Abstract
Fourier transform infrared (FTIR) spectroscopy was carried out to ascertain the mechanism of Ca-alginate and mannitol protection of cell envelope components and secondary proteins of Bifidobacterium animalis subsp. lactis Bb12 after freeze-drying and after 10 weeks of storage at room temperature (25°C) at low water activities (aw) of 0.07, 0.1, and 0.2. Preparation of Ca-alginate and Ca-alginate-mannitol as microencapsulants was carried out by dropping an alginate or alginate-mannitol emulsion containing bacteria using a burette into CaCl2 solution to obtain Ca-alginate beads and Ca-alginate-mannitol beads, respectively. The wet beads were then freeze-dried. The aw of freeze-dried beads was then adjusted to 0.07, 0.1, and 0.2 using saturated salt solutions; controls were prepared by keeping Ca-alginate and Ca-alginate-mannitol in aluminum foil without aw adjustment. Mannitol in the Ca-alginate system interacted with cell envelopes during freeze-drying and during storage at low aws. In contrast, Ca-alginate protected cell envelopes after freeze-drying but not during 10-week storage. Unlike Ca-alginate, Ca-alginate-mannitol was effective in retarding the changes in secondary proteins during freeze-drying and during 10 weeks of storage at low aws. It appears that Ca-alginate-mannitol is more effective than Ca-alginate in preserving cell envelopes and proteins after freeze-drying and after 10 weeks of storage at room temperature (25°C).
INTRODUCTION
Microencapsulation of probiotic bacteria using alginate or alginate fortified with mono-, di-, or polysaccharides or proteins has been widely studied (1, 9, 28, 30, 37, 44, 52). The use of sugars or proteins to fortify alginate was effective in improving bacterial survival; however, Krasaekoopt and others (30) reported that alginate combined with chitosan was not successful in protecting Bifidobacterium in very-low-pH environment. Similarly, Zohar-Perez et al. (60) found that alginate was not effective as a bacterial encapsulant. Stabilization of alginate-based microencapsulated bacteria was influenced by many factors, such as type and concentration of microencapsulants and storage conditions, i.e., temperature, water activity, and the presence of oxygen. The use of mannitol was found to be effective in maintaining bacterial viability during drying and during storage at room temperature (15, 27, 38) and also in stabilizing protein during freeze-drying (26) and during storage (8). Mannitol protected probiotic bacteria during storage, likely due to its role as a hydroxyl radical scavenger (15, 49).
Water activity (aw) is a critical factor for bacterial stabilization during storage at room temperature. Storage at low aws at room temperature maintained the glassy state and minimized chemical reactions, and hence, the survival of bacteria improved (24). Storage at low aws (0.07 and 0.1) maintained high viability of bacteria during long-term storage at room temperature; however, this phenomenon depended on the type of sugars used as protectants: incorporation of mannitol in Ca-alginate improved the survival of Bifidobacterium animalis subsp. lactis Bb12 and preserved some glycolytic enzymes during long-term storage at room temperature and low aw (11, 12).
Even though Ca-alginate is known as an encapsulant for probiotic bacteria (25), the mechanism of its protection of probiotic bacteria has not been established. Changes in functional groups of polypeptides, lipids, and secondary proteins can be observed by Fourier transform infrared (FTIR) spectroscopy (51). FTIR spectroscopy has been successfully applied in identifying microorganisms such as probiotic bacteria and fungi (14, 16, 57, 58). Characterization of lactic acid bacteria (LAB) encapsulated with alginate using FTIR spectroscopy has been carried out by Le-Tien and others (34). The effect of drying and as well as of sugars on bacterial membrane structure and on secondary proteins has been studied by several other groups (26, 40, 45, 46, 47). Trehalose and sorbitol were effective in protecting cell envelopes and secondary proteins of probiotic bacteria during drying (33, 40, 45). However, polysaccharides such as maltodextrin act as inert bulking substances instead of bacterial protectants (33). Lyophilization causes reversible changes to secondary proteins (21) as well as affecting the stability of phosphatidylcholine and liposomes (54). However, freeze-drying is a common method of dehydration of probiotic bacteria. In this study, FTIR spectroscopy was used to establish the mechanism of the protection provided by encapsulants such as Ca-alginate with or without mannitol to cell envelope components and secondary proteins of probiotic bacteria during freeze-drying and after 10-week storage at low aws. Interaction between Ca-alginate and mannitol was also observed in order to understand the effectiveness of mannitol as a protectant in a Ca-alginate matrix.
MATERIALS AND METHODS
B. animalis subsp. lactis Bb12 cultivation.
B. animalis subsp. lactis Bb12 was cultivated out as described by Ding and Shah (13). The freeze-dried pure culture of B. animalis subsp. lactis Bb12 was obtained from Chr. Hansen (Bayswater, Victoria, Australia). The organism was grown in MRS broth (Oxoid Ltd., Hampshire, United Kingdom) supplemented with filter-sterilized 0.05% (wt/vol) l-cysteine-hydrochloride (Sigma Chemical Co., Castle Hill, Australia) at 37°C using a 1% inoculum. The organism was propagated three times successively, and its presence was confirmed by Gram staining.
The cells of B. animalis subsp. lactis Bb12 were concentrated by centrifugation of the broth at 14,000 × g for 15 min at 4°C using a Sorvall centrifuge (56), and the cell pellet was washed twice with 0.85% sterilized saline solution. The cell pellet was then resuspended in one-fourth the original volume (5-ml cell pellet added to 15 ml of saline solution). The initial population of bacteria in the suspension was ∼2.5 × 1010 CFU/ml.
Microencapsulation of probiotic bacteria.
Microencapsulation was carried out using two types of alginate emulsions as per Dianawati and Shah (12). The first formulation contained sodium alginate (at 2.5% [wt/vol] of the emulsion), and the second formulation contained sodium alginate and mannitol (each at 2.5% [wt/vol] of the emulsion). Canola oil containing 0.5% Tween 80 at 10% was used to develop an emulsion system for each formulations. Each emulsion was pasteurized by heating at 70°C for 30 min and cooled to 10°C before B. animalis subsp. lactis Bb12 was incorporated into the emulsion system (250 ml cells in a total of 1,000 ml of emulsion). Each mixture containing the bacteria was then dropped into 0.1 M CaCl2 solution using a burette to create either Ca-alginate beads or Ca-alginate-mannitol beads of a uniform size. Wet beads of both types were then freeze-dried (freeze-drier model FD-300; Airvac Engineering Pty. Ltd., Dandenong, Australia), with the instrument set to achieve −100 torr of internal pressure before freeze-drying at −88°C, including 44 h of primary freeze-drying and 4 h of secondary freeze-drying. Once the freeze-drying process was completed, two types of dehydrated beads, namely Ca-alginate containing B. animalis subsp. lactis Bb12 (CAB) and Ca-alginate-mannitol containing B. animalis subsp. lactis Bb12 (CAMB), were obtained. The samples (CAB and CAMB) were stored for 10 weeks in desiccators with the aw adjusted to 0.07, 0.1, and 0.2 using NaOH, LiCl, and CH3COOK, respectively, at 25°C. NaOH (200 g) was added to 45 ml of water (equivalent to 111.1 M) to achieve an aw of 0.07; LiCl (200 g) was added to 113 ml of water (equivalent to 41.7 M) to achieve an aw of 0.11, and CH3COOK (200 g) was added to 65 ml water (equivalent to 31.4 M) to achieve an aw of 0.23. CAB and CAMB samples kept in aluminum foil at room temperature (without aw adjustment) were used as controls.
