Abstract
The leafhopper Matsumuratettix hiroglyphicus (Matsumura) is the most important vector of a phytoplasma pathogen causing sugarcane white leaf (SCWL) disease. The purpose of this study was to evaluate candidate bacterial symbionts for possible use as vehicles in the control of the disease. 16S rRNA bacterial genes were amplified from whole bodies of M. hiroglyphicus leafhoppers and analyzed by cloning and sequencing. Two dominant groups were found: one belonged to the Betaproteobacteria that did not closely match any sequences in the database and was named bacterium associated with M. hiroglyphicus (BAMH). Another one found to be abundant in this leafhopper is “Candidatus Sulcia muelleri” in the order Bacteroidetes, which was previously reported in the insect members of the Auchenorrhyncha. Most M. hiroglyphicus leafhoppers carry both BAMH and “Ca. Sulcia muelleri.” Fluorescent in situ hybridization showed that BAMH and “Ca. Sulcia muelleri” colocalized in the same bacteriomes. BAMH was present in the midgut and ovaries of the leafhopper and was found in all developmental stages, including eggs, nymphs, and adults. Because BAMH appears to be specific for the SCWL vector, we evaluated it as a candidate for symbiotic control of sugarcane white leaf disease.
INTRODUCTION
Sugarcane white leaf (SCWL) is one of the most destructive of known sugarcane diseases in the Asian region and has been reported in Taiwan, Sri Lanka, the Lao People's Democratic Republic, and Thailand (9, 18, 19, 25, 46). This disease is caused by a phytoplasma, a bacterium without a cell wall, belonging to the class Mollicutes that lives in the phloem of host plants and is transmitted by insect vectors (24). The SCWL phytoplasma belongs to the rice yellow dwarf group 16Sr XI (23). SCWL phytoplasma is transmitted by phloem-feeding leafhoppers [Matsumuratettix hiroglyphicus (Matsumura)] belonging to the Cicadellidae in the suborder Auchenorrhyncha of the Deltocephalinae (9, 18, 19, 25). The infected plant shows leaf chlorosis and tiller proliferation (33, 46). So far there is no sugarcane-resistant variety or effective methods to control SCWL disease, except for elimination of infected plants to decrease the amount of the phytoplasma inoculum. Measures to limit the spread of the disease have focused on control of the insect vector. However, use of insecticides is unsustainable for vector control on large-scale commercial crops, while other traditional control methods are lacking (44).
A new biological control method for plant disease treatment is known as “symbiotic control.” In this method, symbiotic microorganisms are isolated, genetically modified, and then reintroduced to express an antipathogen agent in the insect vectors (5). This approach has been used to control Chagas' disease (5, 6, 7, 13), where a symbiotic bacterium, Rhodococcus rhodnii, was isolated from the vector Rhodnius prolixus and genetically transformed to express an antipathogen agent in the vector gut. The bacterial genus Asaia, found in malarial mosquito vectors, can also be cultivated, genetically manipulated, and reintroduced into the insect host (14). Moreover, the same acetic acid bacterium is reported to be capable of cross-colonizing leafhopper (Scaphoideus titatus), a vector of Flavescence doree, the yellow disease in grapevines which is caused by a phytoplasma (10). Symbiotic control is also being developed for control of Pierce's disease in grapevines caused by the bacterial pathogen Xylella fastidiosa and transmitted by sharpshooters, principally, Homalodisca vitripennis (8). Wolbachia has been reported to induce abnormal reproduction in insects (41, 48), and it is currently being used in symbiotic control of dengue fever (39). These studies have shown that there is a possibility to use symbiotic control as an alternative strategy for SCWL disease control.
Therefore, the purpose of this study was to identify and characterize the candidate bacterial symbionts associated with the leafhopper M. hiroglyphicus with the long-term goal of developing symbiotic control of SCWL.
MATERIALS AND METHODS
Leafhopper collection and DNA extraction.
M. hiroglyphicus adults were collected at light traps from 6 different sugarcane fields in the northeast region of Thailand during the rainy season (July to September 2008). They were kept in 95% ethanol (EtOH) at −20°C until DNA extraction. Twelve males and 12 females from 6 fields were selected for individual DNA extraction by using a phenol-chloroform protocol (3). To remove surface microorganisms, insects were sterilized with 70% EtOH and 6% sodium hypochlorite for 3 min each and then washed 3 times with sterilized water. Individual leafhoppers were ground up in DNA extraction buffer (200 mM Tris, pH 8.0, 250 mM NaCl, 25 mM EDTA, 0.5% SDS, 0.1 mg/ml proteinase K) and incubated at 37°C for 24 h. DNA was extracted with an equal volume of phenol-chloroform-isoamyl alcohol (25:24:1), followed by 5 min of centrifugation at 13,000 rpm. The supernatants were transferred, and an equal volume of chloroform-isoamyl alcohol (24:1) was added, followed by 5 min centrifugation at 13,000 rpm. The supernatants were precipitated with 3 M sodium acetate and isopropanol. The pellet was washed with 70% EtOH, air dried, and resuspended in TE buffer (10 mM Tris, 1 mM EDTA), before storage at −20°C until use.
Bacterial screening by PCR, cloning, and sequencing of 16S rRNA gene.
Identification of the bacteria associated with M. hiroglyphicus was studied by amplification of the 16S rRNA genes with universal primers 27F and 1513R (45) (Table 1). PCR amplification was performed in a final volume of 25 μl containing 2 μl DNA templates, 0.2 mM deoxynucleoside triphosphates (dNTPs), 0.3 μM each primer, 1 U of Taq DNA polymerase, 2.5 mM MgCl2, and 1× reaction buffer (Promega). Cycling conditions consisted of an initial denaturation (95°C, 5 min), followed by 30 rounds of denaturation (95°C, 1 min), annealing (55°C, 1 min), and extension (72°C, 2 min) and then a final extension step at 72°C for 10 min. PCR products were analyzed by gel electrophoresis, and the positives were cloned into the TOPO-TA vector (Invitrogen) following the manufacturer's instructions. Five recombinant plasmid clones were randomly selected from each leafhopper DNA template for sequencing analysis. Nucleotide sequencing was done at the Genome Institute of the National Center of Engineering and Biotechnology, Thailand. All 120 sequences obtained were compared with other 16S rRNA gene sequences by using a nucleotide BLAST search at the National Center for Biotechnology Information (NCBI database).
Table 1.
