Abstract
Xanthomonas campestris pv. campestris strain 8004 contains an orphan quorum-sensing (QS) locus, xccR-pipXcc, in which the proline iminopeptidase (pipXcc) gene (where “Xcc” indicates that the pip gene is from X. campestris pv. campestris) is positively regulated by the LuxR homologue XccR by binding to the luxXc box of the pipXcc promoter. The disruption of pipXcc significantly attenuated the virulence of X. campestris pv. campestris. An imperfect plant-inducible promoter (PIP) box is located in the upstream region of the pipXcc promoter, which is the putative binding site of the transcriptional activator HrpX. To explore whether the expression of the pipXcc gene is regulated by HrpX, the expression level of a pipXcc promoter-gusA fusion gene was assayed in an hrpX disruption mutant. The results showed that the lack of HrpX dramatically decreased the β-glucuronidase (GUS) activity. Further analyses using an electrophoretic mobility shift assay (EMSA) and chromatin immunoprecipitation (ChIP)-PCR indicated that the imperfect PIP box in X. campestris pv. campestris is specifically bound to HrpX. These data demonstrated that the pipXcc gene belongs to the hrp regulon and that the imperfect PIP box of the pipXcc promoter could be a cis element for the HrpX protein. We further showed in a pulldown assay that XccR can bind HrpX, suggesting that these two regulatory proteins coactivate the virulence factor by binding to the different cis elements of the pipXcc gene and adapt to the host environment during X. campestris pv. campestris infection.
INTRODUCTION
Diseases caused by members of the genus Xanthomonas contribute to devastating losses of cultivated crops worldwide (27). Many phytopathogenic bacteria elicit the hypersensitive response (HR) in nonhost plants or pathogenicity in host plants, depending on hrp (hypersensitive reaction and pathogenicity) and hrc (hrp-conserved) genes (2). The hrp gene cluster in phytopathogens is regulated by two types of regulators (2, 13). Group I hrp genes in Erwinia amylovora and Pseudomonas syringae are activated by an alternative sigma factor (30), whereas the group II hrp genes of Ralstonia solanacearum and Xanthomonas campestris are activated by an AraC family regulator (HrpX for Xanthomonas and HrpB for Ralstonia). In many Xanthomonas species, HrpX regulates the expression of a genome-wide regulon, including type II and type III secretion systems (14), which also exist in many bacterial pathogens of humans and animals to secrete effector proteins and degradation enzymes (6, 17). The promoters controlled by HrpX often carry a conserved motif called plant-inducible promoter (PIP) box and a −10 box (11, 12). HrpX regulates the PIP box-containing promoters by directly binding to the conserved cis element (TTCGC-N15-TTCGC) in xanthomonads (11, 33). A similar sequence (TTCG-N16-TTCG), called hrpII box, discovered in R. solanacearum, was regulated by HrpB (7). The central cytidine of each half-site is essential for the function of the cis element, while the other nucleotides are more flexible (7). Notably, genes with an imperfect PIP box or without a PIP box have also been shown to be expressed in an HrpX-dependent manner (7, 20, 26). Thus, HrpX is believed to be a global regulator, and there are more genes belonging to the HrpX regulon than previously expected.
X. campestris pv. campestris is the causal agent of black rot on most cultivated crucifer plants (27). Our previous study showed that the xccR-pipXcc locus (where “Xcc” indicates that the pip gene is from X. campestris pv. campestris) is related to pathogenicity, and disruption of either of the two genes results in significantly attenuated virulence of X. campestris pv. campestris (34). The proline iminopeptidase (pipXcc) gene was regulated by quorum-sensing (QS) LuxR homolog XccR, and the pipXcc promoter-gusA fusion gene was significantly induced when the bacteria grew in planta (34). QS enables bacterial cell-cell communication via signal molecules, and it monitors the density of bacterial populations (10). In Gram-negative bacteria, the classic QS regulation is mediated by N-acylhomoserine lactone (AHL) signal molecules, and LuxI and LuxR are responsible for producing and sensing signals, respectively (10). Only a few isolates of Xanthomonas produced detectable AHLs (5). A genome survey showed that X. campestris pv. campestris strain 8004 has no cognate LuxI synthase for AHLs (28), and in consequence, no AHL activity was detected. Instead, XccR activates the expression of pipXcc, which encodes a hydrolase rather than a LuxI synthase, by binding to the luxXc box, highly similar to the lux box in the promoter region of the LuxI genes.
