Abstract
Legionella species are frequently detected in hot water systems, attached to the surface as a biofilm. In this work, the dynamics of Legionella spp. and diverse bacteria and eukarya associated together in the biofilm, coming from a pilot scale 1 system simulating a real hot water system, were investigated throughout 6 months after two successive heat shock treatments followed by three successive chemical treatments. Community structure was assessed by a fingerprint technique, single-strand conformation polymorphism (SSCP). In addition, the diversity and dynamics of Legionella and eukarya were investigated by small-subunit (SSU) ribosomal cloning and sequencing. Our results showed that pathogenic Legionella species remained after the heat shock and chemical treatments (Legionella pneumophila and Legionella anisa, respectively). The biofilm was not removed, and the bacterial community structure was transitorily affected by the treatments. Moreover, several amoebae had been detected in the biofilm before treatments (Thecamoebae sp., Vannella sp., and Hartmanella vermiformis) and after the first heat shock treatment, but only H. vermiformis remained. However, another protozoan affiliated with Alveolata, which is known as a host cell for Legionella, dominated the eukaryal species after the second heat shock and chemical treatment tests. Therefore, effective Legionella disinfection may be dependent on the elimination of these important microbial components. We suggest that eradicating Legionella in hot water networks requires better study of bacterial and eukaryal species associated with Legionella in biofilms.
INTRODUCTION
Despite increased monitoring and advances in detection methods, there is still a lack of knowledge about the microbial ecology of Legionella and its response to treatment. Therefore, contamination of hot water systems with Legionella remains a persistent environmental challenge and a threat to public health. Inhalation of aerosols contaminated with Legionella increases the incidence of legionellosis, a pulmonary infection that is deadly in 15% of cases (19).
Legionella spp. are waterborne gammaproteobacteria that grow at temperatures ranging from 20°C to 42°C (14, 64). These bacteria are found in natural environments (streams, rivers, and lakes) and in soil. However, most cases of legionellosis can be traced to man-made aquatic environments (shower heads, faucets, water fountains, ice machines, medical devices, air conditioning, hot water systems, and cooling towers) (5, 43, 63, 68), where hot water systems and cooling towers present the major sources of contamination.
Legionellae are found normally attached to these systems surfaces, associated with other bacteria in an extracellular anionic matrix known as biofilm (12, 15, 21, 35, 46). The matrix is extremely hydrated (97% water) and consists mainly of exopolysaccharides (10, 53, 58), biological macromolecules (proteins, lipids, DNA, and RNA), nutrients, metabolites, and inorganic compounds and particles, as well as cellular lysis products (53, 58). Several authors have reported that sessile bacteria (present in the biofilm) become more resistant to environmental stress (22, 50, 58). By adoption of this sessile lifestyle, legionellae, like other bacteria, are far less susceptible to any kind of stress coming from the outside (6, 48, 65). In addition, these bacteria survive as an intracellular parasite of free-living amoebae (3, 29, 47, 62, 69) and can interact with other eukaryal species such as Cyclidium spp., Tetrahymena pyriformi, Dictyostelium discoideum, fungi, and algae (11, 12, 56, 59).
So far, various disinfection processes (thermal, chemical, and physical) have been used to eradicate Legionella (30). Different authors studied these treatments, and at the end the treatments were found to be effective (a few hours after treatment) or rapidly transitorily effective (31, 65, 70) on bacteria in water. However, most of these studies investigated the treatment effect in water; only a few of them were done with hot water biofilm, and these were done by using culture methods.
Most studies concerning Legionella spp. have been done using culture methods (9, 37, 38, 44). However, it is well known that only a small portion of bacteria can be cultivated in all environments (4, 41). The remaining portion is composed of microorganisms for which current culture media are not yet adapted or which are in a viable-but-nonculturable (VBNC) state. In fact, surface-adherent microorganisms and bacteria living in protozoa are unrecognized by conventional methods (8). Alleron et al. (2) have studied the effect of monochloramine on Legionella pneumophila and demonstrated that this bacterium can persist for long periods in biofilms in the VBNC state.
Molecular techniques based on the small-subunit (SSU) rRNA have been demonstrated to be able to characterize the microbial community structure and activity in natural and artificial environments (4, 40, 55). However, only a few studies used the molecular tools to assess the Legionella microflora in order to improve the detection of these bacteria (24, 70). Therefore, little is known about the microbial diversity of in situ biofilm-associated Legionella spp. (12) and the effects of antimicrobial treatments on this microbial diversity.
In previous studies, we have developed a pilot scale 1 system simulating a real hot water system and contaminated it with natural Legionella. We followed Legionella and total flora dynamics in water and biofilm after applying heat shock and chemical treatments. The quantitative results showed rapid recolonization of the biofilm and inefficiency against Legionella and total flora as curative treatments.
The aim of the current work was to investigate the ecology of Legionella and the bacterial community in the biofilm of a hot water system. The dynamics of the microbial diversity structure were studied, and we have produced a qualitative microbial diversity description (SSU ribosomal sequence libraries for Legionella spp. and eukarya) of the biofilm in hot water systems after applying heat shock treatment and chemical treatments. In addition, SSU ribosomal fingerprinting was used to assess the dynamics of the bacterial diversity structure.
MATERIALS AND METHODS
Pilot scale 1 system design.
The present study was performed using a pilot scale 1 system located at the Scientific and Technical Building Centre (CSTB), France). This pilot simulates a real hot water distribution system and consists of two independent stainless steel hot water networks. Farhat et al. presented a full description of the pilot in 2010 (17). The pilot was originally contaminated by Marne river water located in Marne-la-Vallée (France) for over 3 months and was then fed by tap water (17). Throughout this study, the “control loop” was untreated and the “test loop” was used to test the anti-Legionella treatments. The water temperatures in the two loops and the two hot water storage containers were maintained at 35°C and 50°C, respectively. The same water was circulating continuously in the loops and the water storage container, where the water flow was settled at 20 liter min−1. Each day, the total volume of water was automatically renewed in each loop and replaced with tap water in order to provide the network with oxygen and nutrients.
Heat shock treatment.
The heat shock treatment was performed twice in the test loop under the same conditions by increasing the temperature to 70°C and maintaining it for 30 min. The total volume of the test loop water was then renewed as recommended by the High Council for Public Health of France (CSHPF). Test loop biofilm was sampled before the treatment (T0) and 24, 48, 72, and 168 h after the treatment. After each heat shock treatment test, the control and test loops were connected together for 3 weeks (the period of time was tested) to reinstall a biofilm in the test loop.
Chemical treatment.