Storage at low aws at room temperature of 25°C.
An equilibrium between aw of the samples and that of the environment (desiccators) was achieved in 2 weeks, which was considered week 0 of storage. The aw was measured using a water activity meter (CX-2, serial I/O; Decagon, Pullman, WA). Samples were taken at week 10 of storage for measuring changes in cell envelopes and secondary proteins using FTIR spectroscopy.
Sample preparation for FTIR spectroscopy.
Infrared absorption measurements were carried out with an FT-IR spectrometer (IRAffinity-1; Shimadzu Corp., Kyoto, Japan) at room temperature (25°C). Spectra were recorded at a resolution of 4 cm−1 and 20 scans in a wave number range of 4,000 to 500 cm−1. A reference spectrum was measured prior to each experiment to correct the background effects of all the spectra recorded. The instrument was purged with nitrogen gas to reduce the interference of water vapor and CO2 in all the FTIR measurements. The sample preparation was according to Izutsu and Kojima (26) and Sharma and Kalonia (47). Briefly, a 10-mg sample of CAB or CAMB was mixed with 100 mg of dried KBr powder and pressed under a vacuum using 10 tons of hydraulic pressure (JC Hydraulics KBr Beta Press 6010 and 6102; Buck Scientific Inc., East Norwalk, CT) with a 13-mm pellet die (model no. 3000; Specac, Orpington, Kent, Great Britain) to obtain a transparent pellet of CAB or CAMB. Spectra of fresh B. animalis subsp. lactis Bb12 (harvested after 18 h) were used as controls to observe any change in the frequencies of functional groups of cell envelope proteins [PO2−, (CH3)3N+, and C-H] and secondary proteins (C-N and N-H) of microencapsulated B. animalis subsp. lactis Bb12. Samples of fresh bacteria were prepared according to the method of Santivarangkna et al. (45). Briefly, washed cell suspensions were spread onto CaF2 windows and dehydrated at room temperature in vacuum desiccators containing P2O5 for 2 days to reduce any interference of H2O. Spectra were collected from three batches of samples. Smoothing and normalization of second derivatives of deconvoluted spectra were carried out to develop clearer separation of complex bands using IRsolution software (Shimadzu Corp., Kyoto, Japan).
The freeze-dried Ca-alginate-mannitol without bacteria (CAM) was compared with Ca-alginate without bacteria (CA) to study any chemical interaction between Ca-alginate and mannitol as the microencapsulant. Na-alginate (SA) powder was used as a control. To examine changes in cell envelopes and secondary proteins of microencapsulated B. animalis subsp. lactis Bb12, the spectra of CAM and CA were subtracted from those of CAMB and CAB (22; A. Mauerer and G. Lee, presented at the Controlled Release Society German Chapter Annual Meeting, Munich, Germany, 2003). The remaining spectra were then compared with those of the freshly harvested B. animalis subsp. lactis Bb12. All FTIR measurements were repeated three times.
Determination of Ca-alginate-mannitol, cell envelope proteins, and secondary proteins of microencapsulated B. animalis subsp. lactis Bb12 by FTIR spectroscopy.
An interaction between Ca-alginate and mannitol was ascertained by comparing the peaks of CA and CAM at 3,000 to 3,700 cm−1 (broad peak of O-H stretching) and at 1,410 to 1,260 (O-H deformation vibration). Sodium alginate (SA) was used as a control (23, 32). An alteration of COO− stretching symmetric vibration and COO− stretching asymmetric vibration of alginate can be detected at ∼1,420 to 1,300 and ∼1,615 to 1,550, respectively (3, 23, 32, 41).
An investigation of cell envelopes of bacteria in CAMB and CAB was carried out in the frequencies in the FTIR spectra of ∼1,080 cm−1 (P O of PO2− symmetric stretching) and ∼1,240 cm−1 (P
O of PO2− symmetric stretching) and ∼1,240 cm−1 (P O of PO2− asymmetric stretching) (16, 17); ∼2,850 cm−1 and ∼2,925 cm−1 (CH2 symmetric and asymmetric stretching vibrations, respectively) (45, 59), and ∼975 cm−1 [(CH3)3N+ asymmetric stretching vibration] (42). An observation on secondary proteins was carried out by measuring the frequency of amide II at 1,450 to 1,575 cm−1 (CN stretching and NH bending) (33, 35).
O of PO2− asymmetric stretching) (16, 17); ∼2,850 cm−1 and ∼2,925 cm−1 (CH2 symmetric and asymmetric stretching vibrations, respectively) (45, 59), and ∼975 cm−1 [(CH3)3N+ asymmetric stretching vibration] (42). An observation on secondary proteins was carried out by measuring the frequency of amide II at 1,450 to 1,575 cm−1 (CN stretching and NH bending) (33, 35).
ESEM study.
The morphology of freeze-dried microcapsules (CAMB and CAB) was observed after freeze-drying using an FEI Quanta 200 environmental scanning electron microscope (ESEM). The working distance was 10.2 mm, beam energy was 20.0 kV, spot size was 5.0, magnification was ×500, and pressure was 0.98 torr. All scale bars presented 200.0 μm of microcapsule size. The microcapsule bead was loaded on a double-sided carbon tape put on multiple studs before being examined by SEM.
RESULTS
Ca-alginate and mannitol interaction in the gel bead system.
FTIR analysis of CA and CAM was carried out to observe any shift in spectra representing an interaction between alginate and mannitol; thus, its mechanism as a protectant could be predicted. The presence of mannitol may influence the frequency shift indicating its interaction with alginate. In this study, wave number alteration of some functional groups of CA and CAM was identified by comparing the shift in OH stretching and OH deformation, COO− symmetric and asymmetric stretching, and C-O-C asymmetric stretching of freeze-dried CA and CAM (Table 1). Sodium alginate (SA) was used as a control. The alteration of the functional groups of alginate was based on references 55, 32, 59). A decrease in frequency from 3,210 (CA) to 3,207 (CAM) and from 1,257 (CA) to 1,251 (CAM) indicated the presence of OH stretching and OH deformation vibration, respectively; meanwhile, SA in powder form showed a higher frequency of OH stretching and OH deformation vibration. An alteration to a lower frequency indicated an increase in the strength of the hydrogen bond (23), possibly due to the presence of mannitol. In addition, no obvious difference between COO− of CA and CAM (in both symmetric and asymmetric vibrations) was detected; meanwhile, SA demonstrated a lower frequency of COO− than CA and CAM (Table 1). The hydrogen bond increase could be due to the presence of hydroxyl groups of mannitol replacing the moisture availability, as suggested by Aranda et al. (2) and Santivarangkna et al. (45).