In this study, an imperfect PIP box could be found in the xccR-pipXcc intergenic region upstream of the luxXc box by sequence analysis. We provide evidence for direct binding of HrpX to the imperfect PIP box in vivo and in vitro. We further show the in vitro binding of HrpX and XccR in pulldown assays, suggesting that the two proteins are coactivators of pipXcc. This conclusion is consistent with the results that deletion of either hrpX or xccR abolished the pipXcc activity.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The bacteria and plasmids used in this work are listed in Table 1. Escherichia coli strains were grown in Luria-Bertani (24) medium at 37°C. X. campestris pv. campestris strains were cultured at 28°C either in NYG medium (5 g/liter tryptone, 3 g/liter yeast extract, 20 g/liter glycerol, pH 7.2) as a nutrient-rich condition or in MMX [4 g/liter K2HPO4, 6 g/liter KH2PO4, 2 g/liter (NH4)2SO4, 1g/liter citric acid-Na3, 0.2 g/liter MgSO4 · 7H2O, 5 g/liter glucose, pH 7.0] as a minimal medium. Bacterial cell density was monitored by measuring the optical absorbance at 600 nm. Antibiotics were used at the following final concentrations: 50 μg/ml rifampin, 20 μg/ml kanamycin, 100 μg/ml ampicillin, 80 μg/ml spectinomycin, and 3 μg/ml tetracycline for liquid medium and 10 μg/ml for solid medium.
Table 1.
Bacterial strains, plasmids, and primers used in this work
| Strain, plasmid, or primer | Relevant characteristics | Source or reference |
|---|---|---|
| Strains | ||
| 8004 | Wild type, Rifr | 32 |
| ΔhrpX strain | hrpX deletion mutation in strain 8004 | This study |
| Plasmids | ||
| pLAFR6 | pLAFR1 with rho-independent terminator and pUC18 polylinker, Tetr | 11 |
| pFR421 | pLAFR6(PpipXcc-gusA) | 19 |
| pHM1 | Broad-host-range cosmid vector, pSa ori, Spr | 19 |
| pHM1-hrpX-his | pHM1 (hrpX-his) | This study |
| pMAL-p2X | Tac promoter, expression vector, Ampr | New England BioLabs |
| pMAL-p2x/hrpX | pMAL-p2X(hrpX-his) | This study |
| pGEM-T | Cloning vector | Promega Co. |
| pK18mobsacB | Suicide plasmid in X. campestris pv. campestris, Kmr | 29 |
| Primers | ||
| hrpXhisF | 5′cccaagcttATGATCCTTTCGACCTACTTCGC3′a | |
| hrpXhisR | 5′ccggaattctcagtggtggtggtggtggtgGCGTTGCAGGGTTTCCAT3′ | |
| jxhrpXFwd | 5′ttccatatgATGATCCTTTCGACCTACTTCGCA3′ | |
| jxhrpXRev | 5′ccgctcgagGCGTTGCAGGGTTTCCATCGG3′ | |
| hrpX del F1 | 5′gatatcATGATCCTTTCGACCTACTTGA3′ | |
| hrpX del R1 | 5′gtcgacTGGAAGTGGGTCAGCGCCTT3′ | |
| hrpX del F2 | 5′gtcgacCGCCCTGGGGGCTGTGCAA3′ | |
| hrpX del R2 | 5′aagcttTTAGCGTTGCAGGGTTTCCATCG3′ | |
| hrpX outup | 5′GCTCACCGCTGCCCTGCATTGCTGC3′ | |
| hrpX outdown | 5′TACAATCGTTTGCGCCCACCACAAC3′ | |
| box45F | 5′ACGGTGTCGCAATTCGCGCGTTTCGCAATTGCCAACCGGTGTCAT3′ | |
| box45R | 5′ATGACACCGGTTGGCAATTGCGAAACGCGCGAATTGCGA3′ | |
| pip-PF | 5′GTCGAATTCGAAGGCTCAGTTGGTCGGGTTTG3′ | |
| pip-PR | 5′CTCGGTGCACTTCATGACCTGC3′ | |
| pipF1 | 5′GAAGGCTCAGTTGGTCGGGTT3′ | |
| pipR1 | 5′GGCAATTGCGAAACGCGCGAA3′ | |
| pipR2 | 5′GACCTGCGCCCACTTACGG3′ |
The lowercase letters in primer sequences indicate the restriction enzyme recognition sites and fusion tag sequences.
Plasmid construction.
To determine pipXcc promoter activity, plasmid pFR421 was generated, which contains a 438-bp EcoRI-BspHI fragment PCR amplified with primers pip-PF and pip-PR (Table 1) from the X. campestris pv. campestris strain 8004 chromosome (34). The fragment spanned from −438 to −1 relative to the pipXcc translational start site (TSS).
To construct pHM1-hrpX-his, the coding region of the hrpX gene was PCR amplified from the X. campestris pv. campestris 8004 genomic DNA with primers hrpXhisF and hrpXhisR (Table 1). PCR products were first cloned into pGEM-T vector and then digested with HindIII and EcoRI restriction enzymes and ligated into pHM1 vector. The 6×His tag was fused to the C terminus of HrpX. The hrpX HindIII/EcoRI fragment was blunt ended by DNA polymerase I Klenow fragment (NEB, Hitchin, Hertshire, United Kingdom) and ligated into the flush-ended PstI site of pMAL-p2X to generate phrpX-MBP (maltose binding protein).