Nucleotide primers sequences used in the study
| Target organism | Name | Target gene | Sequence | Reference(s) or source |
|---|---|---|---|---|
| Eubacteria | 27F | 16S rRNA | 5′-AGAGTTTGATCMTGGCTCAG-3′ | 45 |
| 1513R | 16S rRNA | 5′-ACGGYTACCTTGTTACGACTT-3′ | ||
| BAMH | MHF | 16S rRNA | 5′-TTATTCTAGTGGCGAACGGG-′3 | This study |
| MHR | 16S rRNA | 5′-GCGGTGTGTACAAGACCAGT-′3 | ||
| “Ca. Sulcia muelleri” of M. hiroglyphicus | SulF | 16S rRNA | 5′-AAACGCTAGCGGAGGGCTTAAC-′3 | This study |
| SulR | 16S rRNA | 5′-CTTACCGCGACTGCTGGCAC-′3 | ||
| SCWL phytoplasma | MLO-X | 16/23S rRNA | 5′-GTTAGGTTAAGTCCTAAAACGAGC-3′ | 18, 19 |
| MLO-Y | 16/23S rRNA | 5′-GTGCCAAGGCATCCACTGTATGCC-3′ | ||
| SCWL phytoplasma | P1 | 16/23S rRNA | 5′-GTCGTAACAAGGTATCCCTACCGG-3′ | 18, 19 |
| P2 | 16/23S rRNA | 5′-GGTGGGCCTAAATGGACTTGAACC-3′ |
Specific primers and PCR for BAMH and “Candidatus Sulcia muelleri” 16S rRNA genes.
A representative of the most common sequences was selected to design specific primers using the Primer-BLAST program at the NCBI home page. The forward primer (MHF) and reverse primer (MHR) specific for the 16S rRNA gene of the bacterium associated with M. hiroglyphicus (BAMH) did not match with any sequences in the NCBI database (Table 1). PCR was performed with primers MHF and MHR according to the amplification program described above, except that the annealing temperature was 58°C. PCR products were checked by 1% agarose gel electrophoresis for an expected 1.3-kb gene. The primer specific for the 16S rRNA gene of “Ca. Sulcia muelleri” (SulF and SulR) of M. hiroglyphicus was also designed, and PCR was performed with the same program used for BAMH detection.
Phylogenetic analysis.
Almost the entire sequences of BAMH and “Ca. Sulcia muelleri” of M. hiroglyphicus were analyzed for phylogenetic relationships with other selected sequences from the NCBI database, including closely related sequences and unrelated sequences. Sequence alignments were performed using the ClustalX program under default settings with a gap opening and gap extension (43). The aligned sequences were analyzed by using the neighbor-joining and Kimura 2-parameter methods with 1,000 bootstraps replications. Phylogenetic trees were generated by using a consensus program within the PHYLIP package (version 3.6) (15). The trees were displayed by using the TREEVIEW program (37).
Frequency prevalence of bacterial symbionts and SCWL phytoplasma in M. hiroglyphicus.
Leafhoppers were collected from three different sugarcane fields during 2010 and 2011. DNA was extracted from whole bodies by the phenol-chloroform protocol for detection of BAMH and “Ca. Sulcia muelleri” with PCR methods. SCWL phytoplasma was detected by nested PCR with two sets of primers designed to amplify the 16S rRNA and 23S rRNA genes, including spacer regions, as previously described (18, 19). The first set of primers was MLO-X and MLO-Y, and the first PCR was performed in 25 μl containing 2 μl leafhopper DNA, 0.2 mM dNTPs, 0.25 μM each primers, 1 U Taq DNA polymerase, 1.5 mM MgCl2, and 1× reaction buffer (Promega). PCR cycling consisted of one cycle of 5 min at 94°C for initial denaturation and 35 cycles of 1 min at 94°C, annealing for 30 s at 60°C, and extension for 1 min at 72°C and then a final extension step of 10 min at 72°C. The second round of PCR was carried out using 1 μl of the first-round PCR product as the template with the second set of primers, P1 and P2. A total of 40 cycles were performed under the same conditions, except that the annealing temperature was 68°C.
Localization of bacterial symbionts in dissected organs.
Eight leafhoppers were dissected with forceps under a light microscope. Dissected organs were fat bodies, ovaries from females, salivary glands, guts, and pairs of bacteriomes. DNA was extracted from each tissue with phenol-chloroform as described above. Each sample was tested for the selected bacterial symbionts, BAMH and “Ca. Sulcia muelleri,” by using specific primers and PCR.
Localization of bacterial symbionts by fluorescent in situ hybridization (FISH).
For whole leafhopper, live specimens were collected and their legs and wings were removed in 70% EtOH under a dissecting microscope. Their cuticle was pricked with a needle in several places to facilitate the infiltration of reagents (22). Specimen fixation and embedding followed standard protocols (3). The specimens were immediately fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) and kept at 4°C overnight. After fixation, the samples were dehydrated by using an ethanol-xylene series and embedded in paraffin wax. Sample tissues were cut into 8-μm sections with a rotary microtome, and the tissue ribbons were floated on a diethylpyrocarbonate (DEPC)-treated water bath at 40 to 42°C and collected on Superfrost plus glass slides (Fisherbrand). The slides were completely dried at room temperature and then dewaxed and rehydrated with a xylene-ethanol series. Then, the specimens were treated with 0.2 M HCl for 20 min and proteinase K (10 μg · ml−1) for 30 min and refixed in 4% paraformaldehyde for 5 min. Slides were air dried before hybridization. A specific probe, MH95 (5′-CCCGTTCGCCACTAGAATAA-3′), was designed for the BAMH 16S rRNA gene and was labeled with 6-carboxyfluorescein (FAM) (excitation, 495 nm; emission, 520 nm). To determine the localization of the endosymbiont “Ca. Sulcia muelleri,” the probe CFB319 (5′-TGGTCCGTGTCTCAGTAC-3′), which perfectly matched positions 319 to 336 of the 16S rRNA of Bacteroidetes species (31), including all of our sequences from “Ca. Sulcia muelleri,” was used. CFB319 was labeled with cyanine 5 (Cy5) (excitation, 646 nm; emission, 669 nm). Two hundred microliters of a hybridization buffer (0.9 M NaCl, 20 mM Tris-HCl [pH 7.4], 0.01% SDS, 30% formamide) containing 100 pmol ml−1 of probes was added to the tissues on the slides. The slides were covered with Parafilm and incubated at 46°C overnight in humid chambers. Unhybridized probes were rinsed with washing buffer (0.2 M NaCl, 20 mM Tris-HCl [pH 7.4], 0.01% SDS) at 46°C for 20 min and then rinsed with 1× PBS buffer and DEPC-treated water. They were air dried and mounted in ProLong antifade reagent (Invitrogen) with coverslips. The slides were observed under a confocal laser scanning microscope (Olympus FV1000) at the Center of Nanoimaging (CNI), Mahidol University, Thailand.