Table 1.
Assignment of some bands found in CA, CAM, and SA spectra
| Assignment of functional group | Frequency (cm−1) in: |
||
|---|---|---|---|
| CA | CAM | SA | |
| OH stretching | 3,210.3 ± 0.3 | 3,207.2 ± 0.2 | 3,237.2 ± 0.2 |
| OH deformation | 1,257.2 ± 0.3 | 1,251.0 ± 0.1 | 1,312.2 ± 0.2 |
| COO symmetric stretching | 1,444.2 ± 0.2 | 1,443.5 ± 0.5 | 1,419.3 ± 0.3 |
| COO asymmetric stretching | 1,609.2 ± 0.2 | 1,609.2 ± 0.3 | 1,609.4 ± 0.4 |
| C—O—C asymmetric stretching | 1,160.3 ± 0.4 | 1,162.3 ± 0.3 | 1,166.2 ± 0.3 |
This suggests that there was no strong interaction between alginate and mannitol due to the stronger ionic bonding of COO− of alginate with cations. On the other hand, frequencies of C-O-C asymmetric vibration altered from 1,160 (CA) to 1,162 (CAM), while SA showed a slightly higher frequency of C-O-C stretching vibration.
Cell envelopes and secondary proteins of microencapsulated Bifidobacterium animalis subsp. lactis Bb12 after freeze-drying and after storage at low aws at room temperature.
Frequencies of some cell envelope components and amide II of freshly harvested B. animalis subsp. lactis Bb12, CAB, and CAMB after freeze-drying and after 10 week storage were demonstrated in Tables 2, 3 and 4, respectively. P O and (CH3)3N+ represented the polar site of phospholipid bilayers, while CH2 was the nonpolar site of phospholipid bilayers. The changes in secondary protein structures were indicated by amide II.
O and (CH3)3N+ represented the polar site of phospholipid bilayers, while CH2 was the nonpolar site of phospholipid bilayers. The changes in secondary protein structures were indicated by amide II.
Table 2.
Assignment of some bands of freshly harvested B. animalis subsp. lactis Bb12
| Assignment of functional group | Frequency in fresh Bb12 (cm−1) |
|---|---|
P O symmetric stretching O symmetric stretching |
1,077.8 ± 0.2 |
P O asymmetric stretching O asymmetric stretching |
1,240.4 ± 0.5 |
| N+(CH3)3 asymmetric stretching | 969.1 ± 0.2 |
| CH2 symmetric stretching | 2,867.0 ± 0.1 |
| CH2 asymmetric stretching | 2,925.9 ± 0.2 |
| Amide II | 1,541.2 ± 0.3 |
Table 3.
Assignment of some bands of CAB after freeze drying and after storage
| Assignment of functional group | Frequency (cm−1) in: |
||||
|---|---|---|---|---|---|
| CAB after FD | FD CAB (aw, 0.07) | FD CAB (aw, 0.1) | FD CAB (aw, 0.2) | FD CAB (in aluminum foil) | |
P O symmetric stretching O symmetric stretching |
1,053.4 ± 0.4 | 1,055.5 ± 0.3 | 1,055.9 ± 0.3 | 1,057.7 ± 0.3 | 1,081.6 ± 0.5 |
P O asymmetric stretching O asymmetric stretching |
1,222.8 ± 0.3 | 1,231.8 ± 0.3 | 1,235.9 ± 0.2 | 1,241.5 ± 0.5 | 1,244.0 ± 0.5 |
| N+(CH3)3 asymmetric stretching | 980.2 ± 0.3 | 996.8 ± 0.3 | 998.8 ± 0.3 | 1,002.8 ± 0.3 | Undetectable |
| CH2 symmetric stretching | 2,866.8 ± 0.3 | 2,902.3 ± 0.3 | 2,870.6 ± 0.5 | 2,875.9 ± 0.2 | 2,906.6 ± 0.5 |
| CH2 asymmetric stretching | 2,924.9 ± 0.3 | 2,947.5 ± 0.5 | 2,944.5 ± 0.5 | 2,946.5 ± 0.5 | 2,963.6 ± 0.5 |
| Amide II | 1,545.8 ± 0.3 | 1,568.5 ± 0.5 | 1,569.8 ± 0.8 | 1,569.8 ± 0.3 | 1,569.3 ± 0.6 |
Table 4.
Assignment of some bands of CAMB after freeze drying and after storage (cm−1)
| Assignment of functional group | Frequency (cm−1) in: |
||||
|---|---|---|---|---|---|
| CAMB after FD | CAMB (aw, 0.07) | CAMB (aw, 0.1) | CAMB (aw, 0.2) | CAMB in aluminum foil | |
P O symmetric stretching O symmetric stretching |
1,043.6 ± 0.5 | 1,043.8 ± 0.2 | 1,042.3 ± 0.3 | 1,043.2 ± 0.3 | 1,047.4 ± 0.4 |
P O asymmetric stretching O asymmetric stretching |
1,218.5 ± 0.5 | 1,226.5 ± 0.5 | 1,225.2 ± 0.3 | 1,226.2 ± 0.3 | 1,227.2 ± 0.3 |
| N+(CH3)3 asymmetric stretching | 978.2 ± 0.3 | 979.8 ± 0.3 | 983.8 ± 0.3 | 994.7 ± 0.3 | 995.9 ± 0.2 |
| CH2 symmetric stretching | 2,867.4 ± 0.1 | 2,854.3 ± 0.6 | 2,855.5 ± 0.5 | 2,853.2 ± 0.3 | 2,849.0 ± 0.0 |
| CH2 asymmetric stretching | 2,923.3 ± 0.2 | 2,920.7 ± 0.6 | 2,921.5 ± 0.6 | 2,920.7 ± 0.6 | 2,931.5 ± 0.5 |
| Amide II | 1,536.4 ± 0.4 | 1,534.5 ± 0.5 | 1,537.8 ± 0.3 | 1,536.5 ± 0.5 | 1,529.8 ± 0.3 and 1,515 ± 0.1 |
A shift of PO2− symmetric stretching vibration of CAB after freeze-drying and after 10-week storage at various low aws at 25°C is shown in Table 3, and that of CAMB after the same treatments is shown in Table 4. There was an interaction between PO2− of cell envelopes and CAMB, as shown by an alteration to lower frequency (1,043.6) (Table 4) from a value of 1,077.8 for freshly harvested B. animalis subsp. lactis Bb12 (Table 2), while CAB demonstrated a shift to a higher frequency (1,053.4) (Table 3) than CAMB. Both frequencies of freeze-dried CAB and CAMB were below that of the control, indicating an interaction of PO2− of the phospholipid site of cell lipids with the microcapsule substances via hydrogen bond, as suggested by several other researchers (2, 33, 40, 45). The similar behaviors were also demonstrated by asymmetric stretching vibration of the P O of PO2− of freeze-dried CAB and CAMB (Tables 3 and 4, respectively) compared to controls (1,240.4 cm−1; Table 2). This indicates that an interaction via hydrogen bond between PO2− of phospholipid bilayers and mannitol was maintained during storage at low aws. In contrast, there was less interaction between alginate and PO2− of phospholipid bilayers of cell envelopes in CAB kept in aluminum foil after 10 weeks of storage, since the frequencies were higher than those of the control (Table 3).