To make the plasmid that expressed the chimeric HrpX protein in E. coli, the hrpX gene was amplified by PCR with primers jxhrpXFwd and jxhrpXRev (Table 1). The fragment was digested with NdeI and XhoI restriction enzyme and cloned into pET30a to generate pET30a-hrpX.
Protein expression, purification, and antibody preparation.
Prokaryotic expression plasmids pET30a-hrpX and phrpX-MBP were transformed into E. coli BL21 or TB1. Fusion proteins were expressed in E. coli cells after induction of an early log culture overnight by isopropyl-β-d-thiogalactopyranoside (IPTG) (0.1 mM) at 16°C. The MBP-tagged protein was purified by affinity chromatography with amylose resin (NEB) and eluted with maltose. The His-tagged protein was purified by Ni-nitrilotriacetic acid (NTA) resin (Novagen) under denaturing conditions. Amicon YM10 (Millipore) was used for protein concentration or for changing the protein suspension buffer. For production of antibody against HrpX, the His-tagged protein expressed from pET30a-hrpX was purified and pooled to immunize and boost rabbits, and serum was taken after the fourth booster injection.
Construction of hrpX mutant strain.
The hrpX deletion mutant was generated using a selection/counterselection suicide vector, pK18mobsacB (21). To construct the strain with disrupted hrpX, two fragments corresponding to the hrpX coding regions 1 to 431 and 1008 to 1431 were PCR amplified from the strain 8004 genomic DNA by two pairs of primers, hrpX del F1/hrpX del R1 and hrpX del F2/hrpX del R2, respectively (Table 1). The resulting EcoRV-SalI and SalI-HindIII fragments were fused into the SmaI/HindIII-cleaved pK18mobsacB vector in one ligation reaction. The hrpX gene in the strain 8004 genome was truncated by homologous recombination. To detect positive clones with the truncated hrpX gene, a PCR primer pair, hrpX outup and hrpX outdown, flanking the hrpX coding region (Table 1), was designed.
GUS assay.
The β-glucuronidase (GUS) activity of different X. campestris pv. campestris strains grown in medium and in planta was measured by the fluorometric method using the substrate 4-methylumbelliferyl β-d-glucuronide (MUG) (34). GUS activity was normalized to bacterial cell numbers. One unit of enzyme activity is defined as the amount of enzyme that releases 1 pmol of 4-methylumbelliferone (MU) min−1 at pH 7.0 at 37°C. The experiments were repeated at least three times for each of the conditions, each time in triplicate.
ChIP-PCR.
Strain 8004 harboring the pHM1-hrpX-his plasmid was grown in 10 ml NYG medium to an optical density at 600 nm of 1.5. The proteins and chromatin DNA were cross-linked by adding formaldehyde to a final concentration of 1% for 10 min. The cross-linking reaction was stopped by the addition of glycine. The assay was performed using a chromatin immunoprecipitation (ChIP) assay kit (Millipore, Billerica, MA), following the manufacturer's instructions. The resulting purified DNA was used for PCR analysis. A small aliquot of untreated sonicated chromatin was reverse cross-linked and used as the total input DNA control. The experiments were repeated at least three times.
EMSA.
MBP-HrpX fusion protein was purified through an amylose resin chromatography column (NEB) according to the manufacturer's instructions. The 45-bp DNA duplex containing the PIP box sequence was generated by annealing the synthetic oligonucleotides box45F and box45R. The product was then end labeled with [α-32P]dATP. For the electrophoretic mobility shift assay (EMSA), 0.65 μg of the labeled probe and the MBP-HrpX protein were incubated in a binding buffer [10 mM Tris-HCl (pH 7.5), 50 mM KCl, 1 mM dithiothreitol (DTT), 2.5% glycerol, and 50 ng ml−1 poly(dI · dC)] for 20 min at room temperature. For competition, a certain amount of the unlabeled probe was coincubated with the labeled probe and the MBP-HrpX protein. Samples were size fractionated by using a 4% nondenaturing polyacrylamide gel in 0.5× TBE buffer (45 mM Tris-borate and 1 mM EDTA) at 4°C. The gel was dried, and the shifted bands were detected by autoradiography.
His pulldown assay.
Purified XccR-MBP, HrpX-His, and MBP were subjected to His tag pulldown analysis. HrpX-his was loaded onto a Ni-NTA column using binding buffer (300 mM NaCl, 50 mM Na-phosphate, pH 8.0, 10 mM imidazole). XccR-MBP was then loaded onto the same column. The column was washed with four volumes of wash buffer (300 mM NaCl, 50 mM Na-phosphate, pH 8.0, 20 mM imidazole), and then the protein was eluted in elution buffer (300 mM NaCl, 50 mM Na-phosphate, pH 8.0, 300 mM imidazole). The eluted proteins were analyzed by SDS-PAGE and verified by Western blotting.
