Abstract
Indoor mold represents an important environmental concern, but a fundamental knowledge of fungal growth stages is needed to limit indoor fungal proliferation on finishing materials used in buildings. The present study focused on the succession of germination stages of the common indoor fungus Penicillium rubens on a gypsum substrate. This substrate is used as a model system representing porous materials that are widely used in indoor environments. Imaging with cryo-scanning electron microscopy showed that the formation of an extracellular matrix (ECM) is a phase of the isotropic growth of P. rubens that is uniquely related to germinating conidia. Furthermore, the ECM is observed only when a dry-state inoculation of the surface is applied, i.e., applying conidia directly from a 7-day-old colony, mimicking airborne contamination of the surface. When inoculation is done by spraying an aqueous conidial suspension, no ECM is observed. Moreover, it is concluded that the formation of an ECM requires active processes in the fungal cell. The porosity of the substrate proved that the ECM substance has high-viscosity characteristics. The present results stress that studies of indoor fungal growth should consider the method of inoculation, knowing that the common aqueous suspension may obscure specific stages in the initial phases of germination.
INTRODUCTION
Indoor mold and moisture and their associated health effects are widespread and important societal problems (11, 27). Without doubt, mold growth requires the presence of water. Water can occur in different forms and may originate from different sources in the indoor environment, i.e., as water vapor in the indoor air—air humidity—or in liquid form in the pore system of a material, originating from external sources such as rain, melting snow, rising dampness, or accidental leakage.
Detailed understanding of the fungal growth phases and the interaction of a substrate, water, and fungi provides information to identify the weakest link in fungal growth. Such knowledge lays the foundation for effective intervention or prevention strategies. The availability of water can be expressed in many ways. Common parameters are water activity (aw), which is widely used in microbiology to describe the water in substrates, and relative humidity (RH), which is typically used to describe water vapor in air. In equilibrium, the two quantities are directly related (26, 39).
Indoor fungi can be grouped according to their water tolerance. Magan and Lacey (38) distinguished hydrophilic (water-loving) and xerophilic (dry-loving) species on the basis of the minimum water activity required for growth. This scheme has been widely accepted and is based on a 0.9 aw or 90% RH threshold value.
It is known that aw or RH influences each of the main phases of the growth cycle. In studies of Aspergillus and Penicillium species (1, 7, 41) on culture media, the minimum value of the aw for germination was lower than that for linear growth, which in turn was usually lower than that for asexual sporulation.
Many studies have been carried out to investigate the susceptibility to indoor molds of construction and finishing materials, including paints, plasters, and wallpapers. The focus of such studies is merely the prevalence of fungal propagules or colony formation, and interpretation of the results on the basis of fungal physiology is generally lacking. These studies led to the conclusion that virtually all indoor molds have optimum conditions for growth in an RH range of 90 to 100%, and there is a consensus in the literature that porous materials are affected by mold growth if the RH is maintained below 80% (4).
Numerous studies have described fungal growth on building materials, wood in particular, as related to temperature, RH, and nutrients (45, 46, 52, 53). The subject has been extensively reviewed (see, for example, the papers by Nielsen et al. [44] and Vereecken and Roels [51]), but only sparse information is available about water in relation to the separate growth stages.
In general, such information is obtained from experiments with artificial growth media such as agar and with conidia that are prepared in standardized spore suspensions (18). However, these experimental approaches may not mimic common indoor situations. First, most finishing and construction materials in the indoor environment are porous. This porosity not only introduces a reservoir for water storage but also exerts capillary forces on liquids. Consequently, in contrast to most artificial culture media, porous material competes with the organism for water as long as the material is unsaturated.
Second, the conditions under which indoor fungi in vivo are dispersed into the air and subsequently land on a substrate will mostly differ from the conditions that are routinely employed in laboratory tests, i.e., with conidial suspensions as the starting point. Airborne spores in vivo have a relatively low water content and a high content of energy reserves, such as lipids, glycogen, or trehalose (19). To obtain a realistic picture of indoor fungal growth, studies should use similar starting points, i.e., porous substrates and spores that have not been in contact with water prior to landing on that substrate.
Only Adan (2) previously studied the occurrence of different developmental phases of fungal growth on building material in more detail by using cryofixed samples and scanning electron microscopy (SEM). He reported conglomerates of amorphous structures on incubated conidia and speculated that they consisted of aqueous residuals containing some hydrophilic exudates.
Observations of amorphous structures on fungal spores are not new; conglomerates have been reported and described as an extracellular matrix (ECM) (37) or spore tip mucilage (STM) (33). In 1980, Nicholson and Moraes (42) investigated the importance of the spore matrix for the survival of spores. The methods used to visualize extracellular materials have been improved over time (23). However, the importance and formation of an ECM by indoor fungi on porous materials have not been investigated yet and its precise function remains unknown.
Because germination is the starting point of the life cycle, the present study concentrated on the initial stages of germination, more specifically, in relation to the role of liquid water during this stage. Gypsum was chosen as a well-defined porous system that is widely used in the construction environment because of properties such as fire resistance and the relative ease of assembly and refinishing within the structure (36). The fungus selected, Penicillium rubens, is one of the most common indoor fungi found in the flora on building materials (5, 48). The purposes of this study were to obtain fundamental insight into the succession of initial germination stages of P. rubens on a porous substrate and to clarify the properties and functions of the ECM in relation to water and germination.
MATERIALS AND METHODS
Fungal cultures.
Conidial suspensions of P. rubens were kept at −80°C and used for inoculation of malt extract agar (MEA) surfaces (Oxoid). One week prior to each experiment, cultures were grown at 21°C in the dark.
P. rubens (strain CBS 401.92; CBS Fungal Biodiversity Centre, Utrecht, The Netherlands) was isolated from a large-scale survey of the fungal flora on affected indoor surfaces in social housing in The Netherlands in 1991, in which it appeared to be a predominant species (2). In many other studies (5, 28, 47, 48, 49), P. rubens is reported to be a common indoor species in both surface and air sampling. The strain used in this study was formerly known as P. chrysogenum (34) and was reclassified in 2011.
Gypsum sample preparation.