O of PO2− of freeze-dried CAB and CAMB (Tables 3 and 4, respectively) compared to controls (1,240.4 cm−1; Table 2). This indicates that an interaction via hydrogen bond between PO2− of phospholipid bilayers and mannitol was maintained during storage at low aws. In contrast, there was less interaction between alginate and PO2− of phospholipid bilayers of cell envelopes in CAB kept in aluminum foil after 10 weeks of storage, since the frequencies were higher than those of the control (Table 3).
The choline chain terminus of the cell surface could provide additional information to help characterize the microencapsulated bacterial cell envelopes. An alteration of frequency of (CH3)3N+ asymmetric stretching vibration of the choline chain terminus of cells within CAB and CAMB after freeze-drying and after 10 week of storage at various low aws is shown in Tables 3 and 4, respectively. After 10 weeks of storage, an increase in frequencies occurred in CAB kept at low aws and in aluminum foil. This may be due to the effect of OH of alginate or OH of residual moisture on choline. Loss in the frequency area of (CH3)3N+ of choline occurred in CAB kept in aluminum foil. This could be due to an increase in the molecular mobility and chemical reactions along with an increase in aw during storage, as suggested by Bell and Labuza (4); therefore, an adverse effect on some substances such as choline could not be avoided in freeze-dried bacteria kept in aluminum foil. This may be the reason why our previous study (12) demonstrated low survival of bacteria in aluminum foil after 10 weeks of storage.
CH2 symmetric and asymmetric stretching vibration of fatty acids of cell envelopes of B. animalis subsp. lactis Bb12 encapsulated within CAB and CAMB after freeze-drying and after storage is also shown in Tables 3 and 4, respectively. The spectrum of CH2 of fatty acids, an apolar site of phospholipid bilayers of the bacterium, was detected at ∼2,867 and 2,926 cm−1. A frequency increase occurred in CAB during storage at various aws and in aluminum foil; a further frequency shift occurred in CAB with aw of 0.07 and CAB kept in aluminum foil. On the other hand, no increase in frequency was observed in fatty acids of cells kept in CAMB with various aws during storage; instead a slight frequency decrease occurred. In addition, frequencies of CH2 asymmetric stretching vibration of fatty acids of CAMB increased after storage in aluminum foil, while those of CAB increased due to storage regardless the presence or an absence of desiccants.
The results of frequency alteration of the secondary protein amide II of B. animalis subsp. lactis Bb12 within CAB or CAMB after freeze-drying and that after storage at various aws at room temperature are shown in Tables 3 and 4, respectively. The control showed a native amide II peak at 1,541 cm−1. Amide II of CAMB after freeze-drying showed lower frequency (1,536 cm−1) (Table 4) than that of the control, while that of CAB after freeze-drying altered to a higher frequency (1,546 cm−1) (Table 3). A further obvious shift of freeze-dried CAB after 10-week storage occurred; the peaks were altered to ∼1,569. On the other hand, the bands of CAMB kept at low aws were relatively unaltered compared to bands of CAMB after freeze-drying, i.e., at 1,534 to 1,538 cm−1, but frequency decreased in CAMB kept in aluminum foil along with a shoulder formation at 1,515. This alteration to ∼1,510 indicates a partially change in secondary proteins from α-helices to β-sheets (50).
Microstructure of microcapsules.
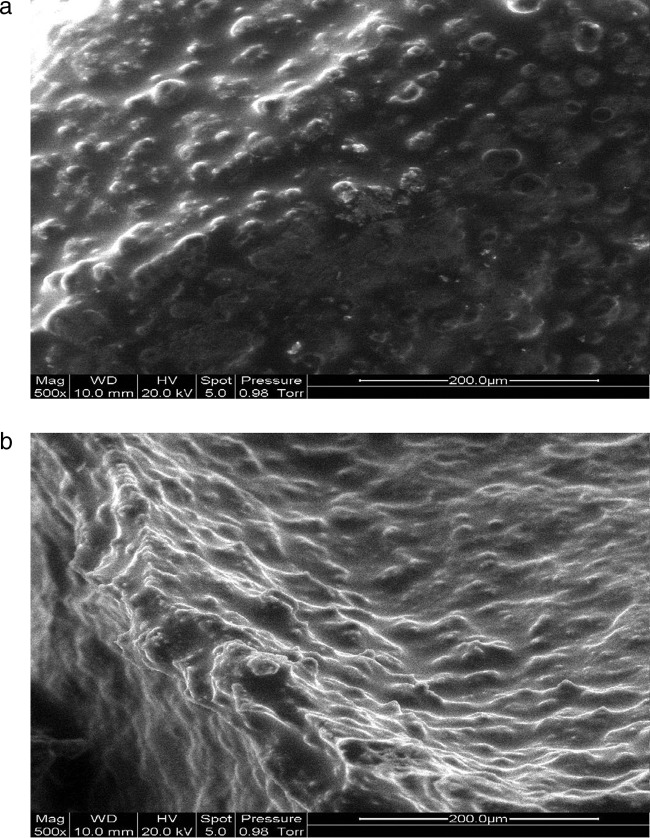
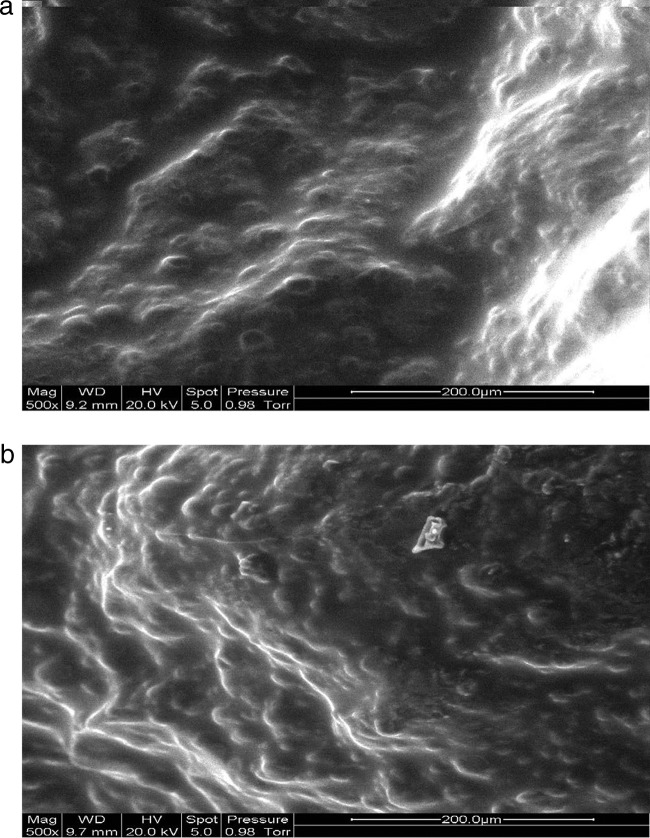
The microstructures of CAB and CAMB microcapsules containing B. animalis subsp. lactis Bb12 after freeze-drying are shown in Fig. 1a and Fig. 2a, respectively, while freeze-dried CAB and CAMB after 10 weeks of storage at an aw of 0.07 are shown in Fig. 2a and 2b. The surfaces of CAB and CAMB microcapsules appeared dense and relatively rough after freeze-drying (Fig. 1a and 2a), with few wrinkles. After 10 weeks of storage, the wrinkles were more obvious (Fig. 1b and 2b), which could be due to residual moisture removal during storage at aw of 0.07. Incorporation of mannitol into alginate gel might soften the bead surface (Fig. 1b and 2b). The bacteria did not appear on the microcapsule surface, indicating that they were trapped within the matrices. The microstructure of our alginate microcapsules was similar to that of Muthukumarasamy et al. (39) and Chen et al. (7). Gbassi et al. (19) found that probiotic bacteria were randomly distributed in the alginate matrices.