RESULTS
Sequence analysis of the pipXcc promoter region.
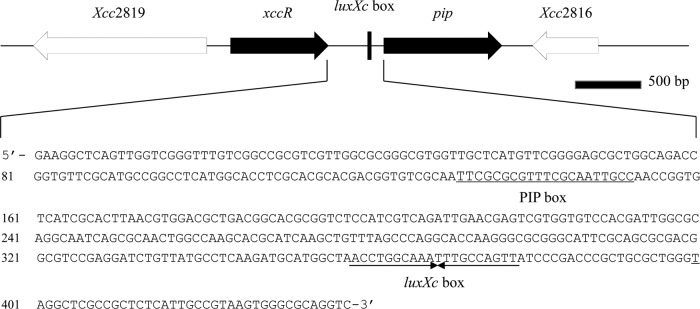
The 438-bp intergenic sequence between xccR and pipXcc in X. campestris pv. campestris 8004 was analyzed, and an imperfect PIP box (TTCGC-N4-TTCGC-N2-TTGCC) was found at positions −307 to −287 relative to the pipXcc translational start site (TSS) (Fig. 1). While the consensus sequence of a perfect PIP box (TTCGC-N15-TTCGC) has two conserved half-sites (TTCGC), the imperfect PIP box comprises not only the two conserved half-sites but also one nonconserved half-site (TTGCC). The three sites were separated in the imperfect PIP box by 4 bp and 2 bp. Including the spacing base pairs, the imperfect PIP box may be presented as TTCGC-N11-TTGCC for HrpX dimer binding.
Fig 1.
Genomic organization of the xccR-pipXcc locus and structure of the intergenic sequence upstream of the pipXcc gene. The imperfect PIP box and luxXc box sequences are indicated.
HrpX is essential for pipXcc expression.
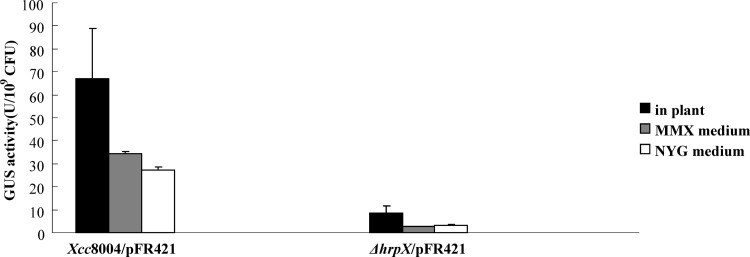
The PIP box is known to be the cis element for HrpX binding in the promoter region that regulates gene expression. The existence of the imperfect PIP box in the pip promoter may indicate that the expression of pip is HrpX dependent. To verify the role of HrpX in pip expression, an hrpX mutant was generated by double crossover steps using the pK18mobsacB vector (21). The GUS reporter plasmid pFR421 that carried a 438-bp pip promoter-gusA fusion (34) was introduced into the wild-type 8004 and hrpX mutant strains. The GUS activity assay showed that disruption of hrpX resulted in decreased GUS activities in NYG medium, MMX medium, and in planta (Fig. 2). Compared with that in strain 8004/pFR421, the GUS activity in ΔhrpX/pFR421 decreased by 6.7-, 12.8-, and 8.2-fold, respectively, under the three growth conditions. These results suggested that HrpX is indispensable for pip expression.
Fig 2.
HrpX is essential for PpipXcc-gusA fusion gene expression. The Xanthomonas campestris pv. campestris ΔhrpX/pFR421[pLAFR6(P438pipXcc-gusA)] strain exhibited reduced GUS activity compared with that of the 8004/pFR421 strain under three conditions. Relative GUS activity units were defined as nM 4-methylumbelliferone/min/109 cells. The graph represents values of experiments from a minimum of three independent samples. Xcc8004, Xanthomonas campestris pv. campestris strain 8004.
HrpX binding to the imperfect PIP box.