Gypsum samples (diameter, 9 mm; height, 3 mm) supplemented with nutrients were produced according to the following method: gypsum [calcium sulfate hemihydrate, (CaSO4)2 · H2O; Acros] was mixed with a Czapek solution at a mass ratio of 3:2. For the solution, Czapek-Dox broth (8.76 g; Sigma) was dissolved in 1 liter of demineralized water (DW); prior to autoclaving, 1 ml of a trace metal solution containing ZnSO4 · 7H2O (0.01 g liter−1; Sigma-Aldrich) and CuSO4 · 5 H2O (0.005 g liter−1; Acros) was added. Gypsum samples were dried for at least 48 h at room temperature in the presence of silica grain to complete the hydration reaction.
For gypsum without nutrients, sterile DW (SDW) was used instead of a Czapek solution at the same mass-to-mass ratio of 2:3. Furthermore, to interfere with the metabolism of P. rubens, specific gypsum samples containing sodium azide were prepared. In this case, sodium azide (NaN3, 50 μM; Sigma) was added as a component of the Czapek solution after the Czapek solution was autoclaved and cooled down to room temperature.
Inoculation and incubation.
To mimic airborne fungal contamination, inoculation of the samples was done with conidia directly from an actively growing colony; these are referred to here as “dry conidia.” Dry conidia were inoculated by gently pressing the gypsum sample onto a 7- to 9-day-old fully grown colony. The hydrophobic nature of the conidia ensured that no fluid was delivered to the gypsum during inoculation.
However, standard laboratory tests are generally performed with spores prepared in a suspension; therefore a conidial suspension was applied by spraying (using a Preval sprayer; Chicago Aerosol) as a reference. Suspensions were prepared by flooding a 7- to 9-day-old colony with SDW supplemented with 0.05% Tween 80. The suspension was filtered through glass wool, subsequently centrifuged three times (1,800 × g) for 5 min, and resuspended in SDW without Tween. The conidial concentration was determined using a hemocytometer, and the concentration was adjusted to 4 × 106 conidia ml−1. The suspension was either used directly for application or stored on ice water and applied within 3 h after preparation.
Both the dry-inoculated and suspension-inoculated samples were incubated in polystyrene boxes in the dark at 21°C. A saturated salt solution of K2SO4 was used to create a constant RH of 97% inside the boxes (30). Such a saturated salt solution creates a stable air humidity that is insensitive to temperature fluctuations.
The effect of transient humidity conditions was investigated in experiments wherein incubation took place for 10 to 14 h at 97% RH until the ECM had formed; samples were then transferred to a polystyrene box filled with a saturated salt solution of MgCl2 to create a constant RH of 33%.
To check whether the formation of the ECM may be an artifact introduced by the experimental procedure, i.e., originating from either the gypsum or the culture medium, specific noninoculated samples were incubated under the same conditions as in the experimental setup. First, the cryofixation procedure was applied to dry gypsum samples and samples that were moistened initially with the same amount of SDW not containing conidia, with and without the addition of 0.05% Tween 80. Second, to check whether residues of the culture medium may induce ECM-like structures, the gypsum sample was gently pressed onto MEA and incubated.
Cryo-SEM.
To study conidial germination and growth, cryo-SEM was used. The majority of the cryo-SEM observations were 5-fold, but all observations were made at least in duplicate. The interval between observations was 2 h during the first 26 h; thereafter, the interval was 24 h. Cryo-SEM was chosen because nonchemical cryofixation enables imaging of conidia in a close-to-natural state. The gypsum samples were prepared in a template that was adapted to the size of the cryosample holder (diameter, 9 mm; height, 3 mm), with the result that the samples would stay in the sample holder without the requirement of any glue.
Incubated gypsum samples were frozen instantaneously by immersion in liquid nitrogen slush (cryofixation at −210°C) and imaged in the frozen state (50). Frozen samples were mounted on a cryostage and placed inside the cryo-SEM apparatus under vacuum. Sublimation (heating to −85°C) was used to remove ice from the sample surface. Subsequently, the sample was flushed three times with argon gas and then sputter coated twice with a gold target for 30 s. Finally, the sample was retransferred onto the cryostage and imaged at −152°C, in a JEOL JSM-5600LV (equipped with an Oxford CT1500 cryostation) using an accelerating voltage of 5 kV.
HPLC sugar detection.
To compare the metabolic state of conidia in suspension with conidia in a dry state, high-performance liquid chromatography (HPLC) was used for the analysis of intracellular sugar content. According to the protocol of Dijksterhuis et al. (22), a conidial suspension was prepared in SDW and its concentration was determined using a hemocytometer. The concentration of the suspension was adjusted to 2 × 108 conidia·ml−1, and it was incubated in an Erlenmeyer flask in a benchtop incubator (150 rpm) at 30°C. Samples were taken at 0 and 210 min. To draw a parallel with metabolically active conidia, a similar suspension was prepared in malt extract broth (MEB) and incubated under the same conditions. To check whether the ECM was dissolved initially during the suspension of conidia in DW and therefore would not be observed, the sugar content of the supernatant of the conidial suspension was determined both directly after suspension and 3.5 h after incubation.
For analysis of the intracellular sugar content of the conidia (incubated in either SDW or MEB), conidia were broken in a Qiagen TissueLyser. To do this, 10 ml of each suspension was centrifuged for 5 min at 1,512 × g and 4°C. The pellet was washed in 1 ml ice-cold SDW and centrifuged again for 3 min at 20,817 × g and 4°C. All samples were stored at −80°C until further preparation. For further preparation, samples were removed from the −80°C storage and conidia were cryoground in a Qiagen TissueLyser for 3 min at 30 strokes·s−1. Subsequently, 500 μl ultrapure water was added and the samples were ground again for 3 min at 30 strokes s−1. The supernatant fraction was transferred to a microcentrifuge tube and centrifuged for 30 min at 20,817 × g and 4°C. After 30 min of heating at 95°C, the supernatant was again centrifuged for 30 min at 20,817 × g and 4°C. Finally, the supernatant was filtered through a syringe filter (polytetrafluoroethylene [PTFE]; diameter, 13 mm; pore size, 0.2 μm; Acrodisc).
For analysis of the supernatant, 500 μl was centrifuged for 3 min at 20,817 × g and 4°C. This was subsequently filtered through a syringe filter (PTFE; diameter, 13 mm; pore size, 0.2 μm; Acrodisc).
