Abstract
Staphylococcus aureus is an important Gram-positive bacterial pathogen producing many secreted and cell surface-localized virulence factors. Here we report that the staphylococcal thiol-disulfide oxidoreductase DsbA is essential for stable biogenesis of the ComGC pseudopilin. The signal peptidase ComC is indispensable for ComGC maturation and optimal cell surface exposure.
TEXT
Staphylococcus aureus is a major Gram-positive bacterial pathogen that causes a broad range of infections. To subvert its mammalian hosts, S. aureus relies on different virulence factors that are localized at the cell surface or secreted into the host milieu (11, 20). For export of newly synthesized virulence factors from the cytoplasm, their translocation across the membrane, and posttranslocational modifications, an intricate secretion machinery has evolved (20). In recent years, the functions of many secretion machinery components of S. aureus have been elucidated (3, 4, 9, 13, 19, 21). Intriguingly however, for several other potential secretion machinery components, no biological functions have been described so far. For example, this applies to the extracytoplasmic thiol-disulfide oxidoreductase (TDOR) DsbA, which is known as one of the strongest bacterial TDORs (12, 15). Likewise, the role of the pseudopilin export machinery of S. aureus has not yet been documented. This machinery is very similar to the Com machinery for DNA binding and uptake in the related Gram-positive bacterium Bacillus subtilis. Interestingly, the genes coding for most Com proteins are present in the sequenced S. aureus strains (20). The transcription of these genes, which are organized in the comG and comE operons, is directed by the staphylococcal alternative sigma factor σH (17).
The biogenesis of the Com pseudopilin system has been well studied in B. subtilis. Among the B. subtilis Com proteins with orthologues in S. aureus are those encoded by the comG operon (1). Specifically, the B. subtilis ComGC, ComGD, ComGE, and ComGG proteins form pilin-like structures that are localized to the cytoplasmic membrane and cell wall (6–8, 22). Assembly of the pseudopilus in B. subtilis requires the specific signal peptidase ComC, which processes the N-terminal signal peptides of ComG proteins upon membrane translocation (6, 22, 23). Furthermore, stability of the B. subtilis ComGC pseudopilin requires posttranslocational TDOR-mediated disulfide bond formation (6, 10, 15, 16).
ComGC of S. aureus is processed by ComC and stabilized by DsbA.
To study the processing and stability of S. aureus ComGC, the expression of the com genes was induced through constitutive expression of sigH from plasmid pRIT:SigH (17). The primers used for strain and plasmid constructions are listed in Table 1. As shown with specific polyclonal antibodies raised against ComGC, exponentially growing cells of the S. aureus strains RN4220, SH1000, RN6911, and Newman produced only the precursor form of this pseudopilin (Fig. 1). ComGC production depended strictly on ectopic expression of σH (Fig. 1). Notably, relatively small amounts of mature ComGC were detectable when the investigated strains were grown to stationary phase (Fig. 1A). This inefficient ComGC processing was due to limited ComC signal peptidase activity, as shown by ComC overexpression from plasmid pCN51:comC. This resulted in close-to-complete ComGC processing (Fig. 1B). Conversely, ComGC processing in the postexponential growth phase was completely abolished by a comC deletion. Consistent with these observations, no comC expression was detectable in exponentially growing S. aureus cells, and low-level comC transcription was detectable in the late stationary growth phase (data not shown). Together, these findings show that ComC is the signal peptidase needed for ComGC processing and that the investigated strains produce limiting amounts of ComC under the tested conditions.
Table 1.