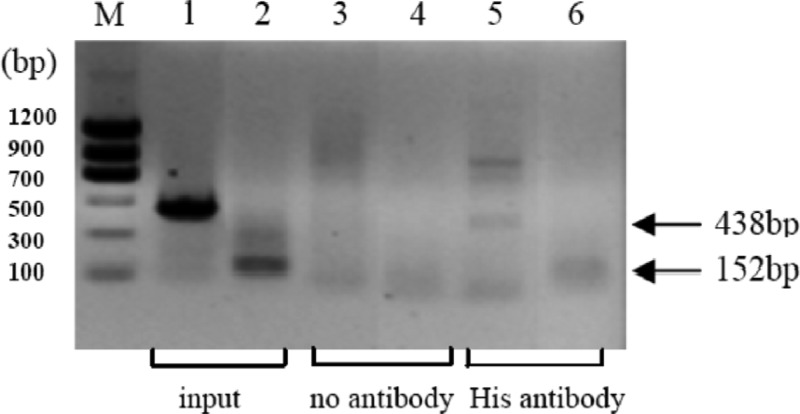
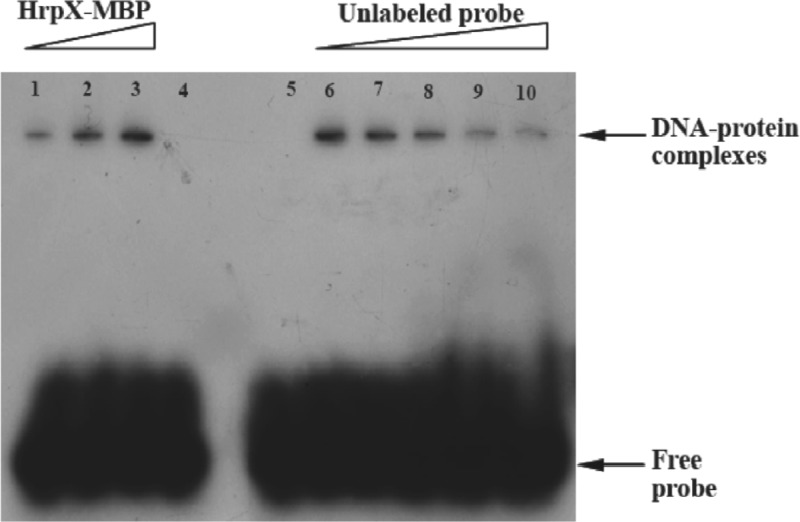
A previous report showed that even an imperfect PIP box could be recognized by cognate regulatory factors (12). As a result, more genes with imperfect cis elements in the promoters could be assigned to the HrpX regulon, such as the pipXcc gene of strain 8004, in which a potential PIP box-like element is located −307 to −287 upstream from the TSS (Fig. 1). In order to detect the interaction between HrpX and this potential PIP box, ChIP-PCR and EMSA were used to assess the presence of the HrpX protein bound to the cis element. ChIP-PCR of strain 8004/pHM1-hrpX-his bacterial extract was performed. The DNA fragments (spanning −438 to −1 and −438 to −287 relative to TSS of pipXcc) immunoprecipitated with the His monoclonal antibody were amplified by PCR. The amplification of a no-antibody sample was the negative control. The results revealed that the HrpX protein is bound to the potential PIP box (Fig. 3). The result of in vitro EMSA was consistent with the ChIP-PCR result. The migrated bands of 45-bp duplex DNA and HrpX-MBP complexes were observed in nondenaturing polyacrylamide gel. Various amounts of unlabeled probe (Fig. 4, right) and various amounts of purified HrpX-MBP (Fig. 4, left) were used as competitors. MBP, as the negative control, did not bind the DNA probe.
Fig 3.
Results of ChIP-PCR assay with or without His tag monoclonal antibody. DNA was amplified by primers designed against the regulatory regions. Lanes 1 and 2 represent the positive controls using chromatin as the template for PCR. The sizes of the resulting fragments are 438 bp (using primers pipF1 and pipR2; Table 1) and 152 bp (using primers pipF1 and pipR1; Table 1), respectively. Negative controls with no antibody are presented in lanes 3 and 4. The results with the addition of anti-His antibody are shown in lanes 5 and 6. The no-template negative control is not shown.
Fig 4.
Binding of HrpX to potential PIP box by EMSA. The interaction of the DNA probe with purified HrpX-MBP is shown. Each lane contains 0.65 μg isotope-labeled probe. Lanes 1 to 3 contain 12.28 μg, 24.56 μg, and 49.12 μg HrpX-MBP, respectively; lane 4 contains MBP (44.48 μg) as the negative control; lane 5 contains the free probe as the positive control; and lanes 6 to 10 contain the same concentration of HrpX-MBP (49.12 μg) and various amounts of unlabeled probe (0.65 μg, 3.23 μg, 13 μg, and 19.5 μg, respectively) as competitors.
HrpX/XccR interplay is responsible for pipXcc induction.