HPLC was used to detect and quantify the sugar content. All samples were taken in duplicate. A 10-μl volume of each sample was injected by a Waters 717 plus autosampler. Sugars were separated on a Waters Sugar Pack-I column at 65°C and detected using a refraction index detector (Waters 241 RI). The eluent was 0.1 mM Ca-EDTA. A Waters 515 HPLC pump and control module (Waters pump control module II) maintained a flow rate of 0.5 ml·min−1. Retention times were compared to standards, at concentrations ranging from 0.01 to 0.5% (wt/vol), of the carbohydrates trehalose, glucose, erythritol, glycerol, mannitol, and arabitol. Peak integration and calculation were performed with Empower software (Waters).
RESULTS
Visualization of conidial germination.
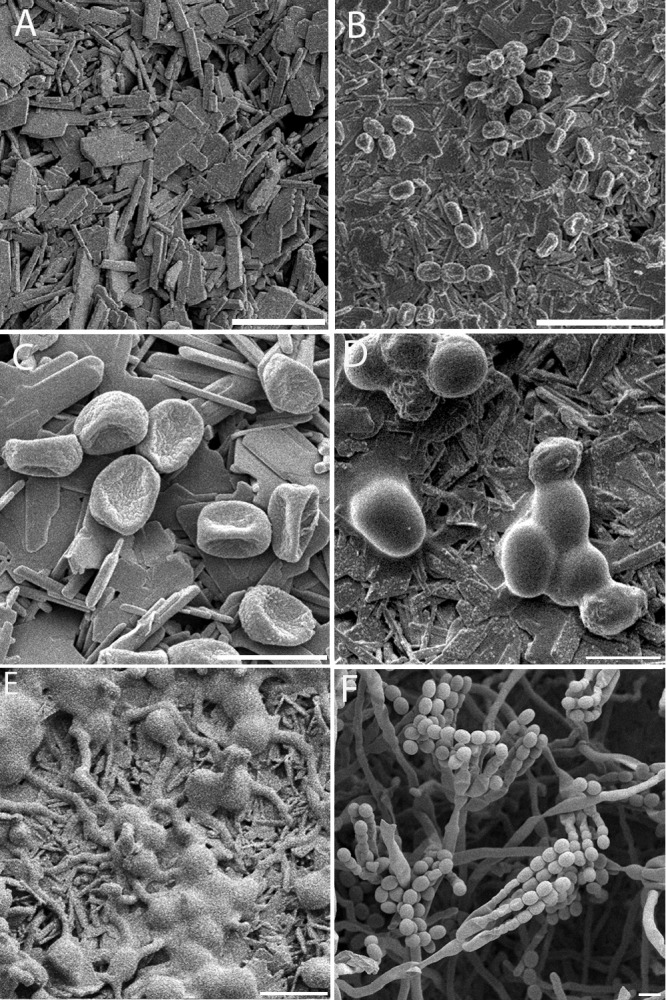
To visualize the conidial germination phases of P. rubens on gypsum in detail, time studies with cryo-SEM were carried out. An overview of all of the results obtained is shown in Fig. 1. Figure 2A shows the typical gypsum surface structure, consisting of needle-shaped crystals, which are distinct from conidial and ECM shapes, which appear smooth and round (Fig. 2E).
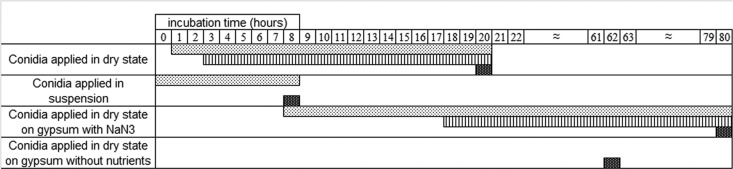
Fig 1.
Overview of early phases of the germination of P. rubens on gypsum with different treatments. Filled areas indicate that a specific germination phase is observed. Germination was taken as the endpoint and was defined as the point when at least 50% of the conidia had a visible germ tube with a length at least equal to the diameter of the conidium. Gray area, rehydrated conidia. Vertical stripes, ECM appearance. Dark dotted area, germination.
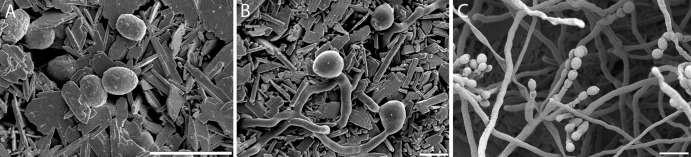
Fig 2.
Early stages in the germination of P. rubens. Conidia were applied in a dry state to gypsum with additional nutrients and incubated at 97% RH and 21°C. (A) Control: gypsum with additional nutrients inoculated with SDW. No alterations of the gypsum surface are visible after 55 h of incubation. The gypsum substrate is visible as sharp and square crystals; this is distinct from panel E, where the conidia and ECM appear smooth and round. (B) After inoculation, conidia appear dehydrated on the surface (t = 5 min). (C) Conidia remain dehydrated on the surface (t = 1 h). (D) ECM is visible on the conidia, and conidia attain an ellipsoidal shape (t = 7 h). (E) Germination tubes are formed, ECM increases with time, and ECM-covered conidia are clustered (t = 26 h). (F) Good germination and development to conidiophores, abundant aerial growth, and conidium formation are visible on the gypsum samples (t = 97 h). Scale bars: 5 μm (C, D, E, F), 10 μm (A), and 20 μm (B). The pictures shown represent specific growth phases and were not necessarily taken at the onset of the growth phase. For the onset, see Fig. 1.
When these stages were examined in detail (Fig. 1), conidia applied in a dry state to the gypsum surface appeared dehydrated, showing collapsed cell walls (Fig. 2C and 3A). After about 1 h of incubation, the conidia started to rehydrate. Hydration of conidia continued over time, and more conidia resumed their original ellipsoidal shape. After approximately 3 h, prior to germ tube formation, some cells started to exhibit an amorphous ECM as small patches on the conidial wall (Fig. 3B). Over time, the amount of ECM on the conidia increased and started to partly cover the adjacent gypsum surface (Fig. 3C). In many cases, the ECM masked the boundaries between neighboring conidia (Fig. 2E and 3B and C). Twenty hours after inoculation, germination tubes started to emerge and the ECM persisted on and around the conidia. After prolonged growth, conidia still remained covered with a substantial amount of ECM, whereas hyphae were not so densely covered (Fig. 2E). After 67 h, ECM, substrate hyphae, and aerial hyphae were prevalent. Conidiophores appeared after 92 h (Fig. 2F).