Primers used in this study
| Primer name and purpose | Sequence (5′→3′)a |
|---|---|
| Construction of S. aureus comC mutant | |
| ComC-F1 | CGAGATGGTCAAACATTTAAG |
| ComC-R1 | TCACGTCAGTCAGTCACCATGGCAATGACAACCTCCTTATGTAAA |
| ComC-F2 | TGCCATGGTGACTGACTGACGTGAAAATTAAAGAAATGGTAA |
| ComC-R2 | AACTGCGATGATTGCATTGGC |
| Construction of S. aureus comGC mutant | |
| ComGC-F1 | GCTCAATAAGATAAACTTTGT |
| ComGC-R1 | CTACGTCAGTCAGTCACCATGGCAATATTAACCTCCATTATTTTA |
| ComGC-F2 | TGCCATGGTGACTGACTGACGTAGAAAGCAGTCAGCATTTAC |
| ComGC-R2 | GATTCATCATTGGTATCAATA |
| Construction of S. aureus dsbA mutant | |
| DsbA-F1 | ATTTCTTTGGATATTTATATT |
| DsbA-R1 | CTACGTCAGTCAGTCACCATGGCAAATAACTCCTATTCATAT |
| DsbA-F2 | TGCCATGGTGACTGACTGACGTAGTCTTAATTGTTGAGATCA |
| DsbA-R2 | CTTTCGTTATAGTTTTCCCAC |
| Construction of pCN51:comC for S. aureus comC overexpression | |
| ComC-F | CAGCCGGATCCCATAAGGAGGTTGTCATTTGGTAG (BamHI) |
| ComC-R | CGGAATTCCTTTAATTTTCAAAAATATACGCCTCC (EcoRI) |
Overlapping parts are shown in boldface. Restriction sites used for cloning are underlined (with the restriction endonuclease given in parentheses.
Fig 1.
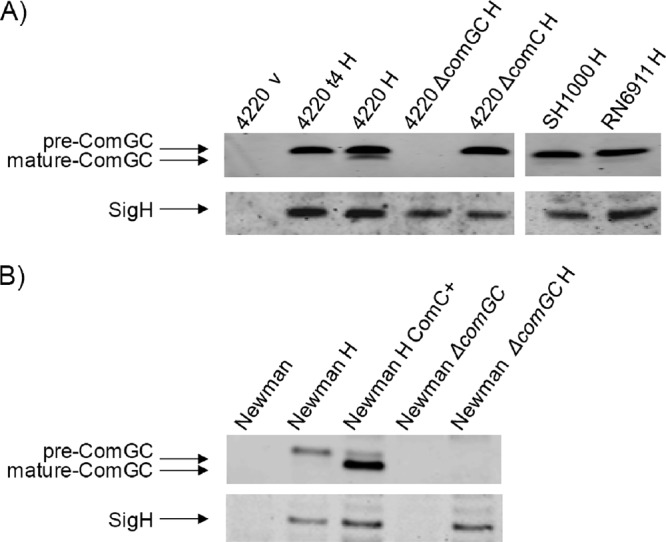
ComGC processing by ComC. S. aureus strains were grown overnight at 37°C in Luria-Bertani (LB) broth supplemented with 12.5 μg/ml chloramphenicol to select for pRIT:sigH or pRITH5 or 5 μg/ml erythromycin to select for pCN51:comC. Samples for Western blotting analyses with polyclonal rabbit antibodies against ComGC or chicken antibodies against SigH were collected after 4 h (t4) or 7 h of growth (A) or after 5 h of growth (B). Cell extracts were prepared as previously described (19). Proteins were separated by SDS-PAGE (NuPage gels; Invitrogen) and blotted onto nitrocellulose membranes (Protran; Schleicher & Schuell). Immunodetection was performed with fluorescent secondary antibodies (IRDye 700 CW goat anti-rabbit and IRDye 800 goat anti-chicken; Li-Cor) in combination with the Odyssey infrared imaging system (LiCor Biosciences). The chromosomal comGC or comC genes were deleted from S. aureus strain RN4220 or Newman Δspa Δsbi (19) as previously described (2, 14). The primers used for strain and plasmid constructions are listed in Table 1. Lanes relating to strains that carry pRIT:sigH for σH production are indicated by “H”; lanes relating to control strains with the empty vector pRIT5H that do not produce σH are indicated by “v”; lanes relating to strains that carry pCN51:comC (5) for ComC production are indicated by “ComC+.”