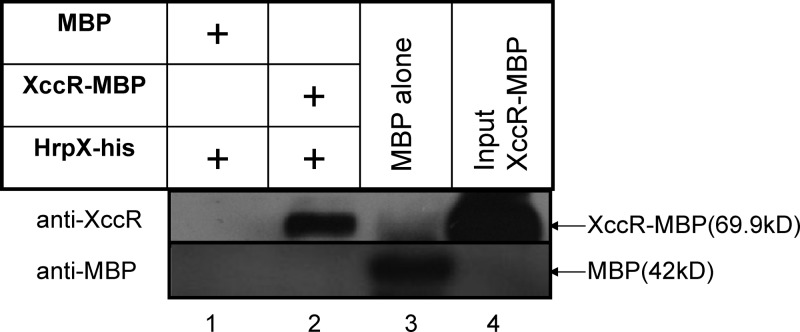
As previously reported, an xccR mutant decreased the induction of the pipXcc promoter in host plants (34). This indicated that the disruption of either xccR or hrpX can result in a failed induction of pipXcc. To investigate the relationship between XccR and HrpX in regulating pipXcc expression, a pulldown assay was carried out to test the binding of the two proteins. The MBP-tagged XccR was expressed in E. coli and X. campestris pv. campestris harboring the plasmid-expressed HrpX-His. The pulldown result showed that the XccR protein interacted with the HrpX protein (Fig. 5). The MBP protein used as a negative control did not bind any of the tested proteins. This result indicates that the complex formed by XccR and HrpX may contribute to the activation of pipXcc.
Fig 5.
XccR interacts with HrpX in vitro. The HrpX-his, MBP, and XccR-MBP strains were used in a His pulldown assay. The precipitated proteins were detected by Western blotting using anti-XccR and anti-MBP antibodies. The recombinant HrpX did not precipitate with MBP (lane 1) but did with XccR-MBP (lane 2). In lane 3, MBP was used as a positive control for immunoblot detection by an anti-MBP antibody. Lane 4 shows an immunoblot of 2 μg purified XccR-MBP.
DISCUSSION
In Xanthomonas species, the essential role of HrpX in virulence-related gene expression, through binding by a plant-inducible element, the PIP box, has been well defined. According to bioinformatics analysis results, an imperfect PIP box (TTCGC-N4-TTCGC-N2-TTGCC) was found in the pipXcc promoter region in strain 8004 (Fig. 1). The X. campestris pv. campestris genome has 12 candidate promoters with perfect PIP boxes (TTCGC-N15-TTCGC) (13), and an earlier study showed that a substitution in the central base C in each of the TTCGC elements will drastically decrease the promoter activity (31). In the case of the imperfect PIP box in the pipXcc promoter, the first two parts were identical to a perfect PIP box half-site consensus sequence, but not the third one. The three parts were separated by 2 and 4 bp. Generally, the separating base stretch between the half-sites ranges from 8 to 16 bp (20), and even a 17-bp spacing retains the promoter activity (12). Thus, the imperfect PIP box in the pipXcc promoter region of X. campestris pv. campestris may be formulated as TTCGC-N11-TTGCC. In this pattern, the central C of the second half-site was replaced by G. We suspect that this replacement may reduce the promoter activity compared with that of the perfect PIP box as previously reported (31). Further experiments need to be conducted to elucidate the imperfect PIP box's contribution to pipXcc gene expression.
To explore whether HrpX contributes to the induction of pipXcc expression, an hrpX mutant was constructed by double crossover. The disruption of HrpX in ΔhrpX/pFR421 decreased the GUS activity by 6.7-, 12.8-, and 8.2-fold in NYG, MMX medium, and in planta, respectively, in comparison with that of the control strain 8004/pFR421, which indicated the importance of HrpX for pipXcc gene expression (Fig. 2). To verify whether HrpX directly binds the imperfect PIP box, we performed ChIP-PCR and EMSA. As expected, both experiments confirmed the direct binding of HrpX to the imperfect PIP box, and this result may expand the gene members of the HrpX regulon in X. campestris pv. campestris. For phytopathogenic bacteria, the HrpG/HrpX regulon responds to the surrounding environment and aids in the nutrient uptake process (16, 33). An acidic pH, low osmotic pressure, and nutritional limitations are thought to partly contribute to the induction of the hrp and hrc genes (30). Several synthetic plant environment mimicking media (such as MMX medium) were used for hrp gene induction and other virulence gene expression experiments (8, 15, 18). The GUS activity of ΔhrpX/pFR421 was lower in the MMX medium than in planta, which indicated that the synthetic medium cannot absolutely mimic the natural conditions. Interestingly, in the nutrient-rich medium NYG (a non-hrp-inducing medium), the GUS activity of ΔhrpX/pFR421 decreased drastically compared with that of strain 8004/pFR421 (Fig. 2). This implied that the basic level of HrpX also affected pipXcc expression in rich medium. Generally, HrpX-regulated genes consist of type III effector protein genes with PIP boxes (12) and genes without PIP boxes (26). As a member of the HrpX regulon, the disruption of pipXcc impaired the virulence of X. campestris pv. campestris in a host plant cabbage. It is worth exploring whether PIPXcc is a secretory virulence protein or a modification enzyme to arrest the other virulence factors.