The chemical treatment was performed in the test loop three times under the same conditions. Fifty milliliters of a biodispersant (based on a tensio-active Ferrofos 5260 [BKG Water Solutions, Düsseldorf, Germany]) was injected at 100 mg liter−1 for a period of 24 h, and then the total volume of the treated water was renewed. Thereafter, 50 ml of biocide (hydrogen peroxide-peracetic acid [Ferrocid 8591; BKG Water Solutions, Düsseldorf, Germany]) was injected at 1,000 mg liter−1 for a period of 3 to 6 h according to the manufacturer's instructions, and the total volume of the test loop water was renewed. The test loop biofilm was sampled (i) before the biodispersant injection and (ii) before and 24, 48, 72, and 168 h after the biocide injection. After each chemical treatment test, the control and test loops were connected together for 3 weeks to reinstall a biofilm in the test loop.
Biofilm sample collection.
A “biofilm box” system composed of 20 stainless steel coupons (16 cm2 of surface) was installed on the test loop and used to collect the biofilm (17). A total of 28 biofilm samplings were performed throughout this study. To recover the biofilm developed on the coupons, each coupon was placed in a sterile 180-ml plastic bottle immersed in 100 ml of sterile water and sonicated at 51 W for 4 min (Branson 2510) (the sonication time has been tested [data not shown]). Analyses were performed on the water resulting from sonication. New stainless steel coupons were cleaned, disinfected, autoclaved (120°C, 20 min), and then installed in the biofilm box after each treatment test.
DNA extraction.
Eighty milliliters of water and biofilm samples was filtered through 0.22-μm polyvinylidene fluoride filters (Millipore), and the filter was cut in half and stored in 2-ml Eppendorf tubes at −80°C until DNA extraction. Each stored filter was kept frozen in dry ice and crushed very well to a powder using a sterile plastic stick. The DNA was extracted from these debris filters using the High Pure PCR template preparation kit (Roche Diagnostics, Meylan, France).
SSCP. (i) Amplification of total bacteria.
In order to study the total bacterial diversity, a portion of the 16S rRNA gene, from base 331 to 533 of Escherichia coli, was amplified by PCR. The primers used were w35 (5′-AGGTCCAGACTCCTACGGG) and w34 (5′-TTACCGCGGCTGCTGGCAC) (70). PCR amplifications were performed with an iCycler (Bio-Rad). The PCR mixture contained 1× Pfu Turbo polymerase buffer, 0.2 mM deoxynucleoside triphosphates (dNTPs), 1.3 μl of each primer (100 ng), 0.5 U of Pfu Turbo DNA polymerase (2.5 U/μl) (Stratagene, La Jolla, CA), 1 μl of genomic DNA, and sterile water to obtain a final volume of 50 μl. The amplification was carried out under the following conditions: initial denaturation step of 2 min at 94°C; 25 cycles of (i) denaturation for 30 s at 94°C, (ii) hybridization for 30 s at 61°C, and (ii) elongation for 30 s at 72°C; and a final elongation for 10 min at 72°C. The amplified products were then stored at −80°C until the single-strand conformation polymorphism (SSCP) analyses. All the PCR products were checked by agarose gel electrophoresis.
(ii) SSCP electrophoresis.
The total bacteria diversity was studied by SSCP fingerprinting from biofilm samples collected during the heat shock and the chemical treatment tests. SSCP analyses are used in order to separate different bacterial species having the same nucleic acid size with different sequences. A mixture of 1 μl of each SSCP PCR product, 17.7 μl of formamide, and 0.3 μl of internal size standard Rox 400 HD (Applied Biosystems, Foster City, CA) was prepared. The samples were placed at 95°C for 5 min, transferred immediately onto ice, and left for 10 min. The high temperature (95°C) separates the DNA double strands into single strands, and then the low temperature (0°C) creates different DNA secondary structures of different DNA sequences. Therefore, DNA sequences migrate in a nondenaturant polyacrylamide gel with different speeds.
All the SSCP analyses were performed using an ABI 310 Genetic Analyzer (Applied Biosystems). Electrophoresis was carried out at 15 kV and 32°C for 40 min per sample. Raw SSCP data were analyzed with Genemapper v4 software (Applied Biosystems).
Identification of Legionella and eukaryal species by cloning and sequencing.
Six biofilm samples were chosen to be sequenced (Table 1): (i) two biofilm samples corresponding to before (T0) and after (72 h) the first heat shock treatment, (ii) two biofilm samples corresponding to before (T0) and after (72 h) the second heat shock treatment, and (iii) two biofilm samples corresponding to before (T0) and after (72 h) the second chemical treatment. For each of those six samples, two libraries of Legionella 16S rRNA gene sequences and eukaryal 18S rRNA gene sequences were built. For Legionella species, the primers and the amplification conditions were the same as described above but with 35 cycles instead of 30 cycles. For the eukaryal community, a 554-bp fragment was amplified using w002 (GNTACCTTGTTACGACTT) and w016 (CTTAATTTGACTCAACACGG) (23). A mixture was prepared of 5 μl of 10× AmpliTaq Gold LD polymerase buffer, 0.2 mM deoxynucleoside triphosphates (dNTPs), 200 ng of each primer, 1 U of AmpliTaq Gold DNA polymerase (1 U/μl) (Sigma-Aldrich, St. Louis, MO), 1 μl of genomic DNA, and sterile water to obtain a final volume of 50 μl. The amplification was carried out under the following conditions: initial denaturation step of 5 min at 95°C; 35 cycles of (i) denaturation for 15 s at 95°C, (ii) hybridization for 15 s at 50°C, and (iii) elongation for 30 s at 72°C; and a final elongation for 7 min at 72°C. All the PCR products were purified with a QIAquick PCR purification kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. The purified products were then sent to Millegen (Toulouse, France) for cloning and sequencing.
Table 1.
Test loop biofilm sample information
| Treatmenta | Date (day/mo/yr) | Code | SSCP analyses (bacteria)b | Bacterial diversity (peak no.) | Sequencing (Legionella and eukarya)b |
|---|---|---|---|---|---|
| Heat shock | |||||
| 1 | 26/05/08 | 11 | + | 13 | + |
| 27/05/08 | 12 | + | 20 | − | |
| 28/05/08 | 13 | + | 18 | − | |
| 29/05/08 | 14 | + | 11 | + | |
| 02/06/08 | 15 | + | 17 | − | |
| 2 | 23/06/08 | 26 | + | 11 | + |
| 24/06/08 | 27 | + | 10 | − | |
| 25/06/08 | 28 | + | 17 | − | |
| 26/06/08 | 29 | + | 11 | + | |
| 30/06/08 | 30 | + | 15 | − | |
| Chemical | |||||
| 1 | 15/07/08 | 44 | + | 19 | − |
| 16/07/08 | 45 | + | 9 | − | |
| 17/07/08 | 46 | + | 15 | − | |
| 18/07/08 | 47 | + | 26 | − | |
| 19/07/08 | 48 | + | 14 | − | |
| 23/07/08 | 49 | + | 16 | − | |
| 2 | 06/10/08 | 69 | + | 18 | + |
| 07/10/08 | 70 | + | 19 | − | |
| 08/10/08 | 71 | + | 16 | − | |
| 09/10/08 | 72 | + | 18 | − | |
| 10/10/08 | 73 | + | 14 | + | |
| 15/10/08 | 74 | + | 14 | − | |
| 3 | 17/11/08 | 94 | + | 17 | − |
| 18/11/08 | 95 | + | 16 | − | |
| 19/11/08 | 96 | + | 14 | − | |
| 20/11/08 | 97 | + | 15 | − | |
| 21/11/08 | 98 | + | 13 | − | |
| 25/11/08 | 99 | + | 15 | − |
Samples were recontaminated between treatments.