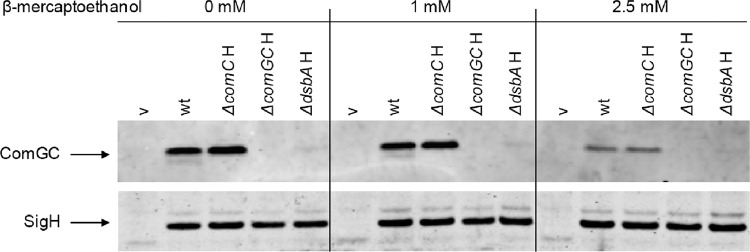
To investigate whether the stability of S. aureus ComGC depends on TDOR activity, the production of this protein was analyzed in strain RN4220 lacking the dsbA gene. Western blotting analyses showed that ComGC was barely detectable in cells lacking DsbA. This effect did not relate to possible changes in the σH levels, which remained unaltered in the dsbA mutant (Fig. 2, lower panel). These observations indicate that the intramolecular disulfide bond of B. subtilis ComGC is conserved in S. aureus ComGC and that the formation of this disulfide bond between Cys46 and Cys87 of S. aureus ComGC is catalyzed by DsbA. Furthermore, this disulfide bond, which is positioned within the predicted extracytoplasmic domain of S. aureus ComGC, would be necessary to stabilize ComGC upon export from the cytoplasm. To test this idea, the reducing agent β-mercaptoethanol was added to the growth medium at concentrations up to 2.5 mM, which is the highest concentration of β-mercaptoethanol that can be added to the cells without affecting growth and cell viability (data not shown). Clearly, in the presence of 2.5 mM β-mercaptoethanol, ComGC was barely detectable (Fig. 2, upper panels), whereas σH production remained unaffected (Fig. 2, lower panels). The simplest explanation for these observations is that the TDOR activity of DsbA is required for disulfide bond formation in ComGC and that this disulfide bond is essential for ComGC stability. Nevertheless, it is possible that DsbA is indirectly involved in the stabilization of ComGC. To our knowledge, this is the first report describing a biological function for DsbA in S. aureus.
Fig 2.
ComGC stabilization by DsbA. S. aureus strains were grown for 7 h in LB broth as described in Fig. 1 in the presence or absence of β-mercaptoethanol (final concentration, 1 or 2.5 mM). The preparation of cell extracts, SDS-PAGE, and Western blotting with specific antibodies against ComGC or σH were performed as described in the legend to Fig. 1. The chromosomal dsbA gene was deleted from S. aureus strain RN4220 as previously described (2, 14). Primers used for strain construction are listed in Table 1. Lanes relating to strains that carry pRIT:sigH for σH production are indicated by “H”; lanes relating to control strains with the empty vector pRIT5H that do not produce σH are indicated by “v.”
ComGC localizes to the membrane, cell wall, and cell surface of S. aureus.