Fig 1.
(a) CAB after freeze-drying; (b) freeze-dried CAB after 10 weeks of storage at an aw of 0.07.
Fig 2.
(a) CAMB after freeze-drying; (b) freeze-dried CAMB after 10 weeks of storage at an aw of 0.07.
DISCUSSION
FTIR analysis of the CA and CAM was carried out to observe any shift in spectra representing an interaction between alginate and mannitol; thus, its mechanism as a protectant could be predicted. The presence of mannitol may influence the frequency shift indicating its interaction with alginate. Some functional groups, such as OH stretching, OH deformation, COO− symmetric and asymmetric stretching, and C-O-C asymmetric stretching, have been used to characterize the Ca-alginate interaction with chitosan or xanthan (32, 41).
The formation of a matrix of alginate and divalent cations such as Ca2+ has been widely studied (36, 41, 48). Such a matrix is known as an “egg-box” formation of alginate-Ca. This matrix formation is mainly due to the interaction between COO− of β-d-mannuronic acid and α-l-guluronic acid of alginate and Ca2+ via an ionic bond. Our result is in agreement with that of Pongjanyakul and Puttipipatkhachorn (41), who stated that a cross-linking matrix of alginate with calcium ions resulted in an alteration to higher frequencies of COO− of alginate than a matrix bound with sodium ions. Besides the ionic bond, a partial covalent bond between calcium and oxygen atom of C-O-C groups of alginate occurs (41); this interaction might cause the difference in C-O-C frequencies of CA and CAM due to the presence of mannitol. In addition, an alteration of the COO− stretching peak to lower frequencies was observed owing to xanthan gum incorporation into CA (41). However, our result demonstrated no obvious difference in frequency alteration of COO− of CA and CAM, indicating that OH of mannitol has a less important role in interacting with COO−. Lack of COO− interaction between CA and mannitol could be due to the effect of an ionic bond between COO− of alginate and Ca2+, forming a strong cross-link (36). A decrease in OH vibration and OH deformation frequencies due to mannitol inclusion is in agreement with the work of Hesse and others (23) and Santivarangkna and others (46), who stated that a stronger hydrogen bond was indicated by a shift to lower vibrational frequencies. However, the presence of bacteria influenced the interaction between functional groups of Ca-alginate and mannitol.
FTIR spectroscopy has been used to determine the chemical components such as lipids, proteins, and polysaccharides of microorganisms (51). FTIR spectroscopy permits us to study the molecular structures of colonies or even single cells in situ without any additional reagents or stains (3, 40, 46). A second derivative method based on mathematical analysis has been applied to improve the level of separation of molecular spectra; thus, specific peaks, such as P O of PO2− of phospholipid bilayers, C-H of fatty acids, (CH3)3N+ of choline, and secondary proteins, can be more easily recognized (29).
O of PO2− of phospholipid bilayers, C-H of fatty acids, (CH3)3N+ of choline, and secondary proteins, can be more easily recognized (29).
PO2− of phospholipid bilayers has been commonly used to recognize any interaction with other substances through hydrogen bonds (2, 45). Variability in frequencies after storage at low water activities could be due to the influence of OH of residual unbound water in CAMB beads, besides OH from mannitol; thus, interaction with PO2− can be varied. However, all PO2− frequencies of cell envelopes of CAMB showed lower frequencies than the control. Frequency changes of PO2− bands to a lower wave number suggested an increase in hydrogen bonds due to the presence of sugars (45). In regard to the lack of interaction between alginate and phospholipid bilayers of CAB, our result is in agreement with that of Oldenhof and others (40). Those authors suggested that high-molecular-weight polysaccharides such as maltodextrin (or alginate) were unable to interact with P O of PO2− of phospholipid bilayers. Alginate interacted with mannitol through hydrogen bonds in the absence of probiotic bacteria (Table 1). However, in the presence of bacteria, mannitol interacted with PO2− of cell envelopes instead of Ca-alginate, as shown by the difference in frequencies between CAB and CAMB (Tables 3 and 4).
O of PO2− of phospholipid bilayers. Alginate interacted with mannitol through hydrogen bonds in the absence of probiotic bacteria (Table 1). However, in the presence of bacteria, mannitol interacted with PO2− of cell envelopes instead of Ca-alginate, as shown by the difference in frequencies between CAB and CAMB (Tables 3 and 4).
An interaction of choline of cell envelopes with OH groups was indicated by an increase in frequencies of N+(CH3)3 (5). A peak at ∼970 cm−1 has been identified as asymmetric stretching for (CH3)3N+ of lipids (42, 51). Grdadolnik and Hadzi (20) found a similar trend in the alteration between hydration and sugar incorporation on choline's trimethylammonium group. They found that sugars, including mannitol, could replace water molecules during dehydration and stabilize the polar head region during storage at room temperature at a controlled aw. CAMB kept at low aws appears to be effective in stabilizing CH3)3N+ of phospholipid bilayers, while after storage in aluminum foil, an increase in frequency along with a broader peak was observed, likely due to a strong effect of unbound water. The interaction of surface-exposed choline with water molecules or sugars caused a shift to higher frequencies due to the sensitivity of (CH3)3N+ asymmetric stretching to dipolar interaction (42). Mannitol kept at a higher aw (such as in aluminum foil) might contribute to the plasticizing effect and cause conformational changes in polar site of phospholipids due to an increased level of OH (53).