+, sample analyzed; −, sample not analyzed.
Each unique sequence was compared using BLASTN (3) and the Ribosomal Database Project II (34). The sequences were clustered into phylotypes using DOTUR software based on at least 99% sequence similarity of Legionella species. For eukarya, 18S rRNA gene sequence alignment was performed using Clustal W and DNA matrix construction was carried out with DNAdist in the Phylip program version 3-68). The sequences were then clustered into phylotype using DOTUR software based at least at 97% of sequence similarity.
Statistical analysis.
Principal-component analysis (PCA) and diversity index determination were performed for SSCP profiles using Statfingerprints software (version 1.2) in order to evaluate relationships between bacterial populations after each treatment test (36). PCA is a projection method that permits visualizing a set of data in reduced dimensions. Relationships between the SSCP profiles could be inferred from visual analysis of the graph plot. The comparison was based on all the points of the profile (scan) and the height of the points. Subjects consisted of each biofilm profile, and variables consisted of each of the points (scan). The first and the second principal component of the PCA plots were chosen because they represent the two components that best highlight the differences between the SSCP fingerprints and represent 39% and 26% of the variability for the first and the second components, respectively.
Nucleotide sequence accession numbers.
The 16S rRNA gene sequences of Legionella spp. and the 18S rRNA gene sequences of eukarya determined in this study were deposited in the GenBank database under accession numbers GQ861542 to GQ861583.
RESULTS
A portion of biofilm samples collected from the test loop during heat shock and chemical treatment tests were chosen and analyzed for Legionella and eukaryal diversities by SSU ribosomal cloning. In addition, the dynamics of bacteria diversity structure were assessed by SSCP fingerprinting. Sample information is given in Table 1.
Spatial variation of the biofilm composition throughout the biofilm box axis.
Before the beginning of the treatment test period, the test loop biofilm was sampled from the 10 coupons extracted from the biofilm box and analyzed by PCR-SSCP after 3 weeks of growth. The water loop was subject to the same conditions as in the period of treatments (water temperature, 35°C; water flow, 20 liter min−1; daily renewal of the total volume of the water loop). Two of the SSCP profiles are presented in Fig. 1. The goal was to test the qualitative homogeneity of the biofilm throughout the biofilm box axis. Based on the peak number and the peak position, the results showed that the 10 profiles superimposed perfectly. Consequently, the microbial populations are distributed in a homogenous way on the biofilm box axis, and therefore the qualitative reproducibility of the analytical methods (biofilm sampling, DNA extraction, PCR amplification, and SSCP analysis) used in this study is valid.
Fig 1.
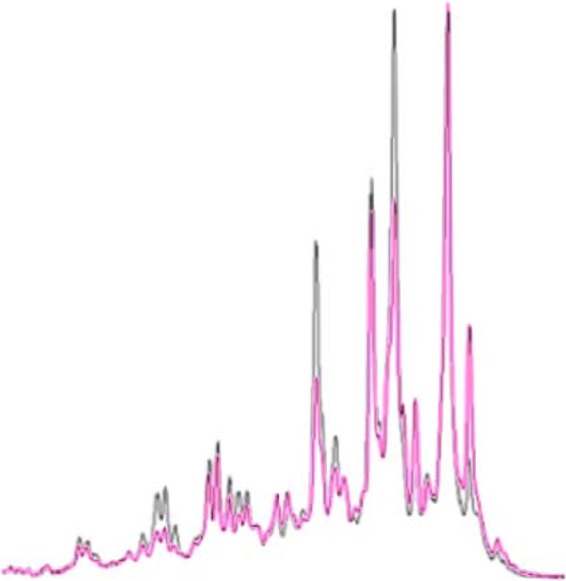
Bacterial SSCP fingerprint of the biofilm sampled from 2 of the 10 coupons of the biofilm box.
Legionella sp. diversity in biofilm before and after treatments.
A total of 164 16S rRNA sequences obtained from four DNA libraries were analyzed in order to characterize the Legionella diversity in biofilm before (T0) and after (72 h) treatment in order to evaluate the possible shift of Legionella diversity after the two heat shock treatments. The 164 sequences were distributed across four phylotypes on the basis of 99% similarity.
For the first heat shock treatment, four phylotypes were obtained before the treatment (Table 2). The sequences were related to cultivable Legionella spp. (L. anisa [T0_HS1_f.02], 44% of the sequences; L. taurinensis [T0_HS1_e.01], 30% of the sequences; L. pneumophila [T0_HS1_f.01], 22% of the sequences; and L. drancourtii [T0_HS1_g.02], 4% of the sequences). After treatment (72 h), the sequencing results showed a decrease of Legionella diversity to a single phylotype related to L. taurinensis (72h_HS1_e.03) (Table 2).
Table 2.
Legionella clones and distribution of operational taxonomic units before and 72 h after the two heat shock treatments and the second chemical treatment
| Test and time point | Operational taxonomic unit |
Organism with best-matching sequence |
||||
|---|---|---|---|---|---|---|
| Name | No. of clones | % Similarity | Affiliation | Accession no. | Name | |
| First heat shock treatment | ||||||
| Before treatment | T0_HS1_g.02 | 1 | 96.8 | Legionella drancourtii | X97366.2 | Legionella drancourtii |
| T0_HS1_f.02 | 12 | 99.84 | Legionella anisa | AY744776.1 | Legionella anisa | |
| T0_HS1_f.01 | 6 | 99.08 | Legionella pneumophila subsp. pneumophila | AE017354.1 | Legionella pneumophila subsp. pneumophila | |
| T0_HS1_e.01 | 8 | 100 | Legionella taurinensis | DQ667196.1 | Legionella taurinensis | |
| Total no. | 4 | 27 | ||||
| 72 h after treatment | 72h_HS1_e.03 | 30 | 99.69 | Legionella taurinensis | DQ667196.1 | Legionella taurinensis |
| Total no. | 1 | 30 | ||||
| Second heat shock treatment | ||||||
| Before treatment | T0_HS2_14 | 23 | 99.84 | Legionella pneumophila | EU054324.1 | Legionella pneumophila |
| T0_HS2_12 | 7 | 99.08 | Legionella taurinensis | DQ667196.1 | Legionella taurinensis | |
| Total no. | 2 | 30 | ||||
| 72 h after treatment | 72h_HS2_26 | 16 | 99.84 | Legionella taurinensis | DQ667196.1 | Legionella taurinensis |
| 72h_HS2_25 | 16 | 99.84 | Legionella pneumophila subsp. pneumophila | AE017354.1 | Legionella pneumophila subsp. pneumophila | |
| Total no. | 2 | 32 | ||||
| Second chemical treatment | ||||||
| Before treatment | T0_C2_h.04 | 2 | 97.11 | – | EU557015.1 | Uncultured bacterium |
| T0_C2_f.04 | 2 | 100 | Legionella pneumophila subsp. pneumophila | AE017354.1 | Legionella pneumophila subsp. pneumophila | |
| T0_C2_d.02 | 11 | 99.84 | Legionella anisa | AY744776.1 | Legionella anisa | |
| T0_C2_d.01 | 10 | 99.84 | Legionella pneumophila | EU054324.1 | Legionella pneumophila | |
| Total no. | 4 | 26 | ||||
| 72 h after treatment | 72h_C2_e04 | 19 | 99.84 | Legionella anisa | AY744776.1 | Legionella anisa |
| Total no. | 1 | 19 | ||||
For the second heat shock treatment, the same two phylotypes were found before and after treatment but in different proportions. Sequences were related to L. taurinensis (T0_HS2_12 [23% of the sequences] and 72h_HS2_26 [50% of the sequences]) and L. pneumophila (T0_HS2_14 [77% of the sequences] and 72h_HS2_25 [50% of the sequences]) before and after heat shock treatment, respectively.
