Abstract
Wolbachia, a maternally transmitted endosymbiont of insects, is increasingly being seen as an effective biological control agent that can interfere with transmission of pathogens, including dengue virus. However, the mechanism of antiviral protection is not well understood. The density and distribution of Wolbachia in host tissues have been implicated as contributing factors by previous studies with both mosquitoes and flies. Drosophila flies infected with five diverse strains of Wolbachia were screened for the ability to mediate antiviral protection. The three protective Wolbachia strains were more closely related and occurred at a higher density within whole flies than the two nonprotective Wolbachia strains. In this study, to further investigate the relationship between whole-fly Wolbachia density and the ability to mediate antiviral protection, tetracycline was used to decrease the abundance of the high-density, protective Wolbachia strain wAu prior to viral challenge. Antiviral protection was lost when the density of the protective Wolbachia strain was decreased to an abundance similar to that of nonprotective Wolbachia strains. We determined the Wolbachia density and distribution in tissues of the same five fly-Wolbachia combinations as used previously. The Wolbachia density within the head, gut, and Malpighian tubules correlated with the ability to mediate antiviral protection. These findings may facilitate the development of Wolbachia biological control strategies and help to predict host-Wolbachia pairings that may interfere with virus-induced pathology.
INTRODUCTION
Wolbachia, an intracellular endosymbiont of insects, is increasingly being seen as an effective biological control agent that can interfere with the transmission of pathogens that are vectored by insects (7, 13, 14, 19, 23, 34, 38, 42, 43). Wolbachia is a maternally transmitted bacterium which is predicted to infect up to 70% of insect species (17, 24, 45). Wolbachia bacteria are best known for their ability to manipulate host reproductive systems, leading to an increase in their frequency within insect populations over successive generations (20, 35, 40, 44). Wolbachia infection can also reduce the ability of insects to transmit viruses such as dengue and Chikungunya viruses and can protect the insect against viral, bacterial, and protozoan parasites (2, 12, 15, 22, 26, 27, 34, 36, 39). However, the mechanism by which Wolbachia protects against pathogens is not well understood. A better understanding of endosymbiont characteristics and antipathogen protection may facilitate the development of improved strategies for use of Wolbachia as a biocontrol agent.
Wolbachia endosymbiosis has been well characterized within the model organism Drosophila, in which protection against viral challenge has been observed (15, 36, 39). Wolbachia-mediated antiviral protection has been described to occur in Drosophila melanogaster and Drosophila simulans, flies which exhibit delayed mortality when challenged with RNA viruses, including Drosophila C virus (DCV), cricket paralysis virus, Flock House virus, and Nora virus (15, 36, 39). In some Wolbachia-host combinations, a delay in the accumulation of viral particles was observed concurrently with a delay in mortality (15, 36, 39). Interestingly, some Wolbachia-containing flies, such as the D. simulans CO line which contains the Wolbachia strain wAu (CO-wAu), were better able to tolerate high titers of virus than Wolbachia-free flies (36). It is not yet understood how the presence of Wolbachia delays virus accumulation or how it contributes to host tolerance of viral infection. Furthermore, not all Wolbachia-fly pairings are protected against virus-induced mortality (29, 36). When five D. simulans-Wolbachia combinations were screened for antiviral protection by viral challenge, three combinations were protected (CO-wAu, Me29-wMel, and DSR-wRi), whereas the other two were not (DSH-wHa and N7NO-wNo) (36). Notably, when the same fly combinations were challenged with mortality-inducing bacteria, including Erwinia carotovora, none of the Wolbachia strains mediated antibacterial protection (47). The difference in response between antiviral and antibacterial protection in the same fly-Wolbachia combination indicates that the Wolbachia-mediated mechanism of antiviral protection differs from the mechanism of antibacterial protection (47).
There are a number of Wolbachia-virus, or Wolbachia-host-virus, interactions that could result in antiviral protection. Examples of these mechanisms could include competition between Wolbachia and virus for a shared resource, steric hindrance between Wolbachia and the virus within the internal cellular environment, altered cell membrane characteristics, or altered cell behaviors such as viral uptake. Limited data are available on the influence of Wolbachia on these factors. If these factors contribute to host susceptibility to virus, the abundance of Wolbachia would likely impact the ability to mediate antiviral protection. Wolbachia density may be important at a cellular level, at the tissue level, or within the whole host organism (10, 34, 36). For example, particular tissues may play a role in the host's viral response or be relevant to viral pathogenesis. If viral pathogenesis relies on the infection of a particular cell or tissue type and Wolbachia can limit or modulate viral infection within that tissue, the outcome of virus-induced disease may be modified. Although the majority of Wolbachia distribution studies have focused on the reproductive organs, Wolbachia has also been identified in a number of somatic tissues across insect species (4, 5, 8, 11, 32–34, 42). Previous studies used different Wolbachia strains, host backgrounds, and techniques to identify Wolbachia in tissue types (2, 4, 5, 8, 34). Intrinsic host and Wolbachia strain factors have been shown to alter Wolbachia distribution within tissues (8, 41). To our knowledge, no study has yet compared Wolbachia tissue distribution and density in natural host-Wolbachia combinations to identify tissues of potential importance in Wolbachia-mediated antiviral protection.
Data from previous studies suggest that Wolbachia density and distribution may be important for antiviral protection to occur (10, 34, 36). When diverse strains of Wolbachia in D. simulans host backgrounds were screened for the ability to mediate antiviral protection, a correlation was identified between Wolbachia density in whole flies and the ability to mediate antiviral protection. The Wolbachia strains that mediated antiviral protection (wAu, wMe29, and wRi) occurred at a higher abundance within individual flies and were more closely related than the Wolbachia strains that did not mediate antiviral protection (wHa and wNo) (36, 48). The importance of Wolbachia density has also been implicated in mosquito and mosquito cell line studies (10, 34). This study investigated the importance of Wolbachia density and distribution within the host on the ability of the strain to mediate antiviral protection across the five fly-Wolbachia combinations. Then the abundance of the protective wAu strain was decreased and flies were challenged with the mortality-inducing DCV to determine if Wolbachia abundance is an important factor in antiviral protection.