Our previous observations showed that the pipXcc gene could be induced in host plants and that the increased GUS activity of strain 8004/pFR421 depends on the activation of XccR by binding to the luxXc box in planta (34). Disruption of xccR on the 8004 chromosome (strain 8515/pFR421) resulted in failure of the in planta induction of gusA (34). In this study, the pipXcc promoter activity also decreased drastically in the hrpX mutant. The results of the pulldown assay indicated that XccR can bind HrpX directly. It is likely that HrpX and XccR form a complex as coactivators to regulate pipXcc expression and that HrpX/XccR interplay is responsible for pipXcc induction.
Quorum-sensing systems exist widely in bacteria, and QS-dependent functions include virulence, sporulation, plasmid transfer, biosynthesis of antibiotics, and plant nodulation (3, 4, 25). In the xccR-pipXcc locus, XccR is an unpaired LuxR homolog of QS because the cognate LuxI synthase gene is lacking. The LuxR orphans, such as ExpR of Sinorhizobium, BisR of Rhizobium, QscR of Pseuodomonas, and SdiA in Salmonella, Escherichia, and Klebsiella, respond to AHL signals (1, 9, 22, 23). However, XccR could not respond to AHLs. Instead, XccR activates the expression of pipXcc, which encodes a hydrolase, by binding the luxXc box (34). In this study, XccR recruited HrpX to coregulate pipXcc expression, which indicated that at least two transcriptional factors mediate the function of the xccR-pipXcc locus.
ACKNOWLEDGMENTS
This work was supported by grants from the National Natural Science Foundation of China (grant no. 31030008) and the National Basic Research Program of China (2011CB100700).
We thank Liu Shangjiang (Institute of Microbiology, CAS, China) for reviewing the manuscript.
Footnotes
Published ahead of print 3 August 2012
REFERENCES
- 1. Ahmer BM. 2004. Cell-to-cell signalling in Escherichia coli and Salmonella enterica. Mol. Microbiol. 52: 933–945 [DOI] [PubMed] [Google Scholar]
- 2. Alfano JR, Collmer A. 1997. The type III (Hrp) secretion pathway of plant-pathogenic bacteria: trafficking hairpins, Avr proteins, and death. J. Bacteriol. 179: 5655–5662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bassler BL, Losick R. 2006. Bacterially speaking. Cell 125: 237–246 [DOI] [PubMed] [Google Scholar]
- 4. Cao H, et al. 2009. Complex quorum-sensing regulatory systems regulate bacterial growth and symbiotic nodulation in Mesorhizobium tianshanense. Arch. Microbiol. 191: 283–289 [DOI] [PubMed] [Google Scholar]
- 5. Cha C, Gao P, Chen YC, Shaw PD, Farrand SK. 1998. Production of acyl-homoserine lactone quorum-sensing signals by gram-negative plant-associated bacteria. Mol. Plant Microbe Interact. 11: 1119–1129 [DOI] [PubMed] [Google Scholar]
- 6. Cornelis GR, Van Gijsegem F. 2000. Assembly and function of type III secretory systems. Annu. Rev. Microbiol. 54: 735–774 [DOI] [PubMed] [Google Scholar]
- 7. Cunnac S, Boucher C, Genin S. 2004. Characterization of the cis-acting regulatory element controlling HrpB-mediated activation of the type III secretion system and effector genes in Ralstonia solanacearum. J. Bacteriol. 186: 2309–2318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Daniels MJ, Barber CE, Turner PC, Cleary WG, Sawczyc MK. 1984. Isolation of mutants of Xanthomonas campestris pathovar campestris showing altered pathogenicity. J. Gen. Microbiol. 130: 2447–2455 [Google Scholar]
- 9. Danino VE, Wilkinson A, Edwards A, Downie JA. 2003. Recipient-induced transfer of the symbiotic plasmid pRL1JI in Rhizobium leguminosarum bv. viciae is regulated by a quorum-sensing relay. Mol. Microbiol. 50: 511–525 [DOI] [PubMed] [Google Scholar]
- 10. Engebrecht J, Nealson K, Silverman M. 1983. Bacterial bioluminescence: isolation and genetic analysis of functions from Vibrio fischeri. Cell 32: 773–781 [DOI] [PubMed] [Google Scholar]
- 11. Fenselau S, Bonas U. 1995. Sequence and expression analysis of the hrpB pathogenicity operon of Xanthomonas campestris pv. vesicatoria which encodes eight proteins with similarity to components of the Hrp, Ysc, Spa, and Fli secretion systems. Mol. Plant Microbe Interact. 8: 845–854 [DOI] [PubMed] [Google Scholar]
- 12. Furutani A, et al. 2006. Identification of novel HrpXo regulons preceded by two cis-acting elements, a plant-inducible promoter box and a −10 box-like sequence, from the genome database of Xanthomonas oryzae pv. oryzae. FEMS Microbiol. Lett. 259: 133–141 [DOI] [PubMed] [Google Scholar]
- 13. Gophna U, Ron EZ, Graur D. 2003. Bacterial type III secretion systems are ancient and evolved by multiple horizontal-transfer events. Gene 312: 151–163 [DOI] [PubMed] [Google Scholar]
- 14. Guo Y, Figueiredo F, Jones J, Wang N. 2011. HrpG and HrpX play global roles in coordinating different virulence traits of Xanthomonas axonopodis pv. citri. Mol. Plant Microbe Interact. 24: 649–661 [DOI] [PubMed] [Google Scholar]
- 15. He SY, Huang HC, Collmer A. 1993. Pseudomonas syringae pv. syringae hairpin Pss: a protein that is secreted via the Hrp pathway and elicits the hypersensitive response in plants. Cell 73: 1255–1266 [DOI] [PubMed] [Google Scholar]
- 16. Huang DL, et al. 2009. The Zur of Xanthomonas campestris is involved in hypersensitive response and positively regulates the expression of the hrp cluster via hrpX but not hrpG. Mol. Plant Microbe Interact. 22: 321–329 [DOI] [PubMed] [Google Scholar]
- 17. Hueck CJ. 1998. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol. Mol. Biol. Rev. 62: 379–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reference deleted.