The universal bacterial probe EUB338 (5′-GCTGCCTCCCGTAGGAGT-3′) (2) was labeled with Cy5 (excitation, 646 nm; emission, 669 nm) and used as a positive control. Slides without the probe and hybridization with sense-strand probes were used as a negative control of the experiments.
BAMH detection in each life stage of M. hiroglyphicus.
Adult M. hiroglyphicus leafhoppers were collected at light traps from sugarcane fields in the northeast of Thailand from July to September 2010. They were maintained on sugarcane plants that tested negative for the presence of BAMH. The plants were prepared by cutting the first and last sugarcane buds from plant stocks, and genomic plant DNA was extracted by the cetyltrimethylammonium bromide method (3). BAMH was detected by PCR, and the stocks which tested negative were used for planting by using the rest of the buds. The leafhoppers were maintained in cages on this plant stock until they produced eggs and nymphs. Some of the eggs and nymphs were collected and kept in 95% ethanol at −20°C until DNA extraction, and some of them were transferred to a new plant if the old plant became yellow. Some of the adults of the first generation were transferred to a new plant and maintained until they produced the second generation of offspring. DNA was extracted from individual specimens, and the presence of the target bacterial symbiont was assessed using PCR and specific primers, as described above. The plants used for rearing the insects were rechecked for the target bacterial symbiont.
Prevalence of bacterial symbionts and SCWL phytoplasma in different species of leafhoppers.
Leafhoppers were collected at light traps from 3 different sugarcane fields in the northeast of Thailand from July to September 2010 and 2011. They were identified by species and sex and kept in 95% EtOH at −20°C until use. The leafhopper species identified were Recilia dorsalis (18 females), Recilia sp. nr. vetus (41 females), Exitianus indicus (36 females and 10 males), Yamatotettix flavovittatus (48 females and 38 males), Bhatia olivacea (11 females and 10 males), and Balclutha sp. nr. impicta (40 females and 25 males). DNA was extracted from individual insects using the phenol-chloroform method. Each sample was tested for the selected bacterial symbionts, BAMH and “Ca. Sulcia muelleri,” by using specific PCR as described above. The same specimens were also tested for SCWL phytoplasma by using specific nested PCR.
Nucleotide sequence accession numbers.
Representatives of the 16S rRNA bacterial genes sequenced from M. hiroglyphicus were submitted to the GenBank database; the accession numbers are JF836007 for BAMH and JQ898318 for “Ca. Sulcia muelleri” of M. hiroglyphicus.
RESULTS
16S rRNA bacterial gene sequencing and BLAST search.
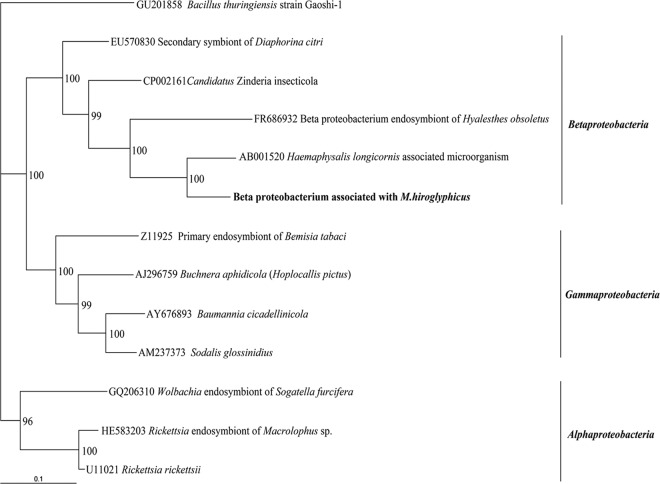
Our first experiment was to identify candidate bacterial symbionts in M. hiroglyphicus using nonculture methods. The DNA of 24 individual leafhoppers was extracted, and PCR was performed using universal primers for the bacterial 16S rRNA gene, followed by cloning, sequencing, and a BLAST search. Nucleotide sequencing was performed on 5 clones randomly picked from each leafhopper DNA template using the forward primer of the plasmid vector. Sequencing results were obtained from 116 clones, whereas 4 clones did not yield any sequence. The sequences were 500 to 750 bp long and separated into two main groups, based on the BLAST search. Results are presented in Table 2. The largest group was made up of 76 clones (66% of total sequenced clones): 36 clones from female leafhoppers and 40 clones from male leafhoppers. Sequences from this group showed low similarity, on average, 85%, with a tick (Haemaphysalis longicornis)-associated microorganism 16S rRNA gene (34) (GenBank accession no. AB001520; class Betaproteobacteria). These sequences were amplified from all 24 tested leafhoppers from different sugarcane fields. These data suggest that they are dominant and stably associated with M. hiroglyphicus. Because of the low similarity to the closest-matching sequence in the database, these 76 clones are likely to come from an unknown species that was named bacterium associated with M. hiroglyphicus (BAMH) in this paper. Almost the entire sequence of BAMH (1,428 bp) was obtained by sequencing the entire inserts with forward and reverse primers of their plasmid vector. A phylogenetic tree of BAMH and other selected bacterial symbionts from three different classes, including Alpha-, Beta-, and Gammaproteobacteria, was generated with the neighbor-joining method. The BAMH sequence was ordered in Betaproteobacteria with 99% bootstrap value support within the group and is most closely related to the bacterium associated with the tick H. longicornis with 100% bootstrap value support. Moreover, BAMH has a phylogenetic position close to the Betaproteobacteria, an endosymbiont of leafhopper (Hyalesthes obsoletus; GenBank accession no. FR686932 [17]) and “Candidatus Zinderia insecticola” (GenBank accession no. CP002161) living in spittlebug (Clastoptera arizonana) (27) (Fig. 1).
Table 2.
Sequencing and BLAST search results for 16S rRNA bacterial gene from leafhopper Matsumuratettix hiroglyphicus (Matsumura)
| No. of positive clones/total no. of clones | Most similar known bacterium | Class | % similarity | No. of positive leafhoppers/total no. tested |
|---|---|---|---|---|
| 76/116 | Bacterium associated with tick Hemaphysalis longicornis | Betaproteobacteria | 85 | 24/24 |
| 38/116 | “Candidatus Sulcia muelleri” | Flavobacteria | 98 | 24/24 |
| 1/116 | Burkholderia sp. | Betaproteobacteria | 99 | 1/24 |
| 1/116 | Rickettsia sp. | Alphaproteobacteria | 90 | 1/24 |
Fig 1.