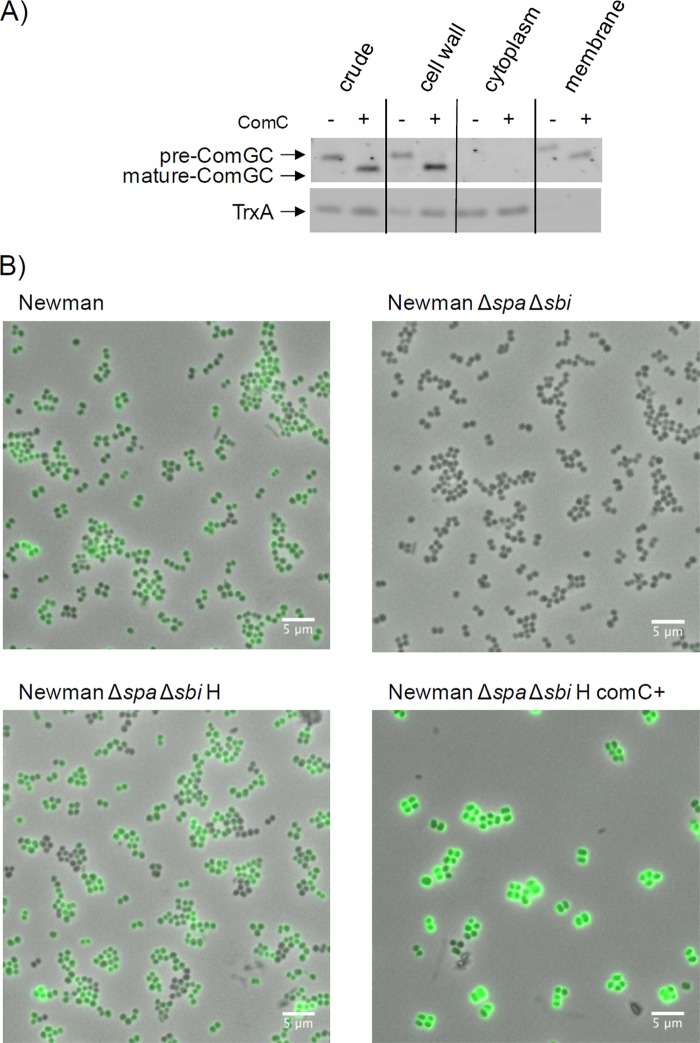
To determine the localization of ComGC, cells of S. aureus strain RN4220 or strain RN4220 overproducing ComC were subjected to subcellular fractionation. Cells were first protoplasted. Next, the protoplasts were separated from liberated cell wall proteins (i.e., the cell wall fraction) by centrifugation. The collected protoplasts were then disrupted by osmotic shock, and cytosolic proteins were separated from the membranes by ultracentrifugation as previously described (24). Proteins in all collected fractions were separated by SDS-PAGE, and the presence of ComGC and thioredoxin A (TrxA) in each fraction was analyzed by Western blotting with specific antibodies (Fig. 3A). TrxA was used as a cytoplasmic control protein. This analysis showed that both pre-ComGC and mature ComGC from S. aureus localize to the cytoplasmic membrane and cell wall. This dual localization is consistent with the localization of the homologous protein in B. subtilis. Furthermore, in S. aureus cells overproducing the ComC protein, we observed slightly increased amounts of ComGC in the cell wall fraction compared to cells of the parental control strain that do not produce ComC under the tested conditions. Next, we investigated by immunofluorescence microscopy whether ComGC is detectable on the cell surface. For this purpose, we employed cells of strain Newman lacking the IgG-binding proteins Spa and Sbi, which displayed negligible background fluorescence (Fig. 3B; compare panels for the spa sbi mutant and the parental strain Newman). Importantly, σH-producing cells showed elevated levels of immune fluorescence, and strongly enhanced immune fluorescence was observed when the signal peptidase ComC was overexpressed together with σH. These observations are consistent with the view that the signal peptide of ComGC facilitates membrane translocation and exposure of ComGC to the cell wall, irrespective of signal peptide processing by ComC. Enhanced signal peptide processing upon ComC overproduction would then allow more of the translocated mature ComGC to penetrate the cell wall and to become exposed at the cell surface. These findings thus show that ComC-dependent processing of ComGC is important for optimal cell surface exposure of ComGC. It should be noted that, for unknown reasons, cells overproducing ComC have a larger diameter, which seems to relate mostly to a thickened cell wall (Fig. 3B).
Fig 3.