Fatty acids of phospholipid bilayers have also been used to recognize changes in cell envelope characteristics (10). Vibration of CH2 of fatty acids of phospholipid bilayers can be determined from the frequency around 2,850 cm−1 and 2,930 cm−1 (51); this can be different due to differences between bacterial strains. Freeze-drying showed no apparent effect on stability of fatty acids of freeze-dried cells within either CAB or CAMB (Tables 3 and 4, respectively). This indicated that CAB or CAMB was effective in maintaining apolar site of lipids due to a protection effect of CAB or CAMB on the surface site of phospholipid bilayers, as shown by PO2− and choline frequency alteration. However, long-term storage of CAB at room temperature at low aws or in aluminum foil demonstrated an alteration to higher frequencies. The peak alteration to higher frequencies suggests a melting of lipid acyl chains along with a gel-liquid crystalline transition (42). It appears that Ca-alginate was not able to preserve the fatty acid site of phospholipid bilayers of freeze-dried bacterial cell envelopes during storage at room temperature, even at low aws. Conversely, storage of CAMB at low aws and in aluminum foil resulted in lower frequencies than that of the control. The presence of sugars (including mannitol) which interact with polar site of lipids during dehydration inhibits the interior apolar site of lipid changes, such as lipid phase transition and fusion (43), whereas storage at low aws maintained the glassy state of sugars (31). Hence, lipid stability could be preserved. The alteration to a lower frequency (compared to the control frequency of 2,867) could be due to the presence of the membrane proteins and glycolipids as a constituent of cell envelopes (45), which became more obvious on water removal.
This study used amide II band to examine the changes in secondary proteins instead of amide I, which is commonly examined by FTIR (18, 40). The use of amide I is unreliable compared to amide II, as the C O stretching vibration of alginate interferes with amide I bands (35). Amide II bands represent 60% N-H bending and 40% C-N stretching (51). Any changes in amide II bands represent changes in secondary proteins, as reported by Carpenter and Crowe (6), Leslie and others (33), and Marcotte and others (35). The amide II band alteration indicated a change in secondary protein structures, such as a decrease in the number of native α-helices and an increase in the number of β-sheets (50). Encapsulation of the cells within alginate fortified with mannitol was able to preserve the native conformation of proteins of the cells during freeze-drying and during storage at room temperature at low aws. This result was in agreement with that of Leslie and others (33), Garzon-Rodriguez and others, (18) and Thomas and others (53). In contrast, CAB might undergo a failure in protecting secondary proteins of freeze-dried bacteria during long-term storage at room temperature, as indicated by an alteration to higher frequencies (from 1,538 cm−1); this phenomenon appeared to be independent of aw. High-molecular-weight carbohydrates have been found to be ineffective in retarding protein unfolding during lyophilization (18). This result showed the importance of mannitol incorporation in preserving secondary protein conformation of probiotic bacteria during storage at room temperature at low aws. This may be the reason why the survival of B. animalis subsp. lactis Bb12 in CAMB was higher than that in CAB after 10 weeks of storage at aws of 0.1 and 0.2 (12). Our previous study showed that the survival of bacteria in alginate-mannitol microcapsules was 82.6% and 82.0% after 10 weeks of storage at aws of 0.1 and 0.2, respectively, while bacterial survival in alginate microcapsules without mannitol incorporation was 81.1% and 80.2% after storage under the same conditions.
O stretching vibration of alginate interferes with amide I bands (35). Amide II bands represent 60% N-H bending and 40% C-N stretching (51). Any changes in amide II bands represent changes in secondary proteins, as reported by Carpenter and Crowe (6), Leslie and others (33), and Marcotte and others (35). The amide II band alteration indicated a change in secondary protein structures, such as a decrease in the number of native α-helices and an increase in the number of β-sheets (50). Encapsulation of the cells within alginate fortified with mannitol was able to preserve the native conformation of proteins of the cells during freeze-drying and during storage at room temperature at low aws. This result was in agreement with that of Leslie and others (33), Garzon-Rodriguez and others, (18) and Thomas and others (53). In contrast, CAB might undergo a failure in protecting secondary proteins of freeze-dried bacteria during long-term storage at room temperature, as indicated by an alteration to higher frequencies (from 1,538 cm−1); this phenomenon appeared to be independent of aw. High-molecular-weight carbohydrates have been found to be ineffective in retarding protein unfolding during lyophilization (18). This result showed the importance of mannitol incorporation in preserving secondary protein conformation of probiotic bacteria during storage at room temperature at low aws. This may be the reason why the survival of B. animalis subsp. lactis Bb12 in CAMB was higher than that in CAB after 10 weeks of storage at aws of 0.1 and 0.2 (12). Our previous study showed that the survival of bacteria in alginate-mannitol microcapsules was 82.6% and 82.0% after 10 weeks of storage at aws of 0.1 and 0.2, respectively, while bacterial survival in alginate microcapsules without mannitol incorporation was 81.1% and 80.2% after storage under the same conditions.
Conclusions.
FTIR study showed an interaction between Ca-alginate and mannitol, mainly between OH of mannitol and C-O-C groups of alginate via a hydrogen bond. However, mannitol tended to interact with cell components when B. animalis subsp. lactis Bb12 was incorporated; hence, mannitol might act as a protectant instead of an inert bulking substance, like alginate. Mannitol in the alginate system was able to interact with head groups of lipids of cell envelopes of B. animalis subsp. lactis Bb12. CAMB interacted with P O of PO2− of phospholipid bilayers after freeze-drying and after storage at low aws, while CAB was able to protect this functional group only after freeze-drying, not after 10 weeks of storage at low aws. CAMB also showed an interaction with the choline head group of lipids and prevented the fatty acids (apolar site of phospholipid bilayers) from gel-liquid crystalline transition. Similarly, CAMB was effective in protecting secondary proteins of the bacteria during freeze-drying and during storage at low aws, while CAB failed to protect the cells. In general, Ca-alginate was not effective in protecting cell envelopes and secondary proteins of the probiotic bacteria during freeze-drying and during storage at low aws or in aluminum foil. Incorporation of mannitol was required to improve stability of cell envelopes and secondary proteins of the cells.
O of PO2− of phospholipid bilayers after freeze-drying and after storage at low aws, while CAB was able to protect this functional group only after freeze-drying, not after 10 weeks of storage at low aws. CAMB also showed an interaction with the choline head group of lipids and prevented the fatty acids (apolar site of phospholipid bilayers) from gel-liquid crystalline transition. Similarly, CAMB was effective in protecting secondary proteins of the bacteria during freeze-drying and during storage at low aws, while CAB failed to protect the cells. In general, Ca-alginate was not effective in protecting cell envelopes and secondary proteins of the probiotic bacteria during freeze-drying and during storage at low aws or in aluminum foil. Incorporation of mannitol was required to improve stability of cell envelopes and secondary proteins of the cells.
ACKNOWLEDGMENT
D.D. is grateful to the Indonesian Department of Higher Education (DIKTI) for providing financial support.