- 19. Innes RW, Hirose MA, Kuempel PL. 1988. Induction of nitrogen-fixing nodules on clover requires only 32 kilobase pairs of DNA from the Rhizobium trifolii symbiosis plasmid. J. Bacteriol. 170: 3793–3802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Koebnik R, Kruger A, Thieme F, Urban A, Bonas U. 2006. Specific binding of the Xanthomonas campestris pv. vesicatoria AraC-type transcriptional activator HrpX to plant-inducible promoter boxes. J. Bacteriol. 188: 7652–7660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kvitko BH, Collmer A. 2011. Construction of Pseudomonas syringae pv. tomato DC3000 mutant and polymutant strains. Methods Mol. Biol. 712: 109–128 [DOI] [PubMed] [Google Scholar]
- 22. Lequette Y, Lee JH, Ledgham F, Lazdunski A, Greenberg EP. 2006. A distinct QscR regulon in the Pseudomonas aeruginosa quorum-sensing circuit. J. Bacteriol. 188: 3365–3370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McIntosh M, Krol E, Becker A. 2008. Competitive and cooperative effects in quorum-sensing-regulated galactoglucan biosynthesis in Sinorhizobium meliloti. J. Bacteriol. 190: 5308–5317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Miller JH. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 25. Ng WL, Bassler BL. 2009. Bacterial quorum-sensing network architectures. Annu. Rev. Genet. 43: 197–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Noel L, Thieme F, Nennstiel D, Bonas U. 2001. cDNA-AFLP analysis unravels a genome-wide hrpG-regulon in the plant pathogen Xanthomonas campestris pv. vesicatoria. Mol. Microbiol. 41: 1271–1281 [DOI] [PubMed] [Google Scholar]
- 27. Onsando JM. 1992. Black rot of crucifers, p 243–252 In Chaube HS, Singh US, Mukhopadyay AN, Kumar J. (ed), Plant diseases of international importance, vol II Diseases of vegetables and oil seed crops Prentice Hall, Inc., Englewood Cliffs, NJ. [Google Scholar]
- 28. Qian W, et al. 2005. Comparative and functional genomic analyses of the pathogenicity of phytopathogen Xanthomonas campestris pv. campestris. Genome Res. 15: 757–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schafer A, et al. 1994. Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene 145: 69–73 [DOI] [PubMed] [Google Scholar]
- 30. Tampakaki AP, et al. 2010. Playing the “Harp”: evolution of our understanding of hrp/hrc genes. Annu. Rev. Phytopathol. 48: 347–370 [DOI] [PubMed] [Google Scholar]
- 31. Tsuge S, et al. 2005. Effects on promoter activity of base substitutions in the cis-acting regulatory element of HrpXo regulons in Xanthomonas oryzae pv. oryzae. J. Bacteriol. 187: 2308–2314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Turner P, Barber C, Daniels M. 1984. Behavior of the transposons Tn5 and Tn7 in Xanthomonas campestris pv. campestris. Mol. Gen. Genet. 195: 101–107 [Google Scholar]
- 33. Wengelnik K, Bonas U. 1996. HrpXv, an AraC-type regulator, activates expression of five of the six loci in the hrp cluster of Xanthomonas campestris pv. vesicatoria. J. Bacteriol. 178: 3462–3469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang L, Jia Y, Wang L, Fang R. 2007. A proline iminopeptidase gene upregulated in planta by a LuxR homologue is essential for pathogenicity of Xanthomonas campestris pv. campestris. Mol. Microbiol. 65: 121–136 [DOI] [PubMed] [Google Scholar]