ComGC localizes to the membrane, cell wall, and cell surface of S. aureus. (A) To determine the subcellular localization of ComGC in S. aureus RN4220/pRIT:sigH or S. aureus RN4220/pRIT:sigH containing pCN51:comC, the cells were grown in LB broth for 5 h, collected by centrifugation, and incubated for 1 h at 37°C in protoplast buffer (50 mM Tris-HCl [pH 7.6], 0.145 M NaCl, 30% sucrose, 0.01% DNase, and EDTA-free Complete protease inhibitors [Roche]). The cell wall fraction (i.e., protoplast supernatant) was obtained by centrifugation (20 min, 3,000 × g, 4°C). Protoplasts were disrupted by osmotic shock in 0.05 M Tris-HCl (pH 7.6) with 30 min of incubation on ice and vortexing at 5-min intervals. Cytosolic and solubilized membrane proteins were collected as previously described (24). SDS-PAGE and Western blotting with specific antibodies against ComGC or the cytoplasmic control protein TrxA were performed as described in the legend to Fig. 1. (B) Cell surface exposure of ComGC was assessed in S. aureus Newman Δspa Δsbi or the parental strain (Newman) by immunofluorescence microcopy. For this purpose, cells were grown for 5 h in LB broth, and 1 unit of cells (by optical density at 600 nm) was collected by centrifugation (8,000 rpm, 5 min, 4°C). The cell pellet was resuspended in phosphate-buffered saline-Tween 20 (PBST) plus 2% bovine serum albumin (BSA) and incubated for 10 min on ice. Next, the cells were incubated for 60 min with ComGC-specific polyclonal rabbit antibodies (1:400 in PBST plus 1% BSA). Unbound antibodies were removed by three washes in PBST, and cell-bound ComGC antibodies were visualized using goat-anti-rabbit Alexa Fluor 488 antibodies (Life Technologies) and a Leica DM5500 B microscope. The overlay of phase-contrast and fluorescence microscopy images was done with imageJ. The strains containing pRIT:sigH for σH production are indicated by “H”; the strain containing pCN51:comC for ComC production is indicated by “ComC+.” The magnification is indicated by scale bars.
In summary, we show that biogenesis of the pseudopilin ComGC of S. aureus requires the TDOR DsbA for stability and the signal peptidase ComC for precursor maturation and cell surface exposure. This is thus the first report in which biological functions are demonstrated for S. aureus DsbA and ComC. In B. subtilis, the Com system is needed for DNA uptake during genetic competence. Whether this is also true in S. aureus remains to be demonstrated, but natural competence has been reported for S. aureus (18). Our present findings suggest that expression of comC could be a limiting factor in competence development, even if S. aureus cells overproduce σH for expression of other com genes.
ACKNOWLEDGMENTS
We thank Tarek Msadek for helpful discussions and Kazuya Morikawa for providing plasmids pRIT5H and pRITsigH and anti-σH antibodies.
This research was supported by CEU project LSHG-CT-2006-037469 and the Top Institute Pharma project T4-213.
Footnotes
Published ahead of print 20 July 2012
REFERENCES
- 1. Albano M, Breitling R, Dubnau DA. 1989. Nucleotide sequence and genetic organization of the Bacillus subtilis comG operon. J. Bacteriol. 171:5386–5404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arnaud M, Chastanet A, Debarbouille M. 2004. New vector for efficient allelic replacement in naturally nontransformable, low-GC-content, gram-positive bacteria. Appl. Environ. Microbiol. 70:6887–6891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Biswas L, et al. 2009. Role of the twin-arginine translocation pathway in Staphylococcus. J. Bacteriol. 191:5921–5929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Burts ML, Williams WA, DeBord K, Missiakas DM. 2005. EsxA and EsxB are secreted by an ESAT-6-like system that is required for the pathogenesis of Staphylococcus aureus infections. Proc. Natl. Acad. Sci. U. S. A. 102:1169–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Charpentier E, et al. 2004. Novel cassette-based shuttle vector system for gram-positive bacteria. Appl. Environ. Microbiol. 70:6076–6085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen I, Provvedi R, Dubnau D. 2006. A macromolecular complex formed by a pilin-like protein in competent Bacillus subtilis. J. Biol. Chem. 281:21720–21727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chung YS, Breidt F, Dubnau D. 1998. Cell surface localization and processing of the ComG proteins, required for DNA binding during transformation of Bacillus subtilis. Mol. Microbiol. 29:905–913 [DOI] [PubMed] [Google Scholar]