Footnotes
Published ahead of print 27 July 2012
REFERENCES
- 1. Anal AK, Singh H. 2007. Recent advances in microencapsulation of probiotics for industrial applications and targeted delivery. Trends Food Sci. Technol. 18:240–251 [Google Scholar]
- 2. Aranda FJ, et al. 2007. Domain formation by a Rhodococcus sp. biosurfactant trehalose lipid incorporated into phosphatidylcholine membranes. Biochim. Biophys. Acta 1768:2596–2604 [DOI] [PubMed] [Google Scholar]
- 3. Beekes M, Lasc P, Naumann D. 2007. Analytical applications of Fourier transform-infrared (FT-IR) spectroscopy in microbiology and prion research. Vet. Microbiol. 123:305–319 [DOI] [PubMed] [Google Scholar]
- 4. Bell LN, Labuza TP. 2000. Moisture sorption: practical aspects of isotherm measurement and use, 2nd ed. American Association of Cereal Chemists, Saint Paul, MN [Google Scholar]
- 5. Cacela C, Hincha DK. 2006. Low amounts of sucrose are sufficient to depress the phase transition temperature of dry phosphatidylcholine, but not for lyoprotection of liposomes. Biophys. J. 90:2831–2842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Carpenter JF, Crowe JH. 1989. An infrared spectroscopic study of the interactions of carbohydrates with dried proteins. Biochemistry 28:3916–3922 [DOI] [PubMed] [Google Scholar]
- 7. Chen M-J, Chen K-N, Kuo Y-T. 2007. Optimal thermotolerance of Bifidobacterium bifidum in gellan-alginate microparticles. Biotechnol. Bioeng. 98:411–419 [DOI] [PubMed] [Google Scholar]
- 8. Costantino HR, et al. 1998. Effect of mannitol crystallization on the stability and aerosol performance of a spray-dried pharmaceutical protein, recombinant humanized Anti-IgE monoclonal antibody. J. Pharm. Sci. 87:1406–1411 [DOI] [PubMed] [Google Scholar]
- 9. Cui J-H, Cao Q-R, Choi Y-J, Lee K-H, Lee B-J. 2006. Effect of additives on the viability of bifidobacteria loaded in alginate poly-l-lysine microparticles during the freeze-drying process. Arch. Pharm. Res. 29:707–711 [DOI] [PubMed] [Google Scholar]
- 10. Davis R, Mauer LJ. 2010. Fourier transform infrared (FT-IR) spectroscopy: a rapid tool for detection and analysis of foodborne pathogenic bacteria, p 1582–1594. In Mendez-Vilas A. (ed), Current research, technology and education topics in applied microbiology and microbial biotechnology. FORMATEX microbiology series, no 2, vol 2. FORMATEX, Badajoz, Spain [Google Scholar]
- 11. Dianawati D, Shah NP. 2011. Enzyme stability of microencapsulated Bifidobacterium animalis subsp. lactis bb12 after freeze-drying and during storage in low water activity at room temperature. J. Food Sci. 76:M463–M471 [DOI] [PubMed] [Google Scholar]
- 12. Dianawati D, Shah NP. 2011. Survival, acid and bile tolerance, and surface hydrophobicity of microencapsulated B. animalis subsp. lactis Bb12 during storage at room temperature. J. Food Sci. 76:M592–M599 [DOI] [PubMed] [Google Scholar]
- 13. Ding WK, Shah NP. 2009. An improved method of microencapsulation of probiotic bacteria for their stability in acidic and bile conditions during storage. J. Food Sci. 74:M53–M61 [DOI] [PubMed] [Google Scholar]
- 14. Dziuba B, Babuchowski A, Naecz D, Niklewicz M. 2007. Identification of lactic acid bacteria using FTIR spectroscopy and cluster analysis. Int. Dairy J. 17:183–189 [Google Scholar]
- 15. Efiuvwevwere BJO, Gorris LGM, Smid EJ, Kets EPW. 1999. Mannitol-enhanced survival of Lactococcus lactis subjected to drying. Appl. Microbiol. Biotechnol. 51:100–104 [Google Scholar]
- 16. Erukhimovitch V, Pavlov V, Talyshinsky M, Souprun Y, Huleihel M. 2005. FTIR microscopy as a method for identification of bacterial and fungal infections. J. Pharm. Biomed. Anal. 37:1105–1108 [DOI] [PubMed] [Google Scholar]
- 17. Filip Z, Hermann S, Demnerova K. 2008. FT-IR spectroscopic characteristics of differently cultivated Escherichia coli. Czech J. Food Sci. 26:458–463 [Google Scholar]
- 18. Garzon-Rodriguez W, et al. 2004. Optimizing storage stability of lyophilized recombinant human interleukin-11 with disaccharide/hydroxyethylstarch mixtures. J. Pharm. Sci. 93:684–696 [DOI] [PubMed] [Google Scholar]
- 19. Gbassi GK, Vandamme T, Ennahar S, Marchioni E. 2009. Microencapsulation of Lactobacillus plantarum spp. in an alginate matrix coated with whey proteins. Int. J. Food Microbiol. 129:103–105 [DOI] [PubMed] [Google Scholar]
- 20. Grdadolnik J, Hadzi D. 1998. FT infrared and Raman investigation of saccharide -phosphatidylcholine interactions using novel structure probes. Spectrochim. Acta Part A 54:1989–2000 [DOI] [PubMed] [Google Scholar]
- 21. Griebenow K, Klibanov AM. 1995. Lyophilization-induced reversible changes in the secondary structure of proteins. Proc. Natl. Acad. Sci. U. S. A. 92:10969–10976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Han Y, Jin B-S, Lee SB, Sohn Y, Joung JW, Lee JH. 2007. Effects of sugar additives on protein stability of recombinant human serum albumin during lyophilization and storage. Arch. Pharm. Res. 30:1124–1131 [DOI] [PubMed] [Google Scholar]
- 23. Hesse M, Meier H, Zeeh B. 1997. Spectroscopic methods in organic chemistry. Georg Thieme Verlag, Stuttgart, Germany [Google Scholar]
- 24. Higl B, et al. 2007. Impact of water activity, temperature, and physical state on the storage stability of Lactobacillus paracasei subsp. paracasei freeze-dried in a lactose matrix. Biotechnol. Prog. 23:794–800 [DOI] [PubMed] [Google Scholar]
- 25. Islam MA, Yun CH, Choi YJ, Cho CS. 2010. Microencapsulation of live probiotic bacteria. J. Microbiol. Biotechnol. 20:1367–1377 [DOI] [PubMed] [Google Scholar]
- 26. Izutsu K, Kojima S. 2002. Excipient crystallinity and its protein-structure stabilizing effect during freeze-drying. J. Pharm. Pharmacol. 54:1033–1039 [DOI] [PubMed] [Google Scholar]
- 27. Kets EPW, Galinski EA, Wit MD, Bont JAMD, Heipieper HJ. 1996. Mannitol, a novel bacterial compatible solute in Pseudomonas putida S12. J. Bacteriol. 178:6665–6670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kim S-J, et al. 2008. Effect of microencapsulation on viability and other characteristics in Lactobacillus acidophilus ATCC 43121. LWT Food Sci. Technol. 41:493–500 [Google Scholar]
- 29. Kong J, Yu S. 2007. FTIR spectroscopic analysis of protein secondary structures. Acta Biochim. Biophys. Sin. 39:549–559 [DOI] [PubMed] [Google Scholar]
- 30. Krasaekoopt W, Bhandari B, Deeth H. 2004. The influence of coating materials on some properties of alginate beads and survivability of microencapsulated probiotic bacteria. Int. Dairy J. 14:737–743 [Google Scholar]
