] [PubMed] [Google Scholar]
- 3. Fuerst JA. 1995. The planctomycetes: emerging models for microbial ecology, evolution and cell biology. Microbiology 141(Pt 7):1493–1506 [DOI] [PubMed] [Google Scholar]
- 4. Hirsch P, Müller M. 1985. Planctomyces limnophilus sp. nov., a stalked and budding bacterium from freshwater. Syst. Appl. Microbiol. 6:276–280 [Google Scholar]
- 5. Jetten MS, et al. 2009. Biochemistry and molecular biology of anammox bacteria. Crit. Rev. Biochem. Mol. Biol. 1:1–20 [DOI] [PubMed] [Google Scholar]
- 6. Jogler C, Glockner FO, Kolter R. 2011. Characterization of Planctomyces limnophilus and development of genetic tools for its manipulation establish it as a model species for the phylum Planctomycetes. Appl. Environ. Microbiol. 77:5826–5829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kalb VF, Bernlohr RW. 1977. A new spectrophotometric assay for protein in cell extracts. Anal. Biochem. 82:362–371 [DOI] [PubMed] [Google Scholar]
- 8. Kalyuzhnyi S, Gladchenko M, Mulder A, Versprille B. 2006. New anaerobic process of nitrogen removal. Water. Sci. Technol. 54:163–170 [DOI] [PubMed] [Google Scholar]
- 9. Kirby JR. 2007. In vivo mutagenesis using EZ-Tn5. Methods Enzymol. 421:17–21 [DOI] [PubMed] [Google Scholar]
- 10. Labutti K, et al. 2010. Complete genome sequence of Planctomyces limnophilus type strain (Mu 290). Stand. Genomic Sci. 3:47–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ley RE, et al. 2008. Evolution of mammals and their gut microbes. Science 320:1647–1651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lodge JK, Weston-Hafer K, Berg DE. 1988. Transposon Tn5 target specificity: preference for insertion at G/C pairs. Genetics 120:645–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Østerås M, Finan TM, Stanley J. 1991. Site-directed mutagenesis and DNA sequence of pckA of Rhizobium NGR234, encoding phosphoenolpyruvate carboxykinase: gluconeogenesis and host-dependent symbiotic phenotype. Mol. Gen. Genet. 230:257–269 [DOI] [PubMed] [Google Scholar]
- 14. Qin A, Tucker AM, Hines A, Wood DO. 2004. Transposon mutagenesis of the obligate intracellular pathogen Rickettsia prowazekii. Appl. Environ. Microbiol. 70:2816–2822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rawls JF, Mahowald MA, Ley RE, Gordon JI. 2006. Reciprocal gut microbiota transplants from zebrafish and mice to germ-free recipients reveal host habitat selection. Cell 127:423–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sambrook J, Fritsch F, Maniatis T. 1989. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 17. Santarella-Mellwig R, et al. 2010. The compartmentalized bacteria of the planctomycetes-verrucomicrobia-chlamydiae superphylum have membrane coat-like proteins. PLoS Biol. 8:e1000281 doi: 10.1371/journal.pbio.1000281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Scovill WH, Schreier HJ, Bayles KW. 1996. Identification and characterization of the pckA gene from Staphylococcus aureus. J. Bacteriol. 178:3362–3364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ward N, et al. 2006. The order Planctomycetales, including the genera Planctomyces, Pirellula, Gemmata and Isosphaera and the Candidatus genera Brocadia, Kuenenia and Scalindua, p 757–793. In Dworkin M, Falkow S, Rosenberg E, Schleifer K-H, Stackebrandt E. (ed), The prokaryotes, 3rd ed, vol 7 Springer, New York, NY [Google Scholar]