MATERIALS AND METHODS
Drosophila and Wolbachia.
Five D. simulans lines (CO, Me29, DSR, N7NO, and DSH) were maintained at 25°C with a 12-h light-dark cycle, on standard cornmeal agar media as previously described (36). For each fly line, paired populations of flies were used that either contained a Wolbachia strain (CO-wAu, Me29-wMel, DSR-wRi, N7NO-wNO, and DSH-wHa) or had been previously cured of Wolbachia (CO-T, Me29-T, DSR-T, N7NO-T, and DSH-T) by tetracycline treatment (21, 36). As tetracycline treatment may impact the fly gut microbiota, following tetracycline treatment the flies were placed on food that had been exposed to untreated male flies. With the exception of Me29-wMel, natural fly-Wolbachia combinations were used (18, 21, 31, 35). Me29 had previously been transinfected with the D. melanogaster Wolbachia strain wMel, by microinjection of infected cytoplasm from D. melanogaster Wein 5 embryos into Me29 NHaTC embryos (37).
Preparation of Drosophila histological sections.
Wings and legs were removed from 4- to 7-day-old male anesthetized flies, and insects were preserved in 4% paraformaldehyde plus 0.5% Triton X-100 at 4°C for at least 2 days. Flies were dehydrated in 50% ethanol for 3 h, then 70% ethanol overnight, 90% ethanol for 1 h, and 95% ethanol for 1 h, and rinsed twice in 100% ethanol for 30 min. A 15-min rinse in toluene, followed by overnight storage in cedar wood oil and a 10-min rinse in 1:1 toluene-cedar wood oil, was used to improve wax infiltration. Samples were submerged in paraffin wax (Paraplast-Xtra; McCormick Scientific) at 60°C for 30 min and then twice for 15 min under vacuum, with 5 to 15 insects per wax mold. A rotary microtome was used to obtain 8-μm-thick sections that were adhered to Super Frost Plus slides (Menzel-Gläser). After a minimum of 24 h, slides were deparaffinated by three 3-min xylene washes and hydrated with a series of ethanol washes: 100% ethanol twice for 3 min, 90% ethanol for 3 min, 70% ethanol for 3 min, distilled water for 5 min, and two dips in distilled water. Slides were air dried and stored at room temperature until use.
Fluorescent in situ hybridization (FISH).
Histological slides of flies with and without Wolbachia were treated with hybridization buffer which contained a final concentration of 4× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), 50% (vol/vol) deionized formamide, 0.2 g/ml dextran sulfate, 0.25 mg/ml salmon sperm DNA, 0.25 mg/ml poly(A), 0.25 mg/ml tRNA, 0.1 M dithiothreitol (DTT), and 0.5× Denhardt's solution. Two oligonucleotide probes labeled at the 3′ end with rhodamine were used to target the 16S rRNA region of the Wolbachia species (34). BLAST (NCBI) alignment on the five Wolbachia strains indicated that both probes (5′-CTTCTGTGAGTACCGTCATTATC-3′ and 5′-AACCGACCCTATCCCTTCGAATA-3′) were 100% homologous to all the strains used in this study. Slides were treated with 200 μl of hybridization solution containing a final concentration of 15 nM for both probes and incubated overnight at 37°C. Slides were washed once in 1× SSC and 10 mM DTT for 10 min, twice in 1× SSC and 10 mM DTT at 55°C for 15 min, twice in 0.5× SSC and 10 mM DTT at 55°C for 15 min, once in 0.5× SSC and 10 mM DTT containing 1 pg/ml 4′,6-diamidino-2-phenylindole (DAPI) for 10 min, and then in 0.5× SSC and 10 mM DTT for 10 min. Slides were rinsed in water, allowed to air dry, and mounted with 50 μl of the antifade reagent ProLong (Invitrogen).
Fluorescent labels were visualized using an epifluorescence microscope (Zeiss Axio Imager A.1) and stimulated by a 100-W high-pressure mercury lamp (HBO100) (Zeiss). Red and blue filters (Zeiss) were used to differentiate between DAPI fluorescence (emission, 461 nm) and rhodamine fluorescence (emission, 603 nm). The software program AxioVision, release 4.6.3, was used to record images. By necessity, the length of image exposure differs across tissue sets due to the amount of autofluorescence in different tissues and the magnification used. Exposure and all other microscope settings were kept constant between images of control and Wolbachia-containing flies. As a consequence, images can be compared only within the control-test set and not directly across different tissues or across different fly lines. Autofluorescence of fly tissues was observed in the Wolbachia-free negative-control flies and differed between tissues types. Fluorescence was attributed to Wolbachia presence when surplus to the amount of background fluorescence observed in Wolbachia-free negative-control flies. Both punctate and diffuse fluorescence was interpreted as evidence of Wolbachia, as punctate staining became diffuse when the focal plane was adjusted throughout tissue sections (data not shown). Each image presented is representative of results observed in multiple sections of at least four individual flies.
Tissue dissection.
To dissect tissues, male adult 4- to 7-day-old flies were anesthetized using CO2, chilled, and beheaded to enable dissection. To assist with tissue isolation and to minimize potential contamination between tissues or by hemolymph, flies were dissected submerged in phosphate-buffered saline (PBS) and visually inspected for purity of tissue. Forceps were used to isolate fly heads and gut (including crop), Malpighian tubules, testes, thoracic ganglia, accessory glands, fat body, or muscle tissue. To increase the amount of starting material, isolated tissues were pooled from five individuals for DNA extraction and immediately snap-frozen in a dry ice and ethanol bath. This was repeated across three separate biological cohorts. DNA was extracted from all tissues on the same day as dissection as described below. Wolbachia-free flies were used for negative controls.