Fig 3.
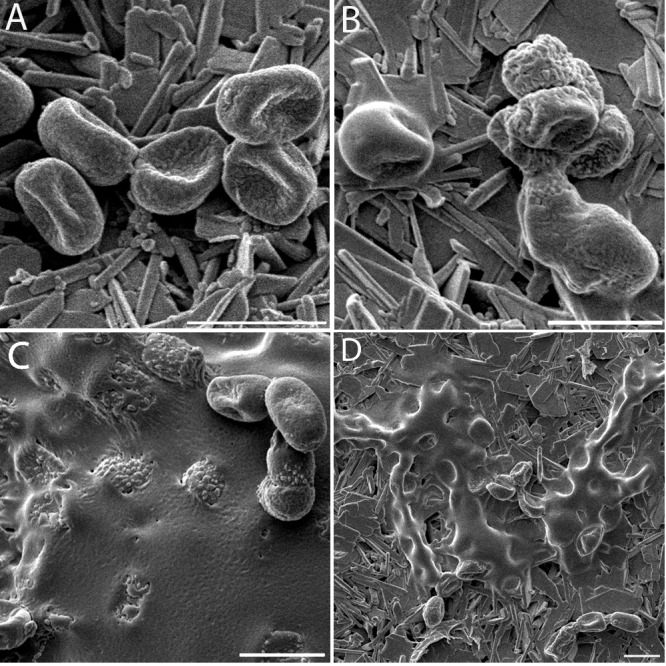
Appearance and increase of the ECM of P. rubens. Conidia in a dry state were applied to gypsum with additional nutrients and incubated at 97% RH and 21°C. (A) At t = 1 h after inoculation, dehydrated conidia on the gypsum surface. (B) ECM appears on the conidia (t = 5 h). (C) ECM increases with time and covers the conidia and the gypsum surface (t = 26 h). (D) Sample incubated for 14 h at 97% RH and subsequently placed for 29 h at 33% RH. The ECM is robust upon drying, and collapsed conidia underneath are visible (t = 43 h). Scale bars, 5 μm. The pictures shown represent specific growth phases and were not necessarily taken at the onset of the growth phase. For the onset, see Fig. 1.
The time of ECM appearance proved to be reproducible in all of the experiments (Fig. 1). Conidia applied in a dry state germinated after approximately 20 h, whereas the ECM started to appear after about 3 h of incubation. The appearance of an ECM was uniquely related to the conidia. Once present on the surface, an ECM volume increase was observed upon exposure to a high RH (Fig. 3).
Effect of an RH transition on conidia and ECM.
The effect of transient humidity conditions on both the fungal structure and the ECM was investigated in experiments wherein incubation took place for 10 to 14 h at 97% RH until an ECM was formed, followed by 28 to 30 h of exposure to 33% RH. Observations showed (Fig. 3D) that as a consequence of drying at a low RH, conidia within the ECM and conidia not covered by the ECM collapsed while the ECM remained present.
Effect of the inoculation method on the ECM.
To investigate the effect of the inoculation method, dry inoculation of conidia directly from a colony was compared with inoculation with aerosolized conidial suspensions (Fig. 1). All samples were incubated at 97% RH and 21°C and monitored by cryo-SEM.
Figure 4A shows that directly after spraying of the suspension, conidia appeared fully hydrated on the surface. These conidia started to form germ tubes after 8 h (Fig. 4B), and the onset of conidium formation occurred after 68 h (Fig. 4C). The presence of surfactant (Tween 80) had no influence on their appearance or on any stage of fungal development. Figure 1 clearly shows that conidia applied in a suspension germinate faster than conidia applied in a dry state and that the ECM is absent from samples that have been inoculated with a suspension.
Fig 4.
Germination of P. rubens conidia applied in a suspension to gypsum with additional nutrients and incubated at 97% RH and 21°C. (A) After inoculation, conidia appear hydrated on the surface (t = 1 h). (B) Elongated germination tubes; no ECM is formed (t = 22 h). (C) Fully developed conidiophores (t = 69 h). Scale bars, 10 μm (A, C) and 5 μm (B). The pictures shown represent specific growth phases and were not necessarily taken at the onset of the growth phase. For the onset, see Fig. 1.
Effects of nutrients and sodium azide on ECM.
To determine whether conidia actively produce an ECM, the gypsum substrate was modified in order to interfere with metabolism. Two types of modified gypsum were used. The first was “standard” gypsum with additional nutrients plus sodium azide (NaN3). Sodium azide is a respiration blocker. It inhibits cytochrome oxidase in the cell through irreversible binding to the heme cofactor. The second was gypsum without nutrients to test whether high humidity alone is sufficient to obtain an ECM or whether an external energy source is required.
We observed that gypsum supplemented with NaN3 delayed the germination of dry-inoculated conidia to approximately 80 h. Moreover, the appearance of an ECM was delayed to 18 h, as shown in Fig. 1.
On gypsum without nutrients, no ECM was produced. Only a small number of germination tubes were formed after approximately 62 h; they were abnormally long and unbranched (results not shown). The overwhelming majority of the conidia on gypsum without nutrients appeared collapsed and unchanged. This collapsed appearance remained for more than 40 days at 97% RH, and no ECM was observed.
Effect of suspending conidia on sugar metabolism.