- 31. Kurtmann L, Carlsen CU, Skibted LH, Risbo J. 2009. Water activity-temperature state diagrams of freeze dried L. acidophilus (La-5): influence of physical state on bacterial survival during storage. Biotechnol. Prog. 25:265–270 [DOI] [PubMed] [Google Scholar]
- 32. Lawrie G, et al. 2007. Interactions between alginate and chitosan biopolymers characterized using FTIR and XPS. Biomacromolecules 8:2533–2541 [DOI] [PubMed] [Google Scholar]
- 33. Leslie SB, Israeli E, Lighthart B, Crowe JH, Crowe LM. 1995. Trehalose and sucrose protect both membranes and proteins in intact bacteria during drying. Appl. Environ. Microbiol. 61:3592–3597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Le-Tien C, Millette M, Mateescu MA, Lacroix M. 2004. Modified alginate and chitosan for lactic acid bacteria immobilization. Biotechnol. Appl. Biochem. 39:347–354 [DOI] [PubMed] [Google Scholar]
- 35. Marcotte L, Kegelaer G, Sandt C, Barbeau J, Lafleur M. 2007. An alternative infrared spectroscopy assay for the quantification of polysaccharides in bacterial samples. Anal. Biochem. 361:7–14 [DOI] [PubMed] [Google Scholar]
- 36. Mohan NN, Prabha D. 2005. Novel porous, polysaccharide scaffolds for tissue engineering applications. Trends Biomater. Artif. Organs 18:219–224 [Google Scholar]
- 37. Mortazavian A, Razavi SH, Ehsani MR, Sohrabvandi S. 2007. Principles and methods of microencapsulation of probiotic microorganisms. Iranian J. Biotechnol. 5:1–18 [Google Scholar]
- 38. Mugnier J, Jung G. 1985. Survival of bacteria and fungi in relation to water activity and the solvent properties of water in biopolymer. Appl. Environ. Microbiol. 50:108–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Muthukumarasamy P, Allan-Wojtas P, Holley RA. 2006. Stability of Lactobacillus reuteri in different types of microcapsules. J. Food Sci. 71:M20–M24 [Google Scholar]
- 40. Oldenhof H, Wolkers WF, Fonseca F, Passot S, Marin M. 2005. Effect of sucrose and maltodextrin on the physical properties and survival of air-dried Lactobacillus bulgaricus: an in situ Fourier transform infrared spectroscopy study. Biotechnol. Prog. 21:885–892 [DOI] [PubMed] [Google Scholar]
- 41. Pongjanyakul T, Puttipipatkhachorn S. 2007. Xanthan-alginate composite gel beads: molecular interaction and in vitro characterization. Int. J. Pharm. 331:61–71 [DOI] [PubMed] [Google Scholar]
- 42. Popova AV, Hincha DK. 2003. Intermolecular interactions in dry and rehydrated pure and mixed bilayers of phosphatidylcholine and digalactosyldiacylglycerol: a Fourier transform infrared spectroscopy study. Biophys. J. 85:1682–1690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ricker JV, et al. 2003. Trehalose maintains phase separation in an air-dried binary lipid mixture. Biophys. J. 84:3045–3051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sabikhi L, Babu R, Thompkinson DK, Kapila S. 2010. Resistance of microencapsulated Lactobacillus acidophilus LA1 to processing treatments and simulated gut conditions. Food Bioproc. Technol. 3:586–593 [Google Scholar]
- 45. Santivarangkna C, Naumann D, Kulozik U, Foerst P. 2010. Protective effects of sorbitol during the vacuum drying of Lactobacillus helveticus: an FT-IR study. Ann. Microbiol. 60:235–242 [Google Scholar]
- 46. Santivarangkna C, Wenning M, Foerst P, Kulozik U. 2007. Damage of cell envelope of Lactobacillus helveticus during vacuum drying. J. Appl. Microbiol. 102:748–756 [DOI] [PubMed] [Google Scholar]
- 47. Sharma VK, Kalonia DS. 2004. Effect of vacuum drying on protein-mannitol interactions: the physical state of mannitol and protein structure in the dried state. AAPS PharmSciTech 5:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sikorski P, Mo F, Skjak-Braek G, Stokke BT. 2007. Evidence for egg-box-compatible interactions in calcium-alginate gels from fiber x-ray diffraction. Biomacromolecules 8:2098–2103 [DOI] [PubMed] [Google Scholar]
- 49. Smirnoff N, Cumbes QJ. 1989. Hydroxyl radical scavenging activity of compatible solutes. Phytochemistry 28:1057–1060 [Google Scholar]
- 50. Sokolowski F, Naumann D. 2005. FTIR study on thermal denaturation and aggregation of recombinant hamster prion protein SHaPrP90-232. Vib. Spectrosc. 38:39–44 [Google Scholar]
- 51. Stuart BH. 2004. Infrared spectroscopy: fundamentals and applications. John Wiley and Sons, Ltd., Chichester, England [Google Scholar]
- 52. Sultana K, et al. 2000. Encapsulation of probiotic bacteria with alginate-starch and evaluation of survival in simulated gastrointestinal conditions and in yoghurt. Int. J. Food Microbiol. 62:47–55 [DOI] [PubMed] [Google Scholar]
- 53. Thomas M, Scher J, Desobry S. 2005. Study of lactose/β-lactoglobulin interactions during storage. Lait 85:325–333 [Google Scholar]
- 54. Tsvetkova NM, Phillips BL, Crowe LM, Crowe JH, Risbud SH. 1998. Effect of sugars on headgroup mobility in freeze-dried dipalmitoylphosphatidylcholine bilayers: solid-state 31P NMR and FTIR studies. Biophys. J. 75:2947–2955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. van Hoogmoed CG, Busscher HJ, de Vos P. 2003. Fourier transform infrared spectroscopy studies of alginate-PLL capsules with varying compositions. J. Biomed. Mater. Res. A 67:172–178 [DOI] [PubMed] [Google Scholar]
- 56. Vinderola CG, Reinheimer JA. 2003. Lactic acid starter and probiotic bacteria, a comparative “in vitro” study of probiotic characteristics and biological barrier resistance. Food Res. Int. 36:895–904 [Google Scholar]
- 57. Vodnar DC, Paucean A, Dulf FV, Socaciu C. 2010. HPLC characterization of lactic acid formation and FTIR fingerprint of probiotic bacteria during fermentation processes. Notulae Bot. Horti Agrobot. Cluj Napoca 38:109–113 [Google Scholar]
- 58. Vodnar DC, Socaciu C, Rotar AM, Stanila A. 2010. Morphology, FTIR fingerprint and survivability of encapsulated lactic bacteria (Streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus) in simulated gastric juice and intestinal juice. Int. J. Food Sci. Technol. 45:2345–2351 [Google Scholar]
- 59. Williams DH, Fleming I. 1989. Spectroscopic methods in organic chemistry, 4th ed. McGraw-Hill Book Company, London, United Kingdom [Google Scholar]
- 60. Zohar-Perez C, Chet I, Nussinovitch A. 2004. Unexpected distribution of immobilized microorganisms within alginate beads. Biotechnol. Bioeng. 88:671–674 [DOI] [PubMed] [Google Scholar]