Phylogenetic relationship of bacterium associated with M. hiroglyphicus with 11 other selected 16S rRNA bacterial gene sequences in the classes Alpha-, Beta-, and Gammaproteobacteria. Numbers at each node indicate the percentage of 1,000 bootstrap replications. Bacillus thuringiensis GU201858 was defined as the outgroup of the tree. Bar, 0.1 substitution per site.
The second group, comprising 38 sequenced clones (20 from females and 18 from males), had 98% similarity with “Ca. Sulcia muelleri” (GenBank accession no. DQ066645; phylum Bacteroidetes, class Flavobacteria [32]). It was reported to be a primary endosymbiont restricted to the bacteriome of insects in the suborder Auchenorrhyncha (32). This sequence was also found in all leafhoppers examined, indicating that it is a common endosymbiont of this leafhopper. The entire sequence (1,479 bp) was obtained with the forward and reverse primers of the plasmid vector. Phylogenetic relationship analysis showed that this sequence could be grouped into a branch of “Ca. Sulcia muelleri” of an insect host in the superfamily Membracoidea with 100% bootstrap support. The phylogenetic position of “Ca. Sulcia muelleri” of M. hiroglyphicus was close (100% bootstrap value support) to the positions of “Ca. Sulcia” of Ecultanus excultus (GenBank accession no. DQ066645) and an Ecultanus sp. (GenBank accession no. DQ066644) belonging to the order Cicadellidae (Fig. 2).
Fig 2.
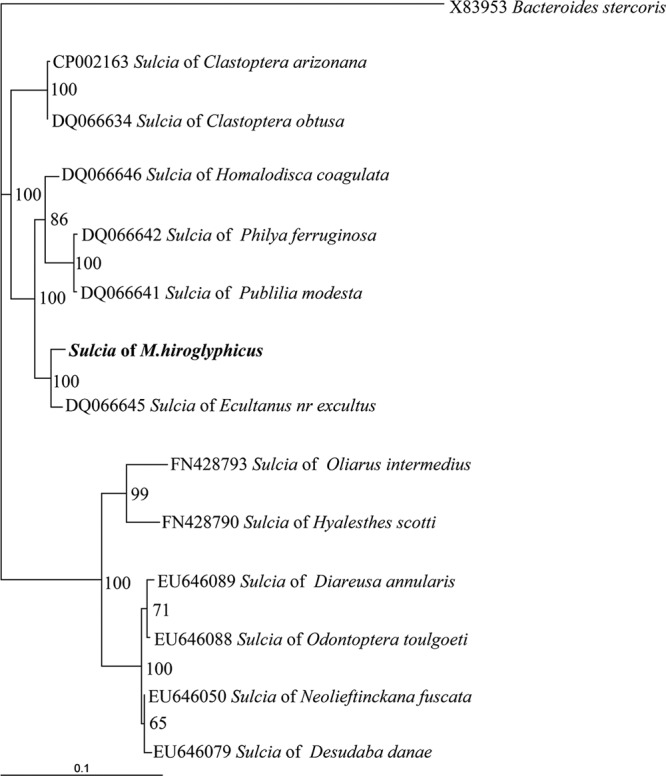
Phylogenetic relationship of “Ca. Sulcia muelleri” in M. hiroglyphicus and other selected “Ca. Sulcia muelleri” clones from three superfamilies of insect hosts. Numbers at each node indicate the percentage of 1,000 bootstrap replications. Bacteroides stercoris X83953 was defined as the outgroup of the tree. Bar, 0.1 substitution per site.
In addition, one sequence from a female leafhopper shared a 99% identity match with Burkholderia vietnamiensis (GenBank accession no. FJ436055), which is grouped in the Betaproteobacteria class. Also, one clone from a female leafhopper showed 90% similarity to a Rickettsia sp. (GenBank accession no. FN550103) belonging to the Alphaproteobacteria class.
Prevalence of BAMH, “Ca. Sulcia muelleri,” and SCWL phytoplasma.
Infection frequencies of two bacterial symbionts and the SCWL phytoplasma in individual leafhoppers collected from three different fields during 2 years were measured by PCR and nested PCR diagnosis. The results are presented in Table 3. BAMH was present in 95% (135 out of 142) of the females tested, while it was shown to be present in 92% (79 out of 86) of male leafhoppers. Tests were positive for “Ca. Sulcia muelleri” in 96% (137 out of 142) of female leafhoppers and 93% (80 out of 86) of male leafhoppers. The test for the SCWL phytoplasma in leafhoppers showed a positive result in an average of 61% of females (87 out of 142) and 52% of males (45 out of 86). In addition, 95% (83 out of 87) of female leafhoppers and 91% (41 out of 45) of male leafhoppers infected with SCWL phytoplasma were also infected with BAMH.
Table 3.
Infection frequency of BAMH, “Ca. Sulcia muelleri,” and SCWL phytoplasma in natural population of M. hiroglyphicus
| Yr | Locationa | No. testedb | No. positive |
|||
|---|---|---|---|---|---|---|
| BAMH | “Ca. Sulcia muelleri” | Phytoplasma | BAMH + phytoplasma | |||
| 2010 | Field 1 | 16 F/17 M | 16 F/15 M | 16 F/15 M | 9 F/14 M | 9 F/13 M |
| Field 2 | 21 F/11 M | 18 F/10 M | 19 F/11 M | 6 F/6 M | 5 F/6 M | |
| Field 3 | 20 F/10 M | 18 F/9 M | 20 F/8 M | 15 F/3 M | 13 F/3 M | |
| 2011 | Field 1 | 36 F/20 M | 35 F/20 M | 34 F/18 M | 25 F/8 M | 25 F/6 M |
| Field 2 | 23 F/14 M | 23 F/12 M | 23 F/14 M | 14 F/8 M | 14 F/7 M | |
| Field 3 | 26 F/14 M | 25 F/13 M | 25 F/14 M | 18 F/6 M | 17 F/6 M | |
| Total | 142 F/86 M | 135 F/79 M | 137 F/80 M | 87 F/45 M | 83 F/41 M | |
Fields 1 and 2 are located in Udonthani province, and field 3 is located in Khon Kaen province.
Number of individuals tested. F, female leafhopper; M, male leafhopper.
Localization of BAMH and “Ca. Sulcia muelleri.”