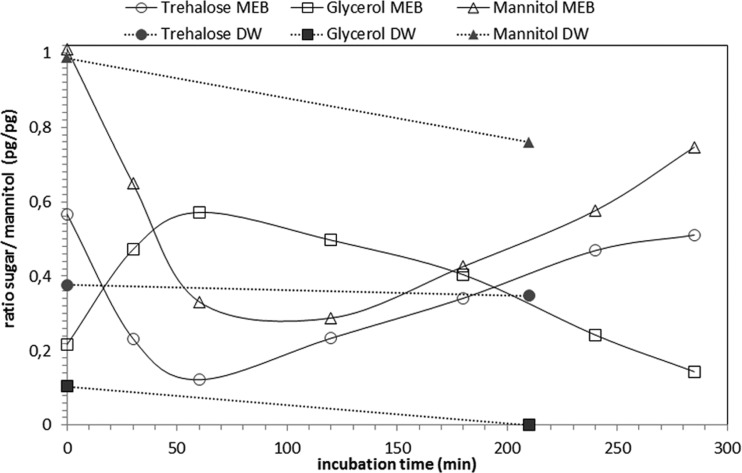
The presence of DW is a major difference between the experiments with different inoculation methods; i.e., conidia directly from a colony, and aerosolized conidia from an aqueous suspension. It is assumed that dry conidia have inactive sugar metabolism at the moment of inoculation. To evaluate the metabolic status of conidia in suspension, the intracellular sugar content of conidia suspended in DW, as well as in in MEB, was analyzed. With HPLC analyses, the initial ratio (i.e., at t = 0) between the measured sugar and mannitol was calculated. Any change in this ratio was considered an indicator of metabolic activity. As mannitol is the main component of the dormant cell, it was used to normalize the data. The results are summarized in Fig. 5.
Fig 5.
Results of HPLC analyses of intracellular sugar content during early germination of P. rubens incubated in MEB (open symbols connected by a solid line) or in DW (solid symbols connected by a dashed line) at 30°C. The change in the sugar-to-mannitol ratio measured at t = 0 was used as an indicator of metabolic activity. The amount of mannitol at t = 0 was taken to normalize the data because this was the principal sugar present in dormant cells. A change in the intracellular sugar content ratio was observed within 30 min of incubation in MEB, whereas no alterations were observed in conidia after 210 min of incubation in DW.
Detection of internal sugar reserves of conidia that were incubated in DW revealed the presence of both trehalose and mannitol. After 210 min, no change in this ratio was determined. In order to compare this with metabolically active conidia, conidia suspended in MEB were also analyzed. Within 30 min, a distinct change in the ratio of internal sugars was observed.
In addition, the sugar content of the supernatant, excluding conidia, was analyzed directly after suspension and again after 210 min of incubation. No sugar was detected in the supernatant at any sample time (Fig. 5).
DISCUSSION
The ECM is a phase of isotropic growth.
In general, spore germination can be described in the following phases. (i) The spore is activated in response to appropriate environmental conditions, i.e., water and a carbon source. (ii) Subsequently, water is taken up and the spore swells, resulting in a morphological change. This is also referred to as isotropic growth, and metabolic activities such as respiration are resumed. Such isotropic growth is usually characterized by a large increase in spore volume. (iii) Growth then becomes polarized and results in the formation of a germ tube (31).
In the case of P. rubens, the formation of a substantial amount of ECM is uniquely related to conidia in a pregermination phase. In addition, this ECM is observed only when conidia are inoculated in a dry state. Different experiments to screen for artifacts introduced by the gypsum used, i.e., gypsum inoculated with SDW, gypsum with MEA gently pressed onto it, and uninoculated gypsum, proved to be negative. All of these experiments showed no alteration in the gypsum surface after incubation at 97% RH (Fig. 2A). Moreover, conidia that were inoculated onto gypsum without additional nutrients did not form an ECM.
The substantial coverage with extracellular material that is observed in conidia was not seen on germ tubes and hyphae in the experiments performed here. Actually, it is known that fungal hyphae may have some type of extracellular material on their surfaces, which is believed to be attributable to adherence to the substrate (25). For most plant pathogens, the ECM is generally assumed to function in the process of adhesion to, and infection of, the host (11, 21, 24). However, the amount of ECM observed in our experiments is many times larger and, moreover, is dependent on the experimental conditions. Thus, its function is believed to be wider than adhesion only, such as the creation of favorable conditions for the fungus by prevention of dehydration.
No previous descriptions of ECM formation by P. rubens in the literature are known. Other fungi that produce an ECM or similar substances, which is also referred to as STM, are generally related to plant or nematode pathogens, including Magnaporthe grisea (33), Blumeria graminis (13, 43), Pestalotia malicola (29), Cochliobolus heterostrophus (9), Uromyces viciae-fabae (15, 21), Botrytis cinerea (16), Colletotrichum graminicola (42, 43), Mycosphaerella graminicola (24), Bipolaris sorokiniana (6), and Drechmeria coniospora (35).
In most studies, formation of the ECM (or STM) starts before spores have germinated (6, 10, 13, 40). The composition of such an extracellular substance appears to be species specific (8, 37). Key components of the ECM mentioned in the literature are proteins (6, 8, 10, 40, 52), polysaccharides (6, 8, 10, 14, 40), or glycoprotein (10).
Our experiments revealed that P. rubens can form germination tubes on gypsum without nutrients. However, germination is poor and the majority of the conidia remain collapsed, even after 40 days at 97% RH. Analysis of water vapor sorption in gypsum (2) shows that the moisture content of gypsum with or without nutrients is approximately 0.06 m3 m−3 (volume of moisture per volume of gypsum) when it is incubated at 97% RH and that equilibration is nearly instantaneous. Therefore, as all of our samples were placed at 97% RH immediately after inoculation, the difference in the germination and development of P. rubens on gypsum without nutrients cannot be due to a moisture content deviation resulting from slow equilibration.
Results of experiments with gypsum containing the respiration blocker sodium azide (NaN3) and gypsum without additional nutrients indicate that active cell processes are involved in ECM formation. In particular at the onset of germination, energy is a limiting factor. Consequently, the formation of an ECM may have a special function in the isotropic growth phase. The requirement of active cell processes implies that the ECM is either produced inside the conidia and transported out or assembled externally, for example, with the aid of enzymes that are transported from inside (32). Such an active process is known for ascospores of Talaromyces macrosporus, in which, during early germination, trehalose reserves are broken down into glucose that is released from the cell (22).