DNA extraction.
To extract DNA from dissected tissues, samples were homogenized in 150 μl of chilled PBS for 90 to 180 s, using two 3-mm glass beads (Sigma-Aldrich) and a Mini Beadbeater-96 (Biospec Products). The QIAamp Viral RNA minikit (Qiagen) was used to extract DNA as per the manufacturer's protocol, with the following adjustments. A centrifuge speed of 14,000 rpm was used at each centrifuge step, and samples were eluted and stored in DNase-free water. As the extraction protocol involves the use of carrier RNA, quantitation of DNA was not performed. Quantitative PCR (qPCR) was used to determine the abundance of the Wolbachia surface protein gene (wsp) (NC_0124161.1) relative to the D. simulans reference gene, Actin 5C (NC_004354.3) (36).
Quantitative PCR.
Quantitative PCR was used to determine the abundance of Wolbachia in a selection of fly tissues. Platinum SYBR green qPCR SuperMix-UDG (Invitrogen) was used as per the manufacturer's protocol, at halved quantities that resulted in 10-μl reaction mixtures that contained 2 μl of DNA for relative Wolbachia quantitation. Two technical replicates were performed for each sample. If the standard deviation between technical replicate takeoff values was greater than 0.5, the samples were retested. Primer sequences correspond to regions of the D. simulans reference gene Actin 5C and the Wolbachia wsp gene (36). The Actin 5C primer sequences used were 5′-GACGAAGAAGTTGCTGCTCTGGTTG-3′ and 5′-TGAGGATACCACGCTTGCTCTGC-3′, while the wsp primer sequences used were 5′-GCATTTGGTTAYAAAATGGACGA-3′ and 5′-GGAGTGATAGGCATATCTTCAAT-3′ (36). Negative controls included template-free qPCRs and the processing of Wolbachia-free flies. Positive controls for the reference gene and Wolbachia were included in each qPCR run. The Rotor-Gene 6000 thermal cycler (Corbett Life Sciences, Qiagen) was used with the following profile: 95°C for 2 min, 50°C for 2 min, and 40 cyclic repeats of 95°C for 10 s, 52°C for 10 s, and 72°C for 20 s. This was followed by a standard melt analysis to confirm that only the expected product had been amplified. The abundance of Wolbachia relative to the host reference gene was determined using qGENE software. To determine if a correlation existed between Wolbachia density and the ability of the strain to mediate protection, a fit model analysis was performed. This was done using the statistical software JMP 8.0.2.2 (SAS), with protection as a fixed variable and strains with protection as a nested variable. A P value of <0.01 was interpreted as statistically significant. Graphical representation was performed using GraphPadPrism 5.0a, and standard error is shown.
Tetracycline treatment to decrease wAu density.
The highly abundant, protective Wolbachia strain wAu was selectively decreased in CO flies by treatment with tetracycline. Flies were allowed to lay eggs on normal fly media which contained tetracycline at 0.3 μg/ml, 3 μg/ml, 9 μg/ml, 27 μg/ml, 81 μg/ml, or 300 μg/ml or no tetracycline (21). Hatched larvae fed on the tetracycline-treated media were removed onto fresh tetracycline-free fly medium as 0- to 3-day-old adults. Individual male flies were collected as 4- to 7-day-olds, and DNA was extracted as per the above protocol before qPCR was used to determine Wolbachia abundance in individual flies.
Survival assays.
After the flies were treated with tetracycline to decrease Wolbachia organisms to desired densities, they were challenged with virus and survival was monitored. Treatment and viral challenge of Wolbachia-free CO flies with 3 μg/ml, 27 μg/ml, and 300 μg/ml or without tetracycline was performed to confirm that antibiotic treatment did not alter survival following viral challenge. Purified DCV isolate EB suspended in Tris (pH 7.4) (16, 25) was maintained at −20°C in aliquots which were used once and then discarded to prevent loss of virus through repeated freeze-thawing. Virus titer was determined by 50% tissue culture infectious dose (TCID50) assay (16). Flies were infected by injection as previously described (36). Briefly, 5 × 103 infectious units of DCV in 50.6 nl was injected into the hemocoels of 4- to 7-day-old male flies. Negative-control flies were injected with 50.6 nl of PBS. Injections were performed using needles pulled from glass capillaries (Drummond Scientific) and a Nanoject II microinjector (Drummond Scientific). Fly survival was monitored daily, deaths within the first day were discounted as needlestick injury, and the data were normalized accordingly. The challenge assay was replicated using independent fly cohorts. Virus-induced mortality across cohorts is representative where similar Wolbachia densities had similar survival outcomes.
RESULTS
Wolbachia distribution in fly tissues.
Fluorescent in situ hybridization was used to qualitatively determine the presence or absence of Wolbachia in fly tissues across the five fly-Wolbachia pairings. Wolbachia was observed in many of the tissues studied, including the head, gut, Malpighian tubules, thoracic ganglion, fat body, and muscle. The Wolbachia distribution differed between the fly-Wolbachia pairings, and Wolbachia was not detected in the tissues of some fly lines (Table 1; see also Fig. S1 to S5 in the supplemental material). In the nonprotective N7NO flies, which have previously been shown to contain the lowest Wolbachia density of the flies studied (36), wNO was not identified by fluorescence microscopy in the fat body or muscle of the individual N7NO flies used in this study (Fig. S5). Wolbachia was observed in 40% of gut tissue and 20% of Malpighian tubules in the N7NO individuals studied (n = 5), indicating that variation in the distribution of detectable Wolbachia exists between individuals. Variation between individuals was also observed in DSR flies, in which Wolbachia was identified in the fat body of only 20% of the individuals studied (n = 5). Despite this, Wolbachia was consistently observed in the head and thoracic ganglion of N7NO flies and in the head, gut, thoracic ganglion, Malpighian tubules, and muscle of DSR flies. Wolbachia was consistently identified in each targeted tissue of the CO, Me29, and DSH flies in this study but was not homogenously distributed through different tissue types (Table 1 and Fig. S1 to S3).
Table 1.
Summary of Wolbachia distribution within fly tissues, across fly-Wolbachia combinations that do or do not mediate antiviral protection
| Tissue | % of flies in which Wolbachia was detected for indicated fly-Wolbachia combinationa |
||||
|---|---|---|---|---|---|
| Protective |
Nonprotective |
||||
| CO-wAu | Me29-wMel | DSR-wRi | DSH-wHa | N7NO-wNo | |
| Head | 100 (4) | 100 (5) | 100 (6) | 100 (5) | 100 (5) |
| Gut | 100 (4) | 100 (6) | 100 (6) | 100 (5) | 40 (5) |
| Malpighian tubules | 100 (4) | 100 (5) | 100 (6) | 100 (5) | 20 (5) |
| Thoracic ganglia | 100 (4) | 100 (4) | 100 (5) | 100 (5) | 100 (5) |
| Fat body | 100 (4) | 100 (6) | 20 (5) | 100 (5) | 0 (5) |
| Muscle | 100 (4) | 100 (5) | 100 (5) | 100 (5) | 0 (5) |
If all individual flies were observed to contain Wolbachia within the tissue of interest, a score of 100% was recorded. Where only some individual flies contained Wolbachia within the tissue of interest, the percentage of flies that contained Wolbachia was recorded. Numbers in parentheses indicate how many individual flies were observed. Percentages do not reflect the Wolbachiaquantity observed within tissues.
Although Wolbachia distribution was not ubiquitous across the five fly-Wolbachia combinations, the presence or absence of Wolbachia from a particular tissue did not correlate with the ability to mediate antiviral protection (Table 1). This was evident as Wolbachia distribution was observed within the same tissues in two protective (CO-wAu and Me29-wMel) and one nonprotective (DSH-wHa) fly-Wolbachia pairing. In these fly lines, Wolbachia was consistently identified in each of the tissues (the head, gut, Malpighian tubules, thoracic ganglion, fat body, and muscle) of each individual in the study (Table 1). This suggests that the presence or absence of Wolbachia in fly tissues is not sufficient to mediate antiviral protection.
Wolbachia density in fly tissues.
Although Wolbachia tissue distribution did not correlate with antiviral protection, a difference in intensity of fluorescence between fly lines was observed. Quantitative PCR was used to determine Wolbachia density in the head, gut, Malpighian tubules, testes, thoracic ganglion, accessory glands, fat body, and muscle dissected from protective and nonprotective fly lines. A correlation was identified between the Wolbachia density and the ability to mediate antiviral protection in three of the tissues studied.
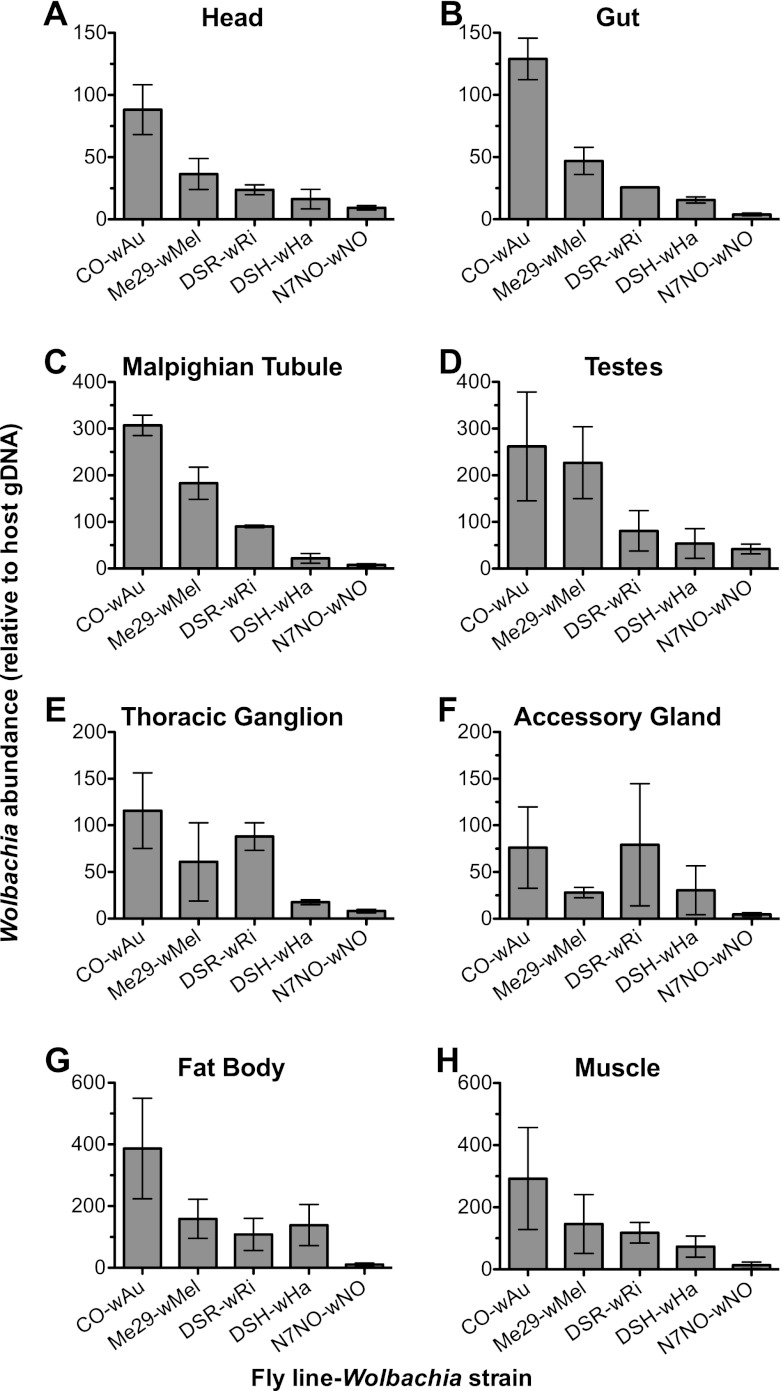
The tissue Wolbachia density across the five strains indicated that Wolbachia tropism differs between the fly-Wolbachia pairings (Fig. 1). For example, in the tissues of CO flies, the greatest mean abundance of Wolbachia (wAu) was identified in the fat tissue, followed by the Malpighian tubules, muscle, and testes, while lower levels of wAu were identified in other tissues. By comparison, DSR contained the most Wolbachia organisms (wRi) in the testes, and similar amounts of wRi organisms were found in the muscle, fat body, Malpighian tubules, thoracic ganglia, and accessory glands, with lower abundances observed in the remaining tissues. The trend of Wolbachia abundance that is seen in whole flies (with highest density in CO, then Me29, DSR, and DSH, and the lowest density in N7NO) was reflected in the head, gut, Malpighian tubules, testes, and muscle, but not in the thoracic ganglia, accessory glands, or fat bodies (Fig. 1).