To determine the location of BAMH and “Ca. Sulcia muelleri” in leafhoppers, selected organs were dissected from female leafhoppers. DNA was extracted and analyzed by PCR using specific primers. BAMH and “Ca. Sulcia muelleri” were detected in all of the dissected organs except the salivary glands of the leafhopper. These two bacterial symbionts were present in the same dissected organs, and the percentage of infection was identical in bacteriomes (100%; 8/8) and in ovaries (88%; 7/8). BAMH was found in 50% of the guts and 50% of the fat bodies, which is slightly different from the rates of infection with “Ca. Sulcia muelleri,” which was found to be present at 88% in the guts and 75% in fat bodies (Table 4).
Table 4.
PCR assays of BAMH and “Ca. Sulcia muelleri” in dissected organs of leafhopper M. hiroglyphicus
| Organism | No. positive/no. testeda |
||||
|---|---|---|---|---|---|
| Gut | Salivary gland | Ovary | Fat body | Bacteriome | |
| BAMH | 4/8 | 0/8 | 7/8 | 4/8 | 8/8 |
| Ca. Sulcia | 7/8 | 0/8 | 7/8 | 6/8 | 8/8 |
All dissected organs are from female leafhoppers.
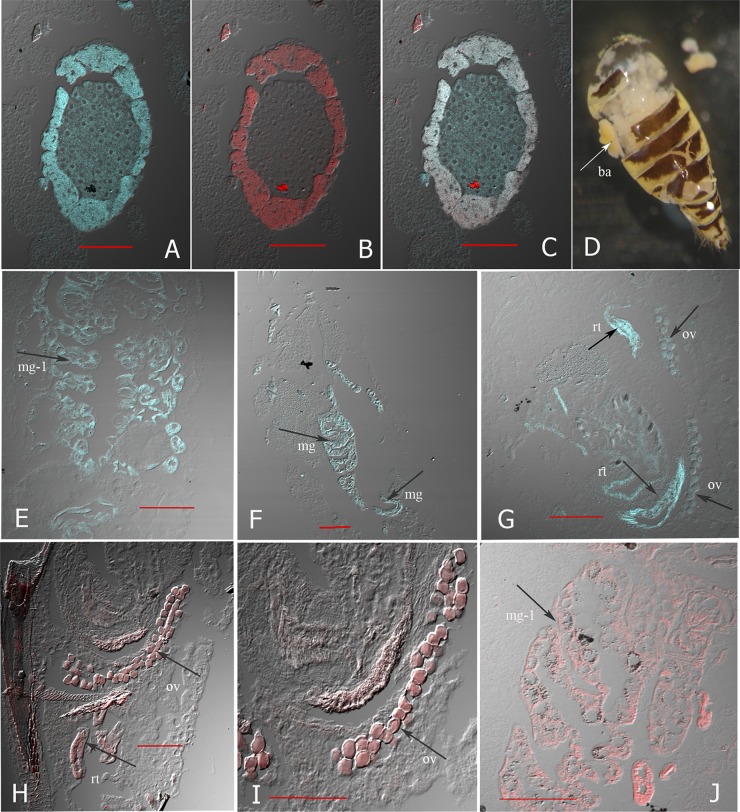
A more detailed localization of BAMH and “Ca. Sulcia muelleri” was revealed by the FISH experiment on tissue sections of adult females and fourth-instar nymphs. Oligonucleotide probe MH95 was designed to target the 16S rRNA gene sequences for BAMH, and a CBF319 probe specific for the 16S rRNA of Bacteroidetes species was used to detect “Ca. Sulcia muelleri” of M. hiroglyphicus. As shown in Fig. 3A to C, hybridization signals from both MH95- and CBF319-specific probes were found in the same bacteriome of the adult leafhopper. The MH95 signals were found all over the bacteriome (Fig. 3A), while the signal of the CBF319 probe for “Ca. Sulcia muelleri” was detected only at the margin of the bacteriome (Fig. 3B). There is colocalization of BAMH and “Ca. Sulcia muelleri” at the margin of the bacteriome (Fig. 3C). The bacteriome is located on each side of the abdomen. It is attached to the leafhopper cuticle and is egg shaped, 0.2 to 0.3 mm, and yellow (Fig. 3D).
Fig 3.
Localization of bacterial symbionts BAMH and “Ca. Sulcia muelleri” of M. hiroglyphicus in longitudinal tissue sections of the leafhopper. (A to C) FISH and confocal laser scanning microscopy image of bacteriome part; (A) hybridization signal of MH95 labeled with FAM (blue) specific for BAMH; (B) hybridization signals of CBF319 labeled with Cy5 (red) specific for “Ca. Sulcia”; (C) merged images of BAMH and “Ca. Sulcia” hybridization signals. (D) Photograph of bacteriome (ba) on each side of leafhopper abdomen. (E to G) The arrows indicate the signal of the MH95 probe specific for BAMH in anterior midgut (mg-1) of adult leafhopper (E), entire midgut (mg) of nymph leafhopper (F), and reproductive tissue (rt) and ovariole (ov) (G). (H and I) The arrows indicate the signal of the CBF319 probe specific for “Ca. Sulcia” in reproductive tissue and ovariole. (J) The arrow indicates the signal from the universal bacterial probe EUB338 (positive control) in anterior midgut (mg-1) of an adult leafhopper. Bars, 100 μm.
Hybridization signals of the MH95 probe were observed in other parts of nymphs and adults. They appeared in the entire midgut of the nymphs (Fig. 3F), whereas in the adults, they appeared only in the anterior midgut (Fig. 3E). The signals in the guts of both nymphs and adults were nonuniform but appeared close to the internal wall of the gut. The signals were also present in the abdominal area, near the reproductive tissues, and were present in parts of the ovarioles of female leafhoppers (Fig. 3G). No BAMH hybridization signals were detected in the salivary glands, thorax, or muscle of the leafhopper, consistent with the PCR results. The CBF319 probe for detection of the “Ca. Sulcia muelleri” signal was also found in reproductive tissues near the ovipositor, oviduct, and ovarioles (Fig. 3H and I). No hybridization signal with the universal probe EUB338 was found in the bacteriome area or the other parts that showed a signal with the probe for the two bacterial symbionts BAMH and “Ca. Sulcia muelleri,” since this probe does not match the 16S rRNA gene of BAMH and “Ca. Sulcia muelleri” of M. hiroglyphicus. However, a signal was obtained with the universal probe EUB338 in the anterior midgut of other leafhoppers that do not harbor BAMH (Fig. 3J). Moreover, no hybridization signals were obtained from tissues without the probe and sense-strand probe control.
Prevalence of BAMH in developmental stages of M. hiroglyphicus leafhoppers.