Comparing the wet and dry inoculation methods, i.e., aerosolized conidial suspensions and direct inoculation from a colony, respectively, revealed marked differences in observations of the conidial state and ECM formation (Fig. 1). First, in accordance with earlier observations of Griffin (31) and Deising et al. (21), the dry-inoculation method showed dehydrated conidia on the surface. These rehydrated, however, after exposure to water vapor. The initial dehydration is not observed, as expected, in the case of the conidial suspension. Second, results presented in this paper show that conidia germinate faster when applied in a suspension. Third, conidia applied in the dry state formed substantial amounts of ECM, in contrast to conidia that originate from the aqueous suspension and which do not have such ECM formation. These observations are in agreement with observations of Apoga and Jansson (6), who reported that the ECM of B. sorokiniana is present when dry-inoculated conidia are used and that no ECM is observed when conidia are inoculated using a drop of water.
Inoculation in a dry state differs from inoculation in a wet state (through spraying of a suspension) not only in terms of ECM appearance but also in terms of germination lag time. The differences in the appearance of an ECM might be related to dissolving effects of the ECM during suspension, but differences in initial metabolic activity may also play a role. Nevertheless, the exact mechanism remains unknown. Both dry conidia and conidia in suspension should be metabolically active in order to start and continue germination. The HPLC results show internal sugar reserves in conidia in the forms of trehalose and mannitol, but over time, no alteration of the sugar content was measured during incubation in DW. This implies that the sugar metabolism of conidia of P. rubens suspended in DW is inactive. As a consequence, ECM production in suspension is not possible. In the supernatant, excluding conidia, no sugars were detected on the basis of HPLC analysis. This indicates that no ECM was dissolved from the surface during the suspension of conidia in DW and that neither dry nor wet conidia had started their sugar metabolism at the moment of inoculation.
The present observations indicate the potential impact of the conditions of conidia prior to landing on a substrate on the developmental stages. It may be that specific germination stages remain unnoticed because of the method of inoculation, which may have consequences for the fundamental understanding of fungal development, including morphology and physiology.
The possible practical consequences are 2-fold. (i) The initial state of aerial conidia in the indoor environment is affected by the indoor climate conditions and determines the chances of germination and subsequent growth. A more thorough knowledge of this relationship may provide insight into control practices to reduce the risk of indoor mold growth. (ii) Building material characteristics play a crucial role in the risk of mold growth and therefore are assessed on the basis of accelerated tests under laboratory conditions. As mimicking of indoor environments should form a starting point in such tests, the method of inoculation seems especially important. However, at present, standard tests (3, 18) are based mostly on the application of fungal suspensions.
ECM characteristics.
The experimental design using a porous substrate introduces the option of deducing some ECM features. It is remarkable that the ECM is not quickly absorbed into the surface. The observations show that the ECM remains on the surface and increases in volume with time. Moreover, conidia that were covered with an ECM during 97% RH incubation and subsequently exposed to a low (i.e., 33%) RH showed that the ECM is resistant to drying (Fig. 3D) and thus behaves as a robust cover or shield.
The gypsum samples are hydrophilic in nature and consist of needle- and plate-shaped crystals (Fig. 2A) that form narrow interlocking edges that exert capillary force on liquids. The typical time it would take for a liquid to be absorbed into gypsum by capillary action is calculated with the following equation (for clarification of the background of this equation, see the supplemental material): t = V2 × [2η/(γ × cosθ × π2 × r5)], where t (in seconds) is the time until the liquid volume (V, in cubic meters) is fully absorbed, η (in pascal seconds) is the viscosity, γ (in pascals per meter) is the liquid-air surface tension, θ is the contact angle of the gypsum with the liquid, and r (in meters) is the radius of a typical pore of the gypsum. This equation shows that if the volume of the liquid increases or if the viscosity is increased, the absorption time is increased. If the surface tension is decreased, then the absorption time increases.
With this equation, we examined whether it is possible that the ECM consists of pure water. For the volume (V), we chose 10−15 m3, which corresponds to a droplet with a diameter of about 10 μm, which is twice the size of a conidium. Furthermore, the viscosity of water (η) is 1 × 10−3 Pa s, the air-water surface tension of water (γ) is 0.07 Pa m−1, and because gypsum is hydrophilic, the contact angle (θ) is 0°. As a typical gypsum pore radius (r), we chose 1 μm. Using these values as a starting point, a volume of 10−15 m3 of water would be fully absorbed in a period of time on the order of 3 ms. Considering this equation, there are three options to retain the ECM at the gypsum surface: (i) the ECM has a high viscosity, (ii) the ECM has a low surface tension, or (iii) the conidium is capable of modifying the contact angle at the gypsum substrate.
The present cryo-SEM observations show that the ECM remains on the surface over longer time periods and even increases with time, suggesting that the ECM is highly viscous. It is known that surface-active proteins, called hydrophobins, may decrease the surface tension of spores (54). For example, Cox et al. (17) reported that the surface tension of Trichoderma reesei can be reduced to 0.03 Pa m−1. Using this value for γ in our equation, the absorption time would be on the order of 6 ms, implying that hydrophobins alone cannot cause the droplet to remain on the surface on the time scale considered. The last option, modifying the contact angle, is very unlikely, as this implies that the fungus turns the surface hydrophobic before contacting it.
These estimations of the order of magnitude of the parameter effects prove that the observed ECM does not consist of pure water and that hydrophobins alone cannot explain the observed phenomenon of ECM retention. The experimental observations indicate a huge increase in absorption time (t), which clearly supports a high viscosity (η) of the observed ECM. This is in agreement with suggestions in the literature about the ECM, namely, that polysaccharides and related substances are hygroscopic and viscous when exposed to water (12, 20).
Our observations shed light on the potential impact of the conditions of conidia prior to their landing on a substrate. The results show that the ECM is a phase in the isotropic growth of P. rubens that is uniquely related to germinating conidia. The ECM is observed only when dry conidia are applied. For the production of an ECM, active processes inside the fungal cell are required. What triggers the conidium to produce an ECM when it is applied in a dry state remains unknown.
The sustained observation of an ECM on porous gypsum proves the highly viscous nature of the substance. Future work may explore the potential function of the ECM in relation to water attraction.
Supplementary Material
ACKNOWLEDGMENT
We thank E. Groenewald for prompt and thorough revision of the manuscript.