In addition, a total of 45 16S rRNA sequences obtained from two DNA libraries were analyzed in order to study Legionella diversity in biofilm before (T0) and after (72 h) chemical treatment in order to evaluate a possible shift in Legionella diversity. The 45 sequences were distributed across four phylotypes on the basis of 99% similarity.
The results showed four phylotypes before the chemical treatment, related to L. anisa (T0_C2_d.02 [42% of the sequences]), L. pneumophila, (T0_C2_d.01 [38% of the sequences] and T0_C2_f.04 [8% of the sequences]), and an uncultured bacterium (T0_C2_h.04 [8% of the sequences]). A decrease of Legionella diversity was observed, and a single group related to L. anisa (72h_C2_e04) remained at 72 h after the treatment.
Pathogenic Legionella spp. such as L. pneumophila and L. anisa represented the major proportion of the total Legionella population and were highly represented before the heat shock and chemical treatments (Table 2).
Dynamics of the bacterial community.
The bacterial community structures in the biofilm obtained from the heat shock and chemical treatment tests throughout a period of 6 months were assessed and compared by SSCP fingerprint analysis in order to assess possible modifications of bacterial community structure after treatment.
For all samples of biofilm considered, before or after heat shock and chemical treatment, a positive response was obtained by PCR. Table 1 showed that all SSCP profiles displayed complex fingerprint patterns (between 9 and 20 peaks).
Bacterial SSCP fingerprints were also compared by principal-component analysis (PCA). In addition, bacterial SSCP fingerprints obtained as described in “Spatial variation of the biofilm composition throughout the biofilm box axis” above were added to this statistical analysis in order to take into consideration the possible error range that could be coming from the coupon position on the biofilm box axis (Fig. 2). The PCA plot of SSCP fingerprints showed the regrouping of the majority of the bacterial communities resulting from the treatment tests and the 10 coupons, which confirms our previous conclusion that the microbial populations are distributed in a homogenous way on the biofilm box axis.
Fig 2.
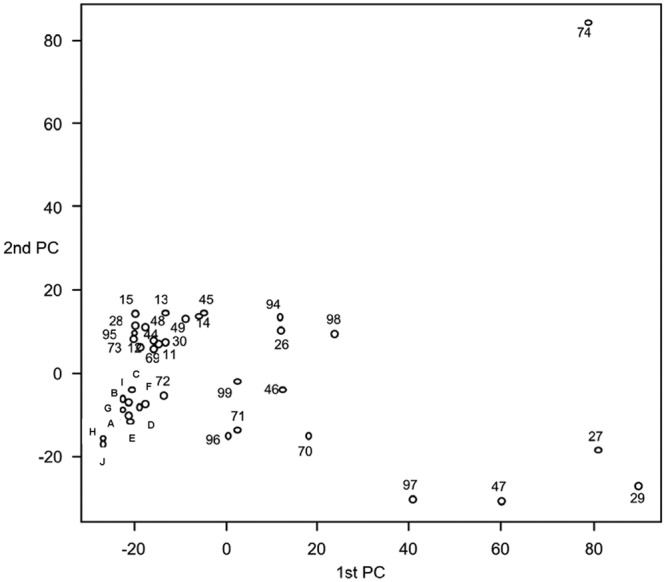
PCA representing the two principal-component axes of SSCP fingerprints obtained from (i) bacteria during the first and second heat shock treatment tests and the first, second, and third chemical treatment tests (codes 11 to 99) and (ii) bacteria sampled from the 10 coupons of the biofilm box before the treatments test period (A to J).
The results also showed no important variation in the bacterial community after treatments, as bacterial profiles remained in one large group except for a few points.
The first heat shock treatment had no effect on the bacterial diversity, as all bacterial profiles (samples 11 to 15) remained in the same group. For the second heat shock treatment, samples 26, 28, and 30 had the same bacterial diversity as the major profiles and were grouped with the main group. However, two profiles (samples 27 and 29, corresponding to the biofilm sampled at 24 h and 72 h, respectively) were located away from the major PCA plots.
For the chemical treatment, the major bacterial diversity at the different sampling times of the three chemical tests remained unchanged, and the profiles were distributed in the large main group. However, this treatment led, in some cases, to different profiles at 48 h (sample 47) after the first chemical treatment, 168 h (sample 74) after the second chemical treatment, and 48 h (sample 97) after the third chemical treatment.
Diversity of the eukaryotic population.
A total of 161 18S rRNA gene sequences obtained from six DNA libraries were analyzed in order to study the eukaryal diversity in the biofilm. In particular, the presence of amoebae was studied before and 72 h after the two heat shock treatments and the second chemical treatment (Table 3). These sequences were distributed across 10 phylotypes on the basis of 97% similarity.
Table 3.
Eukaryotic clones and distribution of operational taxonomic units before and 72 h after the two heat shock and the second chemical treatments.
| Test and time point | Operational taxonomic unit |
Organism with best-matching sequence |
||||
|---|---|---|---|---|---|---|
| Name | No. of clones | % Similarity | Affiliation | Accession no. | Name | |
| First heat shock treatment | ||||||
| Before treatment | T0_HS1_g.03 | 2 | 96.03 | Alveolata | AJ130869.1 | Unidentified eukaryote |
| T0_HS1_g.02 | 1 | 100 | Fungi, Vuilleminia comedens | AF518594.1 | Vuilleminia comedens | |
| T0_HS1_d.02 | 1 | 98.31 | Unidentified eukaryote | AY919786.1 | Uncultured freshwater eukaryote | |
| T0_HS1_d.01 | 2 | 92.90 | Amoebae, Thecamoeba sp. | EF455775.1 | Thecamoeba sp. | |
| T0_HS1_c.02 | 1 | 98.99 | Viridiplantae, Pinus luchuensis | DQ430719.1 | Uncultured eukaryote clone biogas | |
| T0_HS1_b.01 | 1 | 98.85 | Amoebae, Vannella sp. | AY929912.1 | Vannella sp. | |
| T0_HS1_a.02 | 21 | 95.75 | Alveolata | AJ130869.1 | Unidentified eukaryote | |
| Total no. | 7 | 27 | ||||
| 72 h after treatment | 72h_HS1_d.02 | 11 | 100 | Amoebae, Hartmannella vermiformis | AY680840.1 | Hartmannella vermiformis |
| 72h-HS1_a.02 | 14 | 99.67 | Amoebae, Hartmannella vermiformis | AY680840.1 | Hartmannella vermiformis | |
| Total no. | 2 | 25 | ||||
| Second heat shock treatment | ||||||
| Before treatment | T0_HS2_4 | 20 | 95.75 | Alveolata | AJ130869.1 | Unidentified eukaryote |
| T0_HS2_28 | 3 | 93.03 | Amoebae | EF455775.1 | Thecamoeba sp. | |
| T0_HS2_22 | 1 | 96.03 | Alveolata | AJ130869.1 | Unidentified eukaryote | |
| T0_HS2_21 | 1 | 98.92 | Unidentified eukaryote | AY919786.1 | Uncultured freshwater eukaryote | |
| Total no. | 4 | 25 | ||||
| 72 h after treatment | 72h_HS2_32 | 25 | 95.75 | Alveolata | AJ130869.1 | Unidentified eukaryote |
| 72h_HS2_19 | 2 | 96.03 | Alveolata | AJ130869.1 | Unidentified eukaryote | |
| Total no. | 2 | 27 | ||||
| Second chemical treatment | ||||||
| Before treatment | T0_C2_h.04 | 13 | 95.75 | Alveolata | AJ130869.1 | Unidentified eukaryote |
| T0_C2_g.03 | 1 | 100 | Fungi, Davidiella tassiana | EU343116.1 | Davidiella tassiana | |
| T0_C2_f.04 | 1 | 100 | Euglenozoa, Neobodo curvifilus | DQ207577.1 | Neobodo curvifilus | |
| T0_C2_e.01 | 1 | 95.84 | Alveolata, unidentified eukaryote | DQ104583.1 | Uncultured eukaryote | |
| T0_C2_d.04 | 2 | 98.92 | Unidentified eukaryote | AY919786.1 | Uncultured freshwater eukaryote | |
| T0_C2_c.03 | 4 | 96.03 | Alveolata | AJ130869.1 | Unidentified eukaryote | |
| T0_C2_b.03 | 6 | 95.36 | Alveolata | AJ130869.1 | Unidentified eukaryote | |
| T0_C2_b.01 | 1 | 93.03 | Amoebae, Thecamoeba sp. | EF455775.1 | Thecamoeba sp. | |
| T0_C2_a.03 | 1 | 99.83 | Amoebae, Hartmannella vermiformis | AY502961.1 | Hartmannella vermiformis | |
| Total no. | 9 | 30 | ||||
| 72 h after treatment | 72h_C2_g.01 | 6 | 100 | Euglenozoa, Neobodo curvifilus | DQ207577.1 | Neobodo curvifilus |
| 72h_C2_d.02 | 2 | 96.03 | Alveolata | AJ130869.1 | Unidentified eukaryote | |
| 72h_C2_d.01 | 3 | 100 | Euglenozoa, Bodonidae sp. | AY753624.1 | Bodonidae sp. | |
| 72h_C2_c.02 | 16 | 95.75 | Alveolata | AJ130869.1 | Unidentified eukaryote | |
| Total no. | 4 | 27 | ||||
Before the first heat shock treatment, the eukaryal library was dominated by sequences close to Alveolata (protozoa) that represent 78% of the sequences (T0_HS1_a.02). Sequences belonging to Amoebae were also detected: T0_HS1_g.02 and T0_HS1_b.01, which are close to Thecamoebae sp. and Vannella sp., respectively. After the first heat shock, sequencing results showed a decrease of the eukarya diversity, where two phylotypes close to Hartmanella vermiformis (72h_HS1_ld.02 and 72h_HS1_a.02) remained. However, after the second heat shock treatment, two eukaryal species disappeared (Thecamoeba sp. [T0_HS2_28] and an uncultured eukaryote [t0_HS2_21]), and two other species belonging to Alveolata remained after treatment (72h_HS2_32 and 72h_HS2_19).
The second chemical treatment reduced the initial eukaryal diversity to four phylotypes belonging to (i) two flagellates (22% of the sequences belonged to Neobodo curvifilus [72h_C2_g.01] and 11% of the sequences to Bodonidae sp. [72h_C2_d.01]) and (ii) two Alveolata (72h_C2_c.02 [59% of the sequences] and 72h_C2_d.02 [7% of the sequences]).
DISCUSSION
This work gives an overall description of the microbial dynamics in biofilm after heat shock and chemical treatment tests. Legionella and eukaryal diversities were assessed using molecular tools (16S rRNA cloning and sequencing). SSCP fingerprint analyses were also performed for bacteria.
A study by Parthuisot and coworkers in 2010 (42) examined how the diversity and the dynamics of Legionella species along a French river are affected by environmental and anthropogenic factors. This research concluded that dominant Legionella clusters identified by 16S rRNA gene sequencing were most closely related to uncultured bacteria. These findings highlight the importance of using molecular tools in order to better understand Legionella diversity and dynamics in our case. Therefore, the powerful molecular tools used in this project provided us a real picture of Legionella spp. and their biofilm microbial communities.
Few researchers have studied Legionella in hot water networks. Those studies were done by using real hot water networks or laboratory-scale equipment, yet none of them studied Legionella's biofilm in a pilot scale 1 system simulating a real hot water network and by using molecular techniques. Here, for the first time, Legionella diversity in hot water biofilm was assessed in situ by 16S rRNA sequencing.
To our knowledge, the only previous study examining Legionella diversity in hot aquatic biofilm was carried out at Yellowstone National Park with the same approach (52). Three different species belonging to Legionella group were found to be related to L. sainthelensi and L. micdadei, and one was related to a Legionella-like amoebal pathogen (LLAP). Our results showed that of the 56 cultured species of Legionella, four Legionella spp. were associated with the treated biofilm, i.e., L. drancourtii, L. anisa, L. pneumophila, and L. taurinensis, where L. taurinensis is the only nonpathogen species (32). The three other species are implicated in Legionnaires' diseases. According to our findings, biofilm in a hot water system is colonized by Legionella and is a major source of such species in water. Therefore, biofilm elimination in such an environment is a necessary step to effectively reduce the risk caused by Legionella. Other studies have examined Legionella diversity in water and have shown the presence of L. pneumophila and L. anisa in a hospital hot water system (60, 61) and the presence of L. fallonii, L. pneumophila, L. lytica, and several uncultured Legionella spp. in cooling tower water (70).
Heat shock was the first treatment applied. It is very often applied to disinfect hot water systems in hospitals and is recommended by the High Council for Public Health of France (CSHPF). The second treatment was a combination of a biodispersant and a biocide often applied in cooling towers. Legionella spp. were found in all biofilm samples before and after the two treatments, which means that Legionella spp. were not eliminated. On the other hand, treatment impacted Legionella diversity, where some modifications were observed. The number of phylotypes related to Legionella spp. decreased after the first heat shock and the chemical treatment (from four and three, respectively, to one phylotype in each case) and remained the same (two phylotypes) after the second heat shock treatment.
Two of the different resilient Legionella species that remained in biofilm after the second heat shock treatment (Legionella pneumophila) and the second chemical treatment (Legionella anisa) are the most frequent pathogen species of Legionella detected in water distribution systems and constitute an indicator of water contamination (60, 66, 67). Although Legionella pneumophila was not detected by sequencing after the first heat shock, our previous quantitative PCR data showed the presence of L. pneumophila at 1 × 104 genome equivalents (GE)/liter (17). In fact, molecular inventories show dominant species in the microbial ecosystems, so the disappearance of such species in our inventory may be due to their minor presence in the treated biofilm compared to other species. The second heat shock treatment appeared to be totally ineffective. Our previous quantitative data (GVPC counts and quantitative PCR counts) also showed that both total bacteria flora and total and cultivable Legionella spp. were detected at almost the same initial concentrations in the biofilm after treatment (17), which seems to show the rapid transient effect of the heat shock treatment. Indeed, it has been demonstrated that L. pneumophila colonizes biofilms in less than 2 h (13, 39).
Combining a biodispersant and a biocide also had a transient effect on Legionella species in biofilm as showed by our cloning and sequencing results. Although Legionella sp. diversity decreased after the chemical treatment, the resilient species L. anisa is a pathogen used as an indicator of water contamination by Legionella (67). Quantitative PCR counts results done by Farhat and coworkers in 2011 showed that the total Legionella sp. concentration decreased between one and two log units from 24 to 72 h after the biocide injection in the three treatment tests. Therefore, the results in this paper complete the data previously presented by Farhat et al. (16, 17). Our findings here are in perfect agreement with our previous results.
Different studies have evaluated Legionella treatments in water (37, 38, 57), and a few have evaluated treatment in biofilm (49). Most of the studies demonstrated a transient effect on Legionella, where the initial levels were quickly recovered (49, 62, 69).
Moreover, our current findings along with previously published studies have shown that Legionella spp. seem to resist and proliferate in spite of the different treatment procedures applied. In fact, Legionella spp. can withstand temperatures of 5.0°C to 63°C and a pH range of 5.0 to 9.2. Sheehan and colleagues (52) detected at least four Legionella spp. in an extremely acidic biofilm community. In addition, these bacteria have many strategies allowing their survival under different stress conditions, e.g., protection in biofilms (12, 39) or in phagosomes of amoebae and other eukarya (13, 25, 45) and motility. Consequently, understanding Legionella ecology in relation to biofilm communities is of primary importance for controlling Legionella risk.
We assessed the bacterial diversity before and after two treatments. Our SSCP fingerprint comparison by PCA analysis showed that the bacterial community was highly diverse in biofilm and that different bacterial profiles after each treatment were present in one main group, with a transitory effect observed after each treatment.
Based on the PCA plots, bacterial diversity profiles were close, and therefore no persistent change in the bacterial community structure was observed, which means that the initial biofilm was not removed after the two treatments. Murga and coworkers in 2001 (39) studied the role of biofilms in the survival of L. pneumophila in a potable water model system. They demonstrated that the presence of biofilms in potable and health care facility water systems could provide a means for L. pneumophila survival and dissemination. Therefore, in our case, the biofilm presence even after treatments seems to be one of the important reasons for the prevention of Legionella elimination.
Legionella protection against disinfection inside amoebae is well known (1). To our knowledge, this study is the first one to explore amoebae in hot water biofilm after anti-Legionella treatments. Therefore, our results were completed by 18S rRNA gene sequence analyses for the Eukarya domain. Although eukaryal diversity decreased after the heat shock treatment to two sequences close to Hartmannella vermiformis, this species is a thermotolerant amoeba isolated from hot water networks (61), and its protection of Legionella is well documented (7). However, after the second heat shock treatment, the two remained eukaryal species belonged to a group of ciliated protozoa (Alveolata) known as a host cell for Legionella (20, 56, 59), and the closest corresponding sequence in the database came from a continuous culture inoculated with Lake Ketelmeer water (The Netherlands). Indeed, Fields and colleagues in 1984 (20) showed the ingestion and survival of Legionella pneumophila phagosomes in Tetrahymena pyriphormis, a ciliated protozoan affiliated with the Alveolata. Subsequent studies have shown that Legionella may infect several species of these protozoa, and some of them are thermophilic (T. thermophila, T. vorax, and T. tropicalis) (18, 28, 54).
The euglenozoan species (Neobodo curvifilus and Bodonidae sp.) detected after our chemical treatment are two unicellular flagellates often identified in marine and freshwater sites (51). The heat shock and chemical treatment comparison revealed that Alveolata dominated all eukaryal species before treatments. These ciliated protozoa remained as dominant eukarya after the second heat shock treatment and the chemical treatment, unlike after the first heat shock. On the other hand, an unidentified eukaryal species detected before the heat shock treatment and the chemical treatment was eliminated after each treatment. Thus, we may ask here if a succession of heat shock treatments selects eukaryal species that could ensure protection of Legionella against treatment.
In conclusion, the aim of this work was to study how Legionella spp. and diverse bacteria and eukarya associated together in biofilm are impacted by various disinfection strategies. Our results showed that although Legionella diversity was reduced, pathogenic Legionella species (Legionella pneumophila and Legionella anisa) remained after the heat shock and chemical treatments, respectively. The biofilm was not removed, and the bacterial community structure was transitorily affected by the treatments. Moreover, several amoebae were detected in the biofilm before treatments (Thecamoebae sp., Vannella sp., and Hartmanella vermiformis). However, know Legionella eukaryotic hosts (Alveolata) dominated the eukaryal species after the second heat shock treatment and the chemical treatment.
Finally, we suggest that eradication of Legionella requires a better understanding of the ecology of bacterial and eukaryal species associated with Legionella-containing biofilms.
ACKNOWLEDGMENTS
This work was supported by the Industrial Research Training Convention (CIFRE).
We thank Nathalie Wéry and the laboratory of INRA/Narbonne for helpful discussion.
Footnotes
Published ahead of print 20 July 2012
REFERENCES
- 1. Adeleke AA. 1996. Legionella-like amebal pathogens—phylogenetic status and possible rôle in respiratory disease. Emerg. Infect. Dis. 2:225–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alleron L, Merlet N, Lacombe C, Frère J. 2008. Long-term survival of L. pneumophila in the viable but nonculturable state after monochloamine treatment. Curr. Microbiol. 57:497–502 [DOI] [PubMed] [Google Scholar]
- 3. Altschul SF, Gish W, Miller W, Miyers EW, Lipman DJ. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403–410 [DOI] [PubMed] [Google Scholar]
- 4. Amann RI, Ludwig W, Schleifer KH. 1995. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol. Rev. 59:143–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Atlas RM. 1999. Legionella: from environmental habitats to disease pathology, detection and control. Environ. Microbiol. 1:283–293 [DOI] [PubMed] [Google Scholar]
- 6. Borella P, et al. 2004. Legionella infection risk from domestic hot water. Emerg. Infect. Dis. 10:457–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brieland JK, et al. 1997. The role of Legionella pneumophila-infected Hartmanella vermiformis as an infectious particle in a murine model of Legionnaires' disease. Infect. Immun. 65:5330–5333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brown MRW, Barker J. 1999. Unexplored reservoirs of pathogens bacteria: protozoa and biofilms. Trends Microbiol. 7:46–50 [DOI] [PubMed] [Google Scholar]
- 9. Chang CW, Hwang YH, Cheng WY, Chang CP. 2007. Effects of chlorination and heat disinfection on long-term starved Legionella pneumophila in warm water. J. Appl. Microbiol. 102:1636–1644 [DOI] [PubMed] [Google Scholar]
- 10. Costerton JW. 1999. Introduction to biofilm. Int. J. Antimicrob. Agents 11:217–221 [DOI] [PubMed] [Google Scholar]
- 11. Critchley M, Bentham R. 2009. The efficacy of biocides and other chemical additives in cooling water systems in the control of amoebae. J. Appl. Microbiol. 106:784–789 [DOI] [PubMed] [Google Scholar]
- 12. Declerck P. 2010. Biofilms: the environmental playground of Legionella pneumophila. Environ. Microbiol. 2:557–566 [DOI] [PubMed] [Google Scholar]
- 13. Declerck P, et al. 2009. Replication of Legionella pneumophila in biofilms of water distribution pipes. Microbiol. Res. 164:593–603 [DOI] [PubMed] [Google Scholar]
- 14. Diederen BM. 2008. Legionella spp. and Legionnaires' disease. J. Infect. 56:1–12 [DOI] [PubMed] [Google Scholar]
- 15. Donlan RM. 2002. Biofilms: microbial life on surfaces. Emerg. Infect. Dis. 8:881–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Farhat M, et al. 2011. Chemical disinfection of Legionella in hot water systems biofilm: a pilot-scale 1 study. Water Sci. Technol. 64:708–714 [DOI] [PubMed] [Google Scholar]
- 17. Farhat M, et al. 2010. Development of a pilot-scale 1 for Legionella elimination in biofilm in hot water network: heat shock treatment evaluation. J. Appl. Microbiol. 108:1073–1082 [DOI] [PubMed] [Google Scholar]
- 18. Faulkner G, Berk SG, Garduno E, Ortiz-Jimenez MA, Garduno RA. 2008. Passage through Tetrahymena tropicalis triggers a rapid morphological differentiation in Legionella pneumophila. J. Bacteriol. 190:7728–7738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fields BS, Benson RF, Besser RE. 2002. Legionella and Legionnaires's disease: 25 years of investigation. Clin. Microbiol. Rev. 15:506–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fields BS, Shotts EB, Feeley JC, Gorman GW, Martin WT. 1984. Proliferation of Legionella pneumophila as an intracellular parasite of the ciliated protozoan Tetrahymena pyriformis. Appl. Environ. Microbiol. 47:467–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Flemming HC, Walker JT. 2002. Contamination potential of biofilms in water distribution systems. Water Sci. Technol. Water Supply 47:271–280 [Google Scholar]
- 22. Fux CA, Costerton JW, Stewart PS, Stoodley 2005. Survival strategies of infectious biofilms. Microbiology 13:34–40 [DOI] [PubMed] [Google Scholar]
- 23. Godon JJ, Zumstein E, Dabert P, Habouzit F, Moletta R. 1997. Molecular microbial diversity of an anaerobic digestor as determined by small-subunit rDNA sequence analysis. Appl. Environ. Microbiol. 63:2802–2813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gomez-Valero L, Rusniok C, Buchrieser C. 2009. Legionella pneumophila: population genetics, phylogeny and genomics. Infect. Gen. Evol. 9:727–739 [DOI] [PubMed] [Google Scholar]
- 25. Greub G, Raoult D. 2004. Microorganisms resistant to free-living amoebae. Clin. Microbiol. Rev. 17:413–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Reference deleted. [Google Scholar]
- 27. Reference deleted. [Google Scholar]
- 28. Kikuhara H, Ogawa M, Miyamoto H, Nikaido Y, Yoshida S. 1994. Intracellular multiplication of Legionella pneumophila in Tetrahymena thermophila. J. Uoeh. 16:263–275 [DOI] [PubMed] [Google Scholar]
- 29. Kilvington S, Price J. 1990. Survival of Legionella pneumophila within cysts of Acanthamoeba polyphaga following chlorine exposure. J. Appl. Bacteriol. 68:519–525 [DOI] [PubMed] [Google Scholar]
- 30. Kim BR, Anderson JE, Mueller SA, Gaines WA, Kendall AM. 2002. Efficacy of various disinfectants against Legionella in water systems. Water Res. 36:4433–4444 [DOI] [PubMed] [Google Scholar]
- 31. LeChevallier MW, Lowry CD, Lee RG. 1988. Inactivation of biofilm bacteria. Appl. Microbiol. 54:2492–2499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lo Presti F, et al. 1999. Legionella taurinensis sp. nov., a new species antigenically similar to Legionella spiritensis. Int. J. Syst. Bacteriol. 49:397–403 [DOI] [PubMed] [Google Scholar]
- 33. Reference deleted. [Google Scholar]
- 34. Maidak BL, et al. 2001. The RDP-II (Ribosomal Database Project). Nucleic Acids Res. 29:173–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Marrão G, Veríssimo A, Bowker RG, da Costa MS. 1993. Biofilms as major sources of Legionella spp. in hydrothermal areas and their dispersion into stream water. FEMS Microbiol. Ecol. 12:25–33 [Google Scholar]
- 36. Michelland RJ, Combes DS, Fortun-Lamothe L, Cauquil L. 2009. Statfingerprints: a friendly graphical interface program for processing and analysis of microbial fingerprint profiles. Mol. Ecol. Res. 9:1359–1363 [DOI] [PubMed] [Google Scholar]
- 37. Mietzener S, et al. 1997. Efficacy of thermal treatment and copper-silver ionization for controlling Legionella pneumophila in high-volume hot water plumbing systems in hospitals. Am. J. Infect. Control 25:452–457 [DOI] [PubMed] [Google Scholar]
- 38. Mouchtouri V, et al. 2007. Risk factors for contamination of hotel water distribution systems by Legionella species. Appl. Environ. Microbiol. 73:1489–1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Murga R, et al. 2001. Role of biofilms in the survival of Legionella pneumophila in a model potable-water system. Microbiology 147:3121–3126 [DOI] [PubMed] [Google Scholar]
- 40. Oberdorfer K, Müssigbrodt G, Wendt C. 2008. Genetic diversity of Legionella pneumophila in hospital water systems. Int. J. Hyg. Environ. Health 211:172–178 [DOI] [PubMed] [Google Scholar]
- 41. Pace NR. 1997. A molecular view of microbial diversity and the biosphere. Science 276:734–740 [DOI] [PubMed] [Google Scholar]
- 42. Parthuisot N, West NJ, Lebaron P, Baudart J. 2010. High diversity and abundance of Legionella spp. in a pristine river and impact of seasonal and anthropogenic effects. Appl. Environ. Microbiol. 76:8201–8210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pedro-Botet ML, Sabria M. 2005. Leginellosis. Semin. Respir. Crit. Care Med. 26:625–634 [DOI] [PubMed] [Google Scholar]
- 44. Peiro Callizo EF, Darpon Sierra B, Santos Pombo JM, Ezpeleta Baquedano C, Perez Huerta B. 2005. Evaluation of the effectiveness of the postormaster method for disinfection of Legionella in a hospital water distribution system. J. Hosp. Infect. 60:150–158 [DOI] [PubMed] [Google Scholar]
- 45. Philippe C, Blech MF, Hartemann P. 2006. Multiplication intra-amibienne de Legionella pneumophila et rôle potentiel des amibes dans la transmission de la légionellose. Méd. Mal. Infect. 36:196–200 [DOI] [PubMed] [Google Scholar]
- 46. Rogers J, Dowsett AB, Dennis PJ, Lee JV, Keevil CW. 1994. Influence of plumbing materials on biofilm formation and growth of Legionella pneumophila in potable water systems. Appl. Environ. Microbiol. 60:1842–1851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rowbotham TJ. 1980. Preliminary report on the pathogenicity of Legionella pneumophila for freshwater and soil amoebae. J. Clin. Pathol. 33:1179–1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Russell AD. 2003. Biocides use and antibiotic resistance: the relevanceof laboratory findings to clinical and environmental situations. Infect. Dis. 3:794–803 [DOI] [PubMed] [Google Scholar]
- 49. Saby S, Vidal A, Suty H. 2005. Resistance of Legionella to disinfection in hot water distribution systems. Water Sci. Technol. 52:15–28 [PubMed] [Google Scholar]
- 50. Sanderson SS, Stewart PS. 1997. Evidence of bacterial adaptation to monochloramine in Pseudomonas aeruginosa biofilms and evaluation of biocide action model. Biotechnol. Bioeng. 56:201–209 [DOI] [PubMed] [Google Scholar]
- 51. Scheckenbach F, Wylezich C, Mylnikov AP, Weitere M, Arndt H. 2006. Molecular comparisons of freshwater and marine isolates of the same morphospecies of heterotropic flagellates. Appl. Environ. Microbiol. 72:6638–6643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sheehan KB, Henson JM, Ferris MJ. 2005. Legionella species diversity in an acidic biofilm community in Yellowstone National Park. Appl. Environ. Microbiol. 71:507–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Shirtliff ME, Camper AK. 2002. Molecular interactions in biofilms. Chem. Biol. 9:859–871 [DOI] [PubMed] [Google Scholar]
- 54. Smith-Somerville HE, Huryn VB, Walker C, Winters AL. 1991. Survival of Legionella pneumophila in the cold-water ciliate Tetrahymena vorax. Appl. Environ. Microbiol. 57:2742–2749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Stahl DA, Flesher B, Mansfield HR, Montgomery L. 1988. Use of phylogenetically based hybridization probes for studies of ruminal microbial ecology. Appl. Environ. Microbiol. 54:1079–1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Steele TW, McLennan AM. 1996. Infection of Tetrahymena pyriformis by Legionella longbeachae and other Legionella species found in potting mixes. Appl. Environ. Microbiol. 62:1081–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Stout JE, Best MG, Yu VL. 1986. Susceptibility of members of the family Legionellaceae to thermal stress: implications for heat eradication methods in water distribution systems. Appl. Environ. Microbiol. 52:396–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sutherland IW. 2001. Biofilm exopolysaccharides: a strong and sticky framework. Microbiology 147:3–9 [DOI] [PubMed] [Google Scholar]
- 59. Taylor M, Ross K, Bentham R. 2009. Legionella, protozoa, and biofilms: interactions within complex microbial systems. Microb. Ecol. 58:538–547 [DOI] [PubMed] [Google Scholar]
- 60. Thacker WL, Benson RF, Hawes L, Mayberry WR, Brenner DJ. 1990. Characterization of a Legionella anisa strain isolated from a patient with pneumonia. J. Clin. Microbiol. 28:122–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Thomas V, Herrera-Rimann K, Blanc DS, Greub G. 2006. Biodiversity of amoebae and amoena-resisting bacteria in a hospital water network. Appl. Environ. Microbiol. 72:2428–2438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Thomas V, et al. 2004. Amoebae in domestic water systems: resistance to disinfection treatments and implication in Legionella persistence. J. Appl. Microbiol. 97:950–963 [DOI] [PubMed] [Google Scholar]
- 63. Tison DL, Seidler RJ. 1983. Legionella incidence and density in potable drinking water supplies. Appl. Environ. Microbiol. 45:337–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Turetgen I, Sungur EI, Cotuk A. 2005. Enumeration of Legionella pneumophila in cooling tower water systems. Environ. Monit. Assess. 100:53–58 [DOI] [PubMed] [Google Scholar]
- 65. Van der Kooij D, Veenendaal HR, Scheffer WJH. 2005. Biofilm formation and multiplication of Legionella in model warm water system with pipes of cooper, stainless steel and cross-linked polyethylene. Water Res. 39:2789–2798 [DOI] [PubMed] [Google Scholar]
- 66. Van der Mee-Marquet N, et al. 2006. Legionella anisa, a possible indicator of water contamination by Legionella pneumophila. J. Clin. Microbiol. 44:56–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Veronesi L, et al. 2007. Legionella contamination in the water system of hospital dental settings. Acta Biomed. 78:117–122 [PubMed] [Google Scholar]
- 68. Wadowsky RM, et al. 1991. Multiplication of Legionella spp. in tap water contaning Hartmanella vermiformis Appl. Environ. Microbiol. 57:1950–1955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wéry N, et al. 2008. Dynamics of Legionella spp. and bacterial populations during the proliferation of L. pneumophila in a cooling tower facility. Appl. Environ. Microbiol. 74:3030–3037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zumstein E, Moletta R, Godon JJ. 2000. Examination of two years of community dynamics in an anaerobic bioreactor using fluorescence polymerase chain reaction (PCR) single-strand conformation polymorphism analysis. Environ. Microbiol. 2:69–78 [DOI] [PubMed] [Google Scholar]