The leafhopper was reared on sugarcane plant stocks that tested negative for the presence of BAMH. Insects from each stage were collected, and DNA was extracted from single individuals. BAMH was detected in all developmental stages of the subsequent first and second generations by specific PCR. The results showed that BAMH was found in eggs, all nymph stages, and adults of the first generation. The rates of infection were 46% in eggs; 55%, 63%, 90%, 100%, and 100% in the first to fifth nymphal instars, respectively; and 100% in both female and male adults. Furthermore, BAMH was also found in all nymph stages of the second generation from the first to fifth nymphal instars (90%, 83%, 96%, 100%, and 100%, respectively) (Table 5).
Table 5.
Results of PCR amplification of DNA from BAMH in different developmental stages of the leafhopper M. hiroglyphicus
| Stage | No. of leafhoppers positive/no. tested (%) |
|
|---|---|---|
| First generation | Second generation | |
| Egg | 13/28 (46) | None |
| Nymph 1 | 24/44 (55) | 9/10 (90) |
| Nymph 2 | 21/33 (63) | 15/18 (83) |
| Nymph 3 | 36/40 (90) | 27/28 (96) |
| Nymph 4 | 29/29 (100) | 10/10 (100) |
| Nymph 5 | 26/26 (100) | 10/10 (100) |
| Female | 20/20 (100) | None |
| Male | 7/7 (100) | None |
Prevalence of BAMH, “Ca. Sulcia muelleri,” and SCWL phytoplasma in other leafhoppers.
We tested for the presence of two bacterial symbionts and the SCWL phytoplasma in different species of leafhoppers by specific PCR and nested PCR, respectively. As summarized in Table 6, BAMH was present in 2 out of 6 species tested. These were R. dorsalis (100% positive) and Recilia sp. nr. vetus (88% positive). On the other hand, “Ca. Sulcia muelleri” was present in all 6 species tested, with rates of infection ranging from 80 to 100%. The SCWL phytoplasma was found in all 6 species of leafhopper tested, with a variation in the rate of infection in each species ranging from 17 to 100%.
Table 6.
Results of PCR amplification of DNA from BAMH and “Ca. Sulcia muelleri” and nested PCR assays of SCWL phytoplasma in different species of leafhopper
| Leafhopper species | No. testeda | No. positive |
|||
|---|---|---|---|---|---|
| BAMH | “Ca. Sulcia muelleri” | Phytoplasma | BAMH+Phytoplasma | ||
| Recilia dorsalis | 18 F | 18 F | 18 F | 12 F | 12 F |
| Recilia sp. nr. vetus | 41 F | 36 F | 36 F | 28 F | 26 F |
| Exitianus indicus | 36 F/10 M | 0 F/0 M | 36 F/9 M | 16 F/8 M | 0 F/0 M |
| Bhatia olivacea | 11 F/10 M | 0 F/0 M | 11 F/10 M | 9 F/6 M | 0 F/0 M |
| Balclutha sp. nr. impicta | 40 F/25 M | 0 F/0 M | 38 F/23 M | 28 F/14 M | 0 F/0 M |
| Yamatotettix flavovittatus | 48 F/38 M | 0 F/0 M | 46 F/36 M | 43 F/31 M | 0 F/0 M |
Number of individuals tested. F, female leafhopper; M, male leafhopper.
DISCUSSION
We found two dominant bacterial species in the leafhopper M. hiroglyphicus. The largest group of bacterial clones was made up of 66% of total clones with no close match with any sequences in the databases. The low identity (85%) with the closest match, an uncharacterized bacterial symbiont of ticks (34), suggests that it is a new member of the Betaproteobacteria that has not been reported before and was named BAMH. “Ca. Sulcia muelleri” was found in 33% of all clones sequenced and belongs to the phylum Bacteroidetes. “Ca. Sulcia muelleri” is an evolutionarily ancient endosymbiont of sap-feeding insects and plays an important role in the hosts by synthesizing essential amino acids missing from their food sources (26, 27, 30, 32, 47). It has been reported to be the primary endosymbiont, is located inside the bacteriome, and is often associated with another type of bacterial symbiont of insects in the suborder Auchenorrhyncha, such as cicadas, planthoppers, and leafhoppers. For instance, “Ca. Sulcia muelleri” and the gammaproteobacterium “Ca. Baumannia cicadellinicola” are colocalized in the bacteriome of sharpshooter (31, 32), “Ca. Sulcia” is associated with the alphaproteobacterium “Ca. Hodkinia cicadicola” of cicadas (28), and “Ca. Sulcia muelleri” was reported to be associated with the betaproteobacterium “Ca. Zinderia insetticola” in the bacteriome of spittlebugs (27). One exception is “Ca. Sulcia muelleri” in the leafhopper Hyalesthes obsoletus, which was observed in organs like guts, salivary glands, and female and male gonads (17). Our approach was to identify the most prevalent bacterial species associated with the insects. No plant-associated bacteria were found. If we had sequenced a larger number of clones, we could have identified additional bacterial species, including plant bacteria. The clones in each group of BAMH and “Ca. Sulcia muelleri” showed high similarity (97% to 100%) but were not identical. These minor differences in nucleotide sequences may reflect population-to-population variation. However, the variation of nucleotides could not separate these sequences into different groups.
Our findings showed that BAMH and “Ca. Sulcia muelleri” bacteria were found at high infection rates and similar prevalences in natural populations of M. hiroglyphicus. PCR assays found BAMH to be coprevalent with “Ca. Sulcia muelleri” in a variety of tissues, including fat bodies, ovaries, intestines, and bacteriomes, in the same individual leafhoppers. With FISH experiments, BAMH and “Ca. Sulcia muelleri” were found to be colocalized in the same bacteriome. We did not observe the coexistence of BAMH and “Ca. Sulcia muelleri” in other parts of the body. However, the ovariole was stained by the MH95 or the CBF319 probe, but not in the same leafhopper.
BAMH was found in all the developmental stages, including eggs, nymphs, and adults of the first and second generations of leafhoppers that were reared on BAMH-free sugarcane plant stocks, suggesting that it is transmitted vertically in the same way as the SCWL phytoplasma (18). BAHM was found in all individuals tested, from nymphal instars 4 and 5 to adults, but in only a fraction of the eggs and nymphal instars 1 to 3 tested. The lack of detection in all early-stage individuals may be due to the sensitivity of the method, as very little material was obtained from individual eggs and early stages of nymphal development. These data indicated a strong association between BAMH and the leafhopper M. hiroglyphicus. An alternative explanation could be horizontal transmission. However, BAMH is not transmitted from the insect host to sugarcane plants, because BAMH-free plants on which the insect was reared remained free of this bacterium. We believe that BAMH is a candidate bacterial symbiont of the leafhopper M. hiroglyphicus based on the following: (i) it is a predominant bacterium, representing 66% of the clones; (ii) it is associated with the “Ca. Sulcia muelleri” endosymbiont in the bacteriome; and (iii) it is highly prevalent in the leafhopper population and passed from generation to generation.