Footnotes
Published ahead of print 27 July 2012
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1. Abellana M, Sanchis V, Ramos AJ. 2001. Effect of water activity and temperature on growth of three Penicillium species and Aspergillus flavus on a sponge cake analogue. Int. J. Food Microbiol. 71: 151– 157 [DOI] [PubMed] [Google Scholar]
- 2. Adan OCG. 1994. On the fungal defacement of interior finishes. PhD. dissertation. University of Technology, Eindhoven, The Netherlands: [Google Scholar]
- 3. Adan OCG. 2011. The fungal resistance of interior finishing materials, p 335–382 In Adan OCG, Samson RA. (ed), Fundamentals of mold growth in indoor environments and strategies for healthy living, 1st ed. Academic Publishers, Wageningen, The Netherlands: [Google Scholar]
- 4. Adan OCG, Huinink HP, Bekker M. 2011. Water relations of fungi in indoor environments, p 41–65 In Adan OCG, Samson RA. (ed), Fundamentals of mold growth in indoor environments and strategies for healthy living, 1st ed. Academic Publishers, Wageningen, The Netherlands: [Google Scholar]
- 5. Andersen B, Frisvad JC, Søndergaard I, Rasmussen IS, Larsen LS. 2011. Associations between fungal species and water-damaged building materials. Appl. Environ. Microbiol. 77: 4180– 4188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Apoga D, Jansson HB. 2000. Visualization and characterization of the extracellular matrix of Bipolaris sorokiniana. Mycol. Res. 104: 564– 575 [Google Scholar]
- 7. Ayerst G. 1969. The effects of moisture and temperature on growth and spore germination in some fungi. J. Stored Prod. Res. 5: 127– 141 [Google Scholar]
- 8. Beauvais A, Loussert C, Prevost MC, Verstrepen K, Latgé JP. 2009. Characterization of a biofilm-like extracellular matrix in FLO1-expressing Saccharomyces cerevisiae cells. FEMS Yeast Res. 9: 411– 419 [DOI] [PubMed] [Google Scholar]
- 9. Braun EJ, Howard RJ. 1994. Adhesion of Cochliobolus heterostrophus conidia and germlings to leaves and artificial surfaces. Exp. Mycol. 18: 211– 220 [Google Scholar]
- 10. Braun EJ, Howard RJ. 1994. Adhesion of fungal spores and germlings to host plant surfaces. Protoplasma 181: 202– 212 [Google Scholar]
- 11. Cabral JPS. 2010. Can we use indoor fungi as bioindicators of indoor air quality? Historical perspectives and open questions. Sci. Total Environ. 408: 4285– 4295 [DOI] [PubMed] [Google Scholar]
- 12. Callow JA, Osborne MP, Callow ME, Baker F, Donald AM. 2003. Use of environmental scanning electron microscopy to image the spore adhesive of the marine alga Enteromorpha in its natural hydrated state. Colloids Surf. B Biointerfaces 27: 315– 321 [Google Scholar]
- 13. Carver TLW, Kunoh H, Thomas BJ, Nicholson RL. 1999. Release and visualization of the extracellular matrix of conidia of Blumeria graminis. Mycol. Res. 103: 547– 560 [Google Scholar]
- 14. Carzaniga R, Bowyer P, O'Connell RJ. 2001. Production of extracellular matrices during development of infection structures by the downy mildew Peronospora parasitica. New Phytol. 149: 83– 93 [DOI] [PubMed] [Google Scholar]
- 15. Clement JA, Martin SG, Porter R, Butt TM, Beckett A. 1993. Germination and the role of the extracellular matrix in adhesion of urediniospores of Uromyces viciae-fabae to synthetic surfaces. Mycol. Res. 97: 585– 593 [Google Scholar]
- 16. Cooper LLD, Oliver JE, De Vilbiss ED, Doss RP. 2000. Lipid composition of the extracellular matrix of Botrytis cinerea germlings. Phytochemistry 53: 293– 298 [DOI] [PubMed] [Google Scholar]
- 17. Cox AR, Cagnol F, Russell AB, Izzard MJ. 2007. Surface properties of class II hydrophobins from Trichoderma reesei and influence on bubble stability. Langmuir 23: 7995– 8002 [DOI] [PubMed] [Google Scholar]
- 18. Dantigny P, Nanguy SP- 2009. Significance of the physiological state of fungal spores. Int. J. Food Microbiol. 134: 16– 20 [DOI] [PubMed] [Google Scholar]
- 19. Deacon JW. 2006. Fungal spores, spore dormancy, and spore dispersal, p 184–212 In Deacon JW. (ed), Fungal biology, 4th ed. Blackwell Publishing, Oxford, United Kingdom [Google Scholar]
- 20. De Groot PWJ, Ram AF, Klis FM. 2005. Features and functions of covalently linked proteins in fungal cell walls. Fungal Genet. Biol. 42: 657– 675 [DOI] [PubMed] [Google Scholar]
- 21. Deising H, Nicholson RL, Haug M, Howard RJ, Mendgen K. 1992. Adhesion pad formation and the involvement of cutinase and esterases in the attachment of uredospores to the host cuticle. Plant Cell 4: 1101– 1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dijksterhuis J, et al. 2002. Trehalose degradation and glucose efflux precede cell ejection during germination of heat-resistant ascospores of Talaromyces macrosporus. Arch. Microbiol. 178: 1– 7 [DOI] [PubMed] [Google Scholar]
- 23. Dohnalkova AC, et al. 2011. Imaging hydrated microbial extracellular polymers: comparative analysis by electron microscopy. Appl. Environ. Microbiol. 77: 1254– 1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Duncan KE, Howard RJ. 2000. Cytological analysis of wheat infection by the leaf blotch pathogen Mycosphaerella graminicola. Mycol. Res. 104: 1074– 1082 [Google Scholar]
- 25. Epstein L, Nicholson RL. 2006. Adhesion and adhesives of fungi and oomycetes, p 41–57 In Smith AM, Callow JA. (ed), Biological adhesives, 1st ed. Springer-Verlag, Berlin, Germany: [Google Scholar]