Fig 1.
Relative Wolbachia abundance in tissues isolated from D. simulans flies. The graphs show the Wolbachia abundance relative to a host reference gene (Actin 5C) in dissected tissues as determined by qPCR. Tissues are the head (A), gut (B), Malpighian tubules (C), testes (D), thoracic ganglion (E), accessory glands (F), fat body (G), and muscle (H). Dissected tissues were pooled from five individual flies, and the experiment was repeated across three independent fly cohorts. The error bars represent standard errors. P values were interpreted as significant when <0.01. A correlation between the Wolbachia density within a tissue and the mediation of antiviral protection was observed with the head (P = 0.0052), gut (P = 0.0001), and Malpighian tubules (P = 0.0001) but not in the testes (P = 0.0430), thoracic ganglia (P = 0.0117), accessory glands (P = 0.2279), fat body (P = 0.1005), or muscle (P = 0.1055).
The Wolbachia density in tissues across the five fly-Wolbachia pairings was correlated with the ability of the strains to mediate antiviral protection. Using a fit model of analysis, with protection as a fixed variable and fly strain with protection as a nested variable, a statistically significant correlation was identified between the Wolbachia density and the ability of the strain to mediate antiviral protection in the head (P = 0.0052), gut (P = 0.0001), and Malpighian tubules (P = 0.0001). Of these, the Malpighian tubules appeared to have the largest biological difference between the abundance of Wolbachia organisms in strains that do and do not protect, followed by the gut and then the head. Although the testes and the thoracic ganglia (P = 0.043 and 0.012) could have been interpreted as significant if a less stringent test was used, the Wolbachia abundance varied greatly in each of these tissues. High biological variations were also observed in the tissues of some, but not all, fly lines. For example, there was high variation in the absolute Wolbachia abundance within the testes of CO flies but not within the testes of N7NO flies. When flies are challenged with DCV, the resulting response to viral challenge is very robust, with little variation between individuals. This suggests that tissues which exhibit high variability of Wolbachia tropism are not likely to be relevant in Wolbachia-mediated antiviral protection. Likewise, high levels of variation in Wolbachia abundance were seen in tissues that did not correlate with the ability to mediate antiviral protection (Fig. 1). The data from this study suggest that Wolbachia abundance within the head, gut, and Malpighian tubules may be relevant for antiviral protection.
Reduction of wAu density in CO flies leads to loss of antiviral protection.
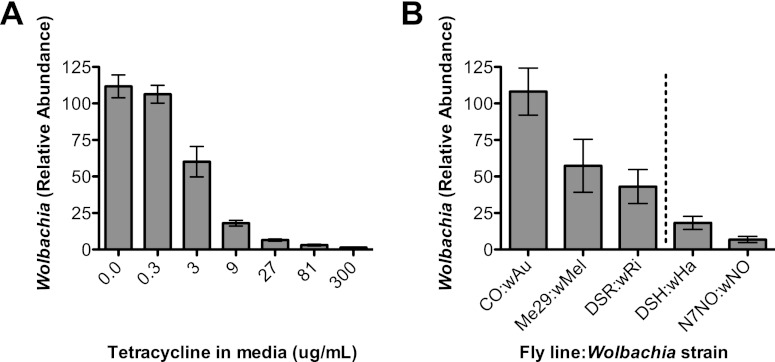
When CO flies containing the protective wAu strain were treated with increasing doses of tetracycline, the Wolbachia density in individual flies was decreased in a dose-dependent manner (nonlinear regression, R2 = 0.8525, correlation, P = 0.0014) (Fig. 2A). Results were consistent between biological replicates of three fly cohorts, and little variation was observed between individual flies. This indicated that Wolbachia could be reproducibly decreased in individual flies. The unaltered density of Wolbachia was also determined in all of the protective (CO-wAu, Me29-wMel, DSR-wRi) and nonprotective (DSH-wHa and N7NO-wNo) fly-Wolbachia combinations using qPCR. Wolbachia was most abundant in CO, followed by Me29, DSR, DSH, and then N7NO (Fig. 2B).
Fig 2.
Wolbachia abundance in whole flies. Relative Wolbachia abundance as determined by qPCR is shown for a high-density, protective Wolbachia strain (wAu) following treatment with different concentrations of tetracycline (A). Treatment with tetracycline was used to decrease Wolbachia abundance in flies and is compiled from three fly cohorts (n = 10 to 12). Error bars show standard errors. Tetracycline treatment decreases Wolbachia abundance in a concentration-dependent manner (nonlinear regression, R2 = 0.8525; correlation, P = 0.0014). (B) Wolbachia in three protective (CO-wAu, Me29-wMel, and DSR-wRi) and two nonprotective (DSH-wHa and N7NO-wNo) fly line-Wolbachia strain pairings. Male 4- to 7-day-old flies were pooled in groups of five and repeated across three individual cohorts. The density of Wolbachia within fly lines correlates with the ability to mediate antiviral protection (P = 0.0005). Error bars show standard errors.
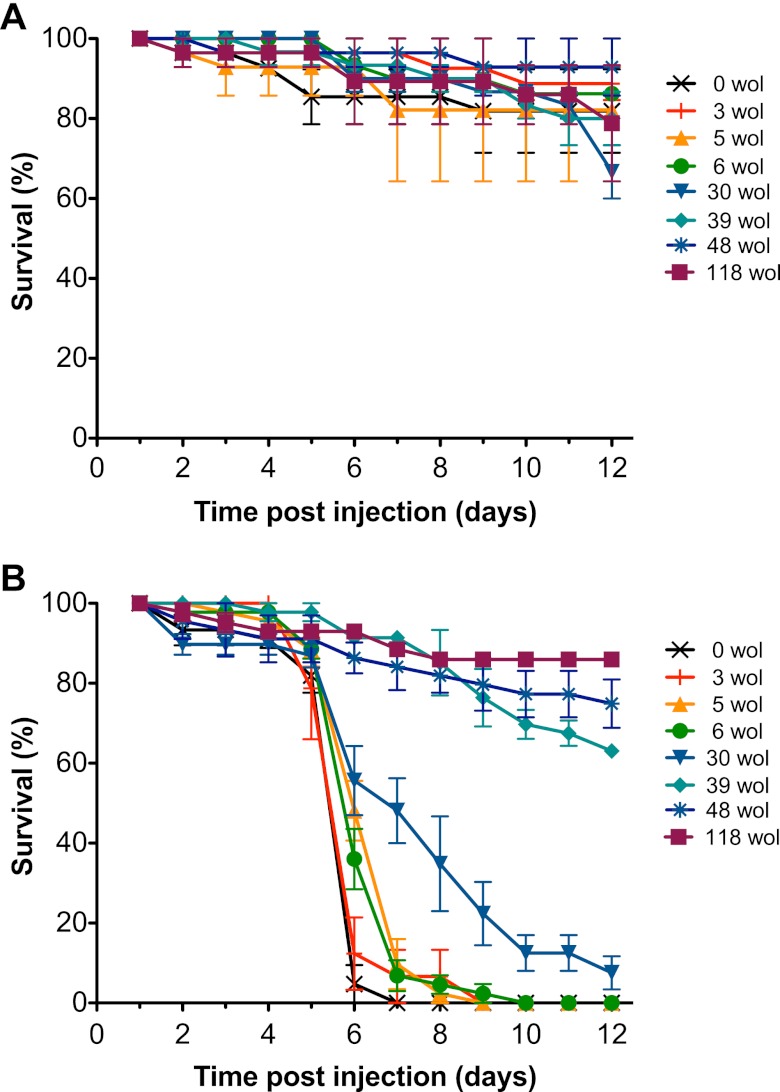
To determine if the abundance of Wolbachia in whole flies was an important factor in antiviral protection, CO flies containing altered levels of the protective wAu were challenged with mortality-inducing DCV (Fig. 3). Wolbachia-free CO flies were used as Wolbachia-negative controls and injected with DCV or PBS. Flies that contained Wolbachia were treated with tetracycline to obtain mean densities of wAu that were equivalent to 3, 5, 6, 30, 39, and 48 copies of wsp relative to the host reference gene, Actin 5C. Wolbachia-containing control flies that were not treated with tetracycline had a Wolbachia density that was equivalent to 118 copies of wsp relative to host Actin 5C. Wolbachia-free positive-DCV control flies injected with virus reached 100% mortality 7 days following DCV challenge, which was similar across all biological replicates. Wolbachia-containing flies not treated with tetracycline had less than 40% mortality across the duration of the experiment, similar to the virus-free PBS-injected control flies, and similar survival rates were observed with CO-wAu flies that contained 48 and 39 relative units of Wolbachia. A moderate delay in virus-induced mortality was observed in CO-wAu flies that contained 30 relative units of Wolbachia. CO-wAu flies with 3 to 6 relative units of Wolbachia were not protected against viral challenge, as virus-induced mortality was similar to that of Wolbachia-free CO flies. Thus, when the abundance of the protective Wolbachia strain wAu was decreased to levels seen in nonprotective fly lines, Wolbachia-mediated antiviral protection was lost.
Fig 3.
Survival of CO flies containing altered abundances of wAu following viral challenge. The abundant, protective Wolbachia strain wAu was decreased in individual CO flies by antibiotic treatment, and then 4- to 7-day-old flies were injected with PBS (negative control) (A) or DCV (B). The graphs show the survival of CO flies which have been treated with tetracycline to decrease the protective Wolbachia strain wAu to densities equivalent to 3, 5, 6, 30, 39, and 48 copies of Wolbachia per host genome (wol) or mock treated (118 wol). Survival of flies is shown from 3 replicates of 15 flies for DCV-injected flies or 2 replicates of 15 flies for PBS controls. CO flies that had previously been cleared of Wolbachia (0 wol) were included as Wolbachia-negative controls. Data shown are representative of independent survival assays, where comparable densities of wAu resulted in similar survival outcomes.
To determine that tetracycline treatment did not alter viral susceptibility, Wolbachia-free flies treated with 300 μg/ml, 27 μg/ml, 3 μg/ml, or no tetracycline were challenged with DCV. Small differences in mortality were observed between Wolbachia-free DCV-injected flies in different treatments. As the onset of mortality did not reflect the concentration of tetracycline doses, and the magnitude of the differences was small and not repeatable across fly cohorts, we concluded that tetracycline treatment does not alter the survival of flies under the conditions of the viral challenge assay. The survival data show that the ability of Wolbachia to mediate antiviral protection was lost when the level of the protective Wolbachia strain wAu was decreased (Fig. 3).
DISCUSSION
Wolbachia-mediated antiviral protection in insects is an intriguing phenomenon, the mechanism of which is not yet understood. The data from this study show that reducing the density of a protective Wolbachia strain results in the loss of Wolbachia-mediated antiviral protection. The Wolbachia strain wAu normally occurs at a high density within the host insect and protects flies against virus-induced mortality (36). When the density of wAu in individual CO flies is reduced by antibiotic treatment, the degree of Wolbachia-mediated antiviral protection is also reduced or lost completely. This suggests that the Wolbachia density in the host is an important factor in the mediation of antiviral protection.
Using five fly-Wolbachia combinations, it has previously been shown that there is a correlation between Wolbachia density in whole flies and antiviral protection (36). However, the five Wolbachia strains are genetically diverse and associated with different D. simulans hosts. Thus, in this study, the density of the abundant wAu strain was decreased to achieve abundances similar to those of other protective or nonprotective Wolbachia strains (36). Wolbachia abundance in host insects varies with host-Wolbachia combination, larval crowding conditions, environmental conditions, and the age of the insect host (3, 6, 8, 9, 18, 46). Determining Wolbachia density under the same conditions as standard viral challenge assays confirms that observations of density correspond with levels of antiviral protection. Interestingly, when the abundance of wAu organisms was decreased to an abundance similar to that of the protective wMel in Me29 and wRi in DSR, wAu continued to mediate antiviral protection. When wAu was decreased to an abundance that was similar to those of the nonprotective DSH-wHa and N7NO-wNo combinations, the mortality rate of flies was similar to that of Wolbachia-free CO flies. Although the influence of other factors, including phylogenetic differences in strains, remains to be determined, the data from this study suggest that the density of organisms of a Wolbachia strain may be important in the ability of the strain to mediate antiviral protection.
Although more work is needed to determine how Wolbachia induces the mechanism of antiviral protection, the data from this paper and from the existing literature suggest that a density-related mechanism of antiviral protection is involved (10, 34, 36). Frentiu et al. (10) have shown increased dengue virus inhibition in cell lines that contain greater Wolbachia abundances than in those with lower Wolbachia abundances, which suggests that Wolbachia density is relevant to viral infection in cell culture. In the whole organism, there is likely to be greater variation in both the presence and absence of Wolbachia within cell types and the number of Wolbachia organisms within particular cells. Despite this variation, the data indicate that the Wolbachia density is still important for antiviral protection at a whole-organism level. Moreira et al. (34) have shown in mosquitoes that when Wolbachia exhibits a tropism for a particular cell type such as cells in the fat body, dengue virus is limited in the Wolbachia-dense tissue. In addition, there is a correlation between Wolbachia density across five D. simulans lines and antiviral protection (36). Taken together with the data from the current study, this suggests that the density of Wolbachia in whole flies is important, possibly as it reflects the Wolbachia density within different host tissue types.
The Wolbachia distribution of across a variety of somatic tissues in this study shows that the Wolbachia strains used here are capable of infecting a wide variety of specialized tissue types. Other studies have identified Wolbachia within either numerous or limited somatic tissues and have reported that tissue distribution varies between the host-Wolbachia combinations studied, and that Wolbachia distribution and density can change when the bacterium is introduced into a novel host (2, 4, 5, 8, 28, 30). As in this study, Dobson et al. (8) have investigated the qualitative distribution of wRi in DSR and identified Wolbachia in the head, midgut, Malpighian tubules, ovaries, testes, and muscle using Western blotting against the Wsp protein. Those findings agreed with the data from this study, which identified Wolbachia distribution using Wolbachia-specific oligonucleotides. As this study identified Wolbachia in the same tissues in individuals of two protective (CO and Me29) and one nonprotective (DSH) fly line, the simple presence or absence of detectable Wolbachia within the tissues studied is not sufficient to mediate antiviral protection. Interestingly, the protective Wolbachia strain wRi was only identified in the fat body of one individual (n = 5) and at a density similar to that in nonprotective pairing DSH-wHa. This indicates that the presence of Wolbachia in the fat body is unlikely to be required for the mediation of antiviral protection. In mosquitoes, Moreira et al. (34) found that high levels of wMelPop-CLA in the fat body greatly reduced dengue virus within this tissue. However, wMelPop and wMelPop-CLA occur at a high abundance within many tissues in comparison to other strains, and it is not known if the Wolbachia abundance in this or other tissues is correlated with protection (33, 34).
As Wolbachia density in whole flies is important for antiviral protection, this study investigated the Wolbachia density within tissues dissected from the five protective or nonprotective host-Wolbachia combinations. Using qPCR, low Wolbachia levels were detected in tissues where Wolbachia was not observed using the FISH technique. This is most likely due to differences in sensitivity of the assays and the use of pooled rather than individual samples. The Wolbachia density in the head, gut, and Malpighian tubules correlated with the ability of the strains to mediate antiviral protection, suggesting that the Wolbachia density at these sites may play a tissue specific role in Wolbachia-mediated antiviral protection. Although tissue tropism varied between fly-Wolbachia pairings, it was interesting to note that these three tissues did not contain the highest relative Wolbachia abundances within individual flies. In fact, across each of the fly lines, the relative Wolbachia abundance observed in the head and gut was less than the relative abundance observed in several other tissues.
Specific tissues may have functions that are involved in the response of the host to viral challenge which are altered by high Wolbachia densities. The gut forms part of the epithelial barrier and therefore is one of the main sites of microbe entry and initial infection. The Malpighian tubules are involved in the regulation of ions and the excretion of waste, much like the mammalian kidney (reviewed in reference 1). Malpighian tubules have been shown to upregulate the production of nitric oxide synthase and potentially regulate the immune response (reviewed in reference 1). It seems plausible that an abundance of Wolbachia organisms in these tissues may alter the host response so that flies with Wolbachia are better able to defend against or survive viral challenge. Furthermore, although an altered response could induce a more effective antiviral response, potentially decreasing the production of self-damaging host responses could also result in an overall improved pathology outcome for the host. Further research is needed to determine what role these tissues may play in the Wolbachia-host-virus interaction or following viral challenge.
Wolbachia is emerging as a useful biocontrol agent to decrease transmission of insect-borne viruses such as dengue virus (7, 13, 14, 19, 23, 34, 38, 42, 43). A better understanding of Wolbachia characteristics required for antiviral protection could be useful for predicting Wolbachia strains that are likely to mediate strong antiviral protection, either in mosquitoes or in other pathogen-carrying insect species of medical or agricultural significance. The data from this study provide strong evidence that Wolbachia density is an important factor required for antiviral protection, although further characterization using different host-Wolbachia combinations, Wolbachia genetic backgrounds, and viral challenge systems should also be investigated. This study has also shown that Wolbachia density within specific tissues (the head, gut, and Malpighian tubules) correlates with the ability to mediate antiviral protection across five diverse Wolbachia strains within D. simulans.
Supplementary Material
ACKNOWLEDGMENTS
We thank Paul Addison for his histological expertise and the use of equipment, David Merritt for his help with dissections, Craig White for his expertise with statistical analysis, and Lauren Hedges for providing reagents.
This research was supported by funding from the Australian Research Council (DP1092492) and from the Foundation for the National Institutes of Health through the Grand Challenges in Global Health Initiative of the Bill and Melinda Gates Foundation.
Footnotes
Published ahead of print 27 July 2012
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1. Beyenbach KW, Skaer H, Dow JA. 2010. The developmental, molecular, and transport biology of Malpighian tubules. Annu. Rev. Entomol. 55:351–374 [DOI] [PubMed] [Google Scholar]
- 2. Bian GW, Xu Y, Lu P, Xie Y, Xi ZY. 2010. The endosymbiotic bacterium Wolbachia induces resistance to Dengue virus in Aedes aegypti. PLoS Pathog. 6:e1000833 doi:10.1371/journal.ppat.1000833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boyle L, O'Neill SL, Robertson HM, Karr TL. 1993. Interspecific and intraspecific horizontal transfer of Wolbachia in Drosophila. Science 260:1796–1799 [DOI] [PubMed] [Google Scholar]
- 4. Cheng Q, Aksoy S. 1999. Tissue tropism, transmission and expression of foreign genes in vivo in midgut symbionts of tsetse flies. Insect Mol. Biol. 8:125–132 [DOI] [PubMed] [Google Scholar]
- 5. Cheng Q, et al. 2000. Tissue distribution and prevalence of Wolbachia infections in tsetse flies, Glossina spp. Med. Vet. Entomol. 14:44–50 [DOI] [PubMed] [Google Scholar]
- 6. Clancy DJ, Hoffmann AA. 1998. Environmental effects on cytoplasmic incompatibility and bacterial load in Wolbachia-infected Drosophila simulans. Entomol. Exp. Appl. 86:13–24 [Google Scholar]
- 7. Cook PE, McMeniman CJ, O'Neill SL. 2008. Modifying insect population age structure to control vector-borne disease. Adv. Exp. Med. Biol. 627:126–140 [DOI] [PubMed] [Google Scholar]
- 8. Dobson SL, et al. 1999. Wolbachia infections are distributed throughout insect somatic and germ line tissues. Insect Biochem. Mol. 29:153–160 [DOI] [PubMed] [Google Scholar]
- 9. Dutton TJ, Sinkins SP. 2004. Strain-specific quantification of Wolbachia density in Aedes albopictus and effects of larval rearing conditions. Insect Mol. Biol. 13:317–322 [DOI] [PubMed] [Google Scholar]
- 10. Frentiu FD, Robinson J, Young PR, McGraw EA, O'Neill SL. 2010. Wolbachia-mediated resistance to dengue virus infection and death at the cellular level. PLoS One 5:e13398 doi:10.1371/journal.pone.0013398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Frydman HM, Li JM, Robson DN, Wieschaus E. 2006. Somatic stem cell niche tropism in Wolbachia. Nature 441:509–512 [DOI] [PubMed] [Google Scholar]
- 12. Glaser RL, Meola MA. 2010. The native Wolbachia endosymbionts of Drosophila melanogaster and Culex quinquefasciatus increase host resistance to West Nile virus infection. PLoS One 5:e11977 doi:10.1371/journal.pone.0011977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hancock PA, Sinkins SP, Godfray HC. 2011. Population dynamic models of the spread of Wolbachia. Am. Nat. 177:323–333 [DOI] [PubMed] [Google Scholar]
- 14. Hancock PA, Sinkins SP, Godfray HC. 2011. Strategies for introducing Wolbachia to reduce transmission of mosquito-borne diseases. PLoS Negl. Trop. Dis. 5:e1024 doi:10.1371/journal.pntd.0001024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hedges LM, Brownlie JC, O'Neill SL, Johnson KN. 2008. Wolbachia and virus protection in insects. Science 322:702. [DOI] [PubMed] [Google Scholar]
- 16. Hedges LM, Johnson KN. 2008. The induction of host defence responses by Drosophila C virus. J. Gen. Virol. 89:1497–1501 [DOI] [PubMed] [Google Scholar]
- 