The role of BAMH and its beneficial effect on the leafhopper host remain to be described. BAMH resides in the bacteriome, suggesting that it is an obligate primary endosymbiont. It is present throughout the life cycle of the leafhopper, suggesting that BAMH is essential for leafhopper growth or part of growth development. In addition to the bacteriome, BAMH is also found in the midgut. It is conceivable that at this location the symbiont provides an essential nutrient to the host, and therefore, interfering with BAMH would impact the viability of the insect. It has been reported that primary endosymbionts which cannot be cultured outside the host are necessary for its normal growth and reproduction (1, 4, 11, 12, 16, 42). In addition, some reports showed that intracellular bacteria can increase the heat tolerance of their host (29, 40) or provide the host with resistance to parasitoid wasps (35, 36). In our case, we speculate that BAMH may provide some benefit or play an important role in the biology of specific hosts.
To determine if BAMH is specific for the leafhopper M. hiroglyphicus, we investigated its presence in 6 other species of leafhoppers in the order Cicadellidae. We found BAMH in Recilia dorsalis and Recilia sp. nr. vetus but not in Exitianus indicus, Yamatotettix flavovittatus, Bhatia olivacea, or Balclutha sp. nr. impicta. Interestingly, two of these insect species (Y. flavovittatus and R. dorsalis) are vectors that transmit phytoplasma to sugarcane and rice, respectively (19, 20, 21, 38), whereas three of the four species that do not carry it are not vectors for phytoplasma. “Ca. Sulcia muelleri” was found in all species tested, including the nonvector species, whereas BAMH appears to be restricted to vector hosts. Therefore, it constitutes a more specific potential target for control.
It is tempting to speculate that BAMH or a related species is required for effective transmission of phytoplasma from insect to plant. The one insect species, Y. flavovittatus, which is a known vector for sugarcane white leaf phytoplasma and does not carry BAMH, may carry another related bacterial species. Further research is required to elucidate the precise role of BAMH in the insect vectors. If it is confirmed that BAMH is essential for host growth or for transmission of the sugarcane white leaf phytoplasma, this may lead to novel approaches for sugarcane white leaf disease control.
ACKNOWLEDGMENTS
This research is supported by the Royal Golden Jubilee Ph.D. program, grant no. PHD/0073/2549, of the Thailand Research Fund and the Research Center of Agricultural Biotechnology for Sustainable Economy, Khon Kaen University, Thailand.
Footnotes
Published ahead of print 13 July 2012
REFERENCES
- 1. Aksoy S. 1995. Wigglesworthia gen. nov. and Wiggleswothia glossinidia sp. nov., taxa consisting of the mycetocyte-associated, primary endosymbionts of tsetse flies. Int. J. Syst. Bacteriol. 45:848–851 [DOI] [PubMed] [Google Scholar]
- 2. Amann RI, et al. 1990. Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl. Environ. Microbiol. 56:1919–1925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ausubel FM, et al. 1994. Current protocols in molecular biology. John Wiley & Sons, Inc., New York, NY [Google Scholar]
- 4. Baumann P. 2005. Biology of bacteriocyte-associated endosymbionts of plant sap-sucking insect. Annu. Rev. Microbiol. 59:155–189 [DOI] [PubMed] [Google Scholar]
- 5. Beard CB, Mason PW, Aksoy S, Tesh RB, Richards FF. 1992. Transformation of an insect symbiont and expression of a foreign gene in the Chagas disease vector Rhodnius prolixus. Am. J. Trop. Med. Hyg. 46:195–200 [DOI] [PubMed] [Google Scholar]
- 6. Beard CB, Durvasula RV, Richards FF. 1998. Bacterial symbiosis in arthropods and the control of disease transmission. Emerg. Infect. Dis. 4:581–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Beard BC, Rosales CC, Durvasula RV. 2002. Bacterial symbiont of the Triatominae and their potential use in control of Chagas disease transmission. Annu. Rev. Entomol. 47:123–141 [DOI] [PubMed] [Google Scholar]
- 8. Bextine B, Lauzon C, Potter S, Lampe D, Miller TA. 2004. Delivery of a genetically marked Alcaligenes sp. to the glass-winged sharpshooter for use in paratransgenic control strategy. Curr. Microbiol. 48:327–331 [DOI] [PubMed] [Google Scholar]
- 9. Chen CT. 1974. Sugarcane white leaf disease in Thailand and Taiwan. Sugarcane Pathologists' Newsl. 11/12:23 [Google Scholar]
- 10. Crotti E, et al. 2009. Asaia, a versatile acetic acid bacterial symbiont, capable of cross-colonizing insects of phylogenetically distant genera and orders. Environ. Microbiol. 11:3252–3264 [DOI] [PubMed] [Google Scholar]
- 11. Douglas AE. 1989. Mycetocyte symbiosis in insects. Biol. Rev. Camb. Philos. Soc. 64:409–434 [DOI] [PubMed] [Google Scholar]
- 12. Douglas AE. 1998. Nutritional interactions in insect-microbial symbioses: aphids and their symbiotic bacteria Bunchnera. Annu. Rev. Entomol. 43:17–37 [DOI] [PubMed] [Google Scholar]
- 13. Durvasula RV, et al. 1997. Prevention of insect-borne disease: an approach using transgenic symbiotic bacteria. Proc. Natl. Acad. Sci. U. S. A. 94:3274–3278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Favia G, et al. 2007. Bacteria of the genus Asaia stably associate with Anopheles stephensi, an Asian malarial mosquito vector. Proc. Natl. Acad. Sci. U. S. A. 104:9047–9051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Felsenstein J. 2004. PHYLIP (Phylogeny Inference Package) version 3.6. Distributed by the author. Department of Genome Sciences, University of Washington, Seattle, WA [Google Scholar]
- 16. Gil R, Latorre A, Moya A. 2004. Bacterial endosymbionts of insects: insights from comparative genomics. Environ. Microbiol. 6:1109–1122 [DOI] [PubMed] [Google Scholar]
- 17. Gonella E, et al. 2011. Bacterial endosymbiont localization in Hyalesthes obsoletus, the insect vector of Bois noir in Vitis vinifera. Appl. Environ. Microbiol. 77:1423–1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hanboonsong Y, Choosai C, Panyim S, Damark S. 2002. Transovarial transmission of sugarcane white leaf phytoplasma in the insect vector Matsumuratettix hiroglyphicus (Matsumura). Insect Mol. Biol. 11:97–103 [DOI] [PubMed] [Google Scholar]
- 19. Hanboonsong Y, Ritthison W, Choosai C, Sirithorn P. 2006. Transmission of sugarcane white leaf phytoplasma by Yamatotettix flavovittatus, a new leafhopper vector. J. Econ. Entomol. 99:1531–1537 [DOI] [PubMed] [Google Scholar]
- 20. Hibino H, Jonson GB, Cruz FC. 1987. Association of mycoplasma like organisms with rice orange leaf in the Philippines. Plant Dis. 71:792–794 [Google Scholar]
- 21. Jung HY, et al. 2003. “Candidatus Phytoplasma oryzae”, a novel phytoplasma taxon associated with rice yellow dwarf disease. Int. J. Syst. Evol. Microbiol. 53:1925–1929 [DOI] [PubMed] [Google Scholar]
- 22. Koga R, Tsuchida T, Fukatsu T. 2009. Quenching autofluorescence of insect tissues for in situ detection of endosymbionts. Appl. Entomol. Zool. 44:281–291 [Google Scholar]
- 23. Lee I-M, Rindal DEG, Davis RE, Bartoszyk IM. 1998. Revised classification scheme of phytoplasmas based on RFLP analysis of 16S rRNA and ribosomal protein gene sequences. Int. J. Syst. Bacteriol. 48:1153–1169 [DOI] [PubMed] [Google Scholar]
- 24. Lee IM, Davis RE, Gundersen DE. 2000. Phytoplasma: phytopathogenic mollicutes. Annu. Rev. Microbiol. 54:221–255 [DOI] [PubMed] [Google Scholar]
- 25. Matsumoto T, Lee CS, Teng WS. 1968. Studies on sugarcane white leaf disease of Taiwan with special reference to transmission by a leafhopper, Epitetti hiroglyphicus Mats. ProSoc Sugarcane Technol. 13:1090–1099 [Google Scholar]
- 26. McCutcheon JP, Moran NA. 2007. Parallel genomic evolution and metabolic interdependence in an ancient symbiosis. Proc. Natl. Acad. Sci. U. S. A. 104:19392–19397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McCutcheon JP, Moran NA. 2010. Functional convergence in reduced genomes of bacterial symbionts spanning 200 My of evolution. Genome Biol. Evol. 2:708–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McCutcheon JP, McDonald BR, Moran NA. 2009. Origin of an alternative genetic code in the extremely small and GC-rich genome of a bacterial symbiont. PLoS Genet. 5:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Montllor CB, Maxmen A, Purcell AH. 2002. Facultative bacterial endosymbionts benefit pea aphids Acyrthosiphon pisum under heat stress. Ecol. Entomol. 27:189–195 [Google Scholar]
- 30. Moran NA. 2007. Symbiosis as an adaptive process and source of phenotypic complexity. Proc. Natl. Acad. Sci. U. S. A. 104:8627–8633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Moran NA, Dale C, Dunbar H, Smith WA, Ochman H. 2003. Intracellular symbionts of sharpshooters (Insecta: Hemiptera: Cicadellinae) form a distinct clade with a small genome. Environ. Microbiol. 5:116–126 [DOI] [PubMed] [Google Scholar]
- 32. Moran NA, Tran P, Gerardo NM. 2005. Symbiosis and insect diversification: an ancient symbiont of sap-feeding insects from the bacterial phylum Bacteroidetes. Appl. Environ. Microbiol. 71:8802–8810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nakashima K, Chaleeprom W, Wongkaew P, Sirithorn P. 1994. Detection of mycoplasma-like organisms associated with white leaf disease of sugarcane in Thailand using DNA probes. Jpn. Int. Res. Ctr. Agric. Sci. J. 1:57–67 [Google Scholar]
- 34. Noda H, Munderloh UG, Kurtti TJ. 1997. Endosymbionts of ticks and their relationship to Wolbachia spp. and tick-borne pathogens of humans and animals. Appl. Environ. Microbiol. 63:3926–3932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Oliver KM, Russell JA, Moran NA, Hunter MS. 2003. Facultative bacterial symbionts in aphids confer resistance to parasitic wasps. Proc. Natl. Acad. Sci. U. S. A. 100:1803–1807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Oliver KM, Moran NA, Hunter MS. 2006. Costs and benefits of a superinfection of facultative symbionts in aphids. Proc. Biol. Sci. 273:1273–1280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Page RDM. 1996. TREEVIEW: an application to display phylogenetic trees on personal computers. Comput. Appl. Biosci. 12:357–358 [DOI] [PubMed] [Google Scholar]
- 38. Rivera CT, Ou S, Pathak MD. 1963. Transmission studies of the orange leaf disease of rice. Plant Dis. Rep. 47:1045–1048 [Google Scholar]
- 39. Ruang-Areerate T, Kittayapong P. 2006. Wolbachia transinfection in Aedes aegypti: a potential gene driver of dengue vectors. Proc. Natl. Acad. Sci. U. S. A. 103:12534–12539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Russell JA, Moran NA. 2006. Costs and benefits of symbiont infection in aphids: variation among symbionts and across temperatures. Proc. Biol. Sci. 273:603–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Stouthamer R, Breeuwer JA, Hurst GD. 1999. Wolbachia pipientis: microbial manipulator of arthropod reproduction. Annu. Rev. Microbiol. 53:71–102 [DOI] [PubMed] [Google Scholar]
- 42. Thao ML, et al. 2000. Cospeciation of psyllids and their primary prokaryotic endosymbionts. Appl. Environ. Microbiol. 66:2898–2905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. 1997. The CLUSTAL_X Windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25:4876–4882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Weintraub PG. 2007. Insect vectors of phytoplasmas and their control—an update. Bull. Insectol. 60:169–173 [Google Scholar]
- 45. Weisburg WG, Barns SM, Pelletier DA, Lane DJ. 1991. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 173:697–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wongkaew P, et al. 1997. Differentiation of phytoplasmas associated with sugarcane and gramineous weed white leaf disease and sugarcane grassy shoot disease by RFLP and sequencing. Theor. Appl. Genet. 95:660–663 [Google Scholar]
- 47. Wu D, et al. 2006. Metabolic complementarity and genomics of the dual bacterial symbiosis of sharpshooter. PLoS Biol. 4:1079–1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zabalou S, et al. 2004. Wolbachia-induced cytoplasmic incompatibility as a means for insect pest population control. Proc. Natl. Acad. Sci. U. S. A. 101:15042–15045 [DOI] [PMC free article] [PubMed] [Google Scholar]