- 8. Chung YS, Dubnau D. 1998. All seven comG open reading frames are required for DNA binding during transformation of competent Bacillus subtilis. J. Bacteriol. 180:41–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cregg KM, Wilding I, Black MT. 1996. Molecular cloning and expression of the spsB gene encoding an essential type I signal peptidase from Staphylococcus aureus. J. Bacteriol. 178:5712–5718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Draskovic I, Dubnau D. 2005. Biogenesis of a putative channel protein, ComEC, required for DNA uptake: membrane topology, oligomerization and formation of disulphide bonds. Mol. Microbiol. 55:881–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dreisbach A, van Dijl JM, Buist G. 2011. The cell surface proteome of Staphylococcus aureus. Proteomics 11:3154–3168 [DOI] [PubMed] [Google Scholar]
- 12. Dumoulin A, Grauschopf U, Bischoff M, Thony-Meyer L, Berger-Bachi B. 2005. Staphylococcus aureus DsbA is a membrane-bound lipoprotein with thiol-disulfide oxidoreductase activity. Arch. Microbiol. 184:117–128 [DOI] [PubMed] [Google Scholar]
- 13. Jongbloed JD, van der Ploeg R, van Dijl JM. 2006. Bifunctional TatA subunits in minimal Tat protein translocases. Trends Microbiol. 14:2–4 [DOI] [PubMed] [Google Scholar]
- 14. Kouwen TR, et al. 2009. The large mechanosensitive channel MscL determines bacterial susceptibility to the bacteriocin sublancin 168. Antimicrob. Agents Chemother. 53:4702–4711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kouwen TR, et al. 2007. Thiol-disulphide oxidoreductase modules in the low-GC Gram-positive bacteria. Mol. Microbiol. 64:984–999 [DOI] [PubMed] [Google Scholar]
- 16. Meima R, et al. 2002. The bdbDC operon of Bacillus subtilis encodes thiol-disulfide oxidoreductases required for competence development. J. Biol. Chem. 277:6994–7001 [DOI] [PubMed] [Google Scholar]
- 17. Morikawa K, et al. 2003. A new staphylococcal sigma factor in the conserved gene cassette: functional significance and implication for the evolutionary processes. Genes Cells 8:699–712 [DOI] [PubMed] [Google Scholar]
- 18. Rudin L, Sjostrom JE, Lindberg M, Philipson L. 1974. Factors affecting competence for transformation in Staphylococcus aureus. J. Bacteriol. 118:155–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sibbald MJ, et al. 2010. Synthetic effects of secG and secY2 mutations on exoproteome biogenesis in Staphylococcus aureus. J. Bacteriol. 192:3788–3800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sibbald MJ, et al. 2006. Mapping the pathways to staphylococcal pathogenesis by comparative secretomics. Microbiol. Mol. Biol. Rev. 70:755–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stoll H, Dengjel J, Nerz C, Gotz F. 2005. Staphylococcus aureus deficient in lipidation of prelipoproteins is attenuated in growth and immune activation. Infect. Immun. 73:2411–2423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tjalsma H, et al. 2004. Proteomics of protein secretion by Bacillus subtilis: separating the “secrets” of the secretome. Microbiol. Mol. Biol. Rev. 68:207–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tjalsma H, Bolhuis A, Jongbloed JD, Bron S, van Dijl JM. 2000. Signal peptide-dependent protein transport in Bacillus subtilis: a genome-based survey of the secretome. Microbiol. Mol. Biol. Rev. 64:515–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zweers JC, Wiegert T, van Dijl JM. 2009. Stress-responsive systems set specific limits to the overproduction of membrane proteins in Bacillus subtilis. Appl. Environ. Microbiol. 75:7356–7364 [DOI] [PMC free article] [PubMed] [Google Scholar]