- 26. Erich BJF, Pel L. 2011. Moisture content measurement, p 305–334 In Adan OCG, Samson RA. (ed), Fundamentals of mould growth in indoor environments and strategies for healthy living, 1st ed. Academic Publishers, Wageningen, The Netherlands [Google Scholar]
- 27. Flannigan B. 2001. Deteriogenic micro-organisms in houses as a hazard to respiratory health. Int. Biodeterior. Biodegradation 48: 41– 54 [Google Scholar]
- 28. Flannigan B, Miller JD. 2001. Microbial growth in indoor environments, p 35–67 In Flannigan B, Samson RA, Miller JD. (ed), Microorganisms in home and indoor work environments, 1st ed. Taylor & Francis, London, United Kingdom: [Google Scholar]
- 29. Gevens AJ, Carver TLW, Thomas BJ, Nicholson RL. 2001. Visualization and partial characterization of the ECM of Pestalotia malicola on artificial and natural substrata. Physiol. Mol. Plant Pathol. 58: 277– 285 [Google Scholar]
- 30. Greenspan L. 1977. Humidity fixed points of binary saturated aqueous solutions. J. Res. Natl. Bur. Stand. 81A: 89– 96 [Google Scholar]
- 31. Griffin DH. 1994. Spore dormancy and germination, p 375–398 In Griffin DH. (ed), Fungal physiology, 2nd ed. Wiley-Liss Inc., New York, NY [Google Scholar]
- 32. Griffin DH. 1994. Primary metabolism, p 238–242 In Griffin DH. (ed), Fungal physiology, 2nd ed. Wiley-Liss Inc., New York, NY: [Google Scholar]
- 33. Hamer JE, Howard RJ, Chumley FG, Valent B. 1988. A mechanism for surface attachment in spores of a plant pathogenic fungus. Science 239: 288– 290 [DOI] [PubMed] [Google Scholar]
- 34. Houbraken J, Frisvad JC, Samson RA. 2011. Fleming's penicillin producing strain is not Penicillium chrysogenum but P. rubens. IMA Fungus 2: 87– 95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jansson HB, Friman E. 1999. Infection-related surface proteins on conidia of the nematophagous fungus Drechmeria coniospora. Mycol. Res. 103: 249– 256 [Google Scholar]
- 36. Karni J, Karni E. 1995. Gypsum in construction: origin and properties. Mater. Struct. 28: 92– 100 [Google Scholar]
- 37. Loussert C, et al. 2010. In vivo biofilm composition of Aspergillus fumigatus. Cell. Microbiol. 12: 405– 410 [DOI] [PubMed] [Google Scholar]
- 38. Magan N, Lacey J. 1984. Effect of temperature and pH on water relations of field and storage fungi. Trans. Br. Mycol. Soc. 82: 71– 81 [Google Scholar]
- 39. Magan N, Lacey J. 1988. Ecological determinants of mould growth in stored grain. Int. J. Food Microbiol. 7: 245– 256 [DOI] [PubMed] [Google Scholar]
- 40. Mahuku GS, Goodwin PH. 1998. Influence of sucrose, mucin and xanthan gum on spore germination of ten different fungi. Eur. J. Plant Pathol. 104: 849– 852 [Google Scholar]
- 41. Nanguy SPM, Perrier-Cornet JM, Bensoussan M, Dantigny P. 2010. Impact of water activity of diverse media on spore germination of Aspergillus and Penicillium species. Int. J. Food Microbiol. 142: 273– 276 [DOI] [PubMed] [Google Scholar]
- 42. Nicholson RL, Moraes WBC. 1980. Survival of Colletotrichum graminicola: importance of the spore matrix. Ecol. Epidemiol. 3: 255– 261 [Google Scholar]
- 43. Nielsen KA, Nicholson RL, Carver TLW, Kunoh H, Oliver RP. 2000. First touch: an immediate response to surface recognition in conidia of Blumeria graminis. Physiol. Mol. Plant Pathol. 56: 63– 70 [Google Scholar]
- 44. Nielsen KF, Holm G, Uttrup LP, Nielsen PA. 2004. Mould growth on building materials under low water activities. Influence of humidity and temperature on fungal growth and secondary metabolism. Int. Biodeterior. Biodegradation 54: 325– 336 [Google Scholar]
- 45. Pasanen AL, Kalliokoski P, Pasanen P, Jantunen MJ, Nevalainen A. 1991. Laboratory studies on the relationship between fungal growth and atmospheric temperature and humidity. Environ. Int. 17: 225– 228 [Google Scholar]
- 46. Pasanen AL, et al. 2000. Fungal growth and survival in building materials under fluctuating moisture and temperature conditions. Int. Biodeterior. Biodegradation 46: 117– 127 [Google Scholar]
- 47. Ponizovskaya VB, Antropova AB, Mokeeva VL, Bilanenko EN, Chekunova LN. 2011. Effect of water activity and relative air humidity on the growth of Penicillium chrysogenum Thom, Aspergillus repens (Corda) Sacc., and Trichoderma viride Pers. isolated from living spaces. Mikrobiologiia 3: 378– 385 [PubMed] [Google Scholar]
- 48. Samson RA. 2011. Ecology and general characteristics of indoor fungi, p 101–116 In Adan OCG, Samson RA. (ed), Fundamentals of mold growth in indoor environments and strategies for healthy living, 1st ed. Academic Publishers, Wageningen, The Netherlands: [Google Scholar]
- 49. Sautour M, et al. 2012. Dynamics of fungal colonization in a new medical mycology laboratory. J. Mycol. Med. 22: 14– 20 [DOI] [PubMed] [Google Scholar]
- 50. Schubert K, et al. 2007. Biodiversity in the Cladosporium herbarum complex (Davidiellaceae, Capnodiales), with standardisation of methods for Cladosporium taxonomy and diagnostics. Stud. Mycol. 58: 105– 156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Vereecken E, Roels S. 2012. Review of mould prediction models and their influence on mould risk evaluation. Build. Environ. 51: 296– 310 [Google Scholar]
- 52. Viitanen H. 1994. Factors affecting the development of biodeterioration in wooden constructions. Mater. Struct. 27: 483– 493 [Google Scholar]
- 53. Viitanen H, Bjurman J. 1995. Mould growth on wood under fluctuating humidity conditions. Mater. Org. 29: 27– 46 [Google Scholar]
- 54. Wessels JGH. 2000. Hydrophobins, unique fungal proteins. Mycologist 14: 153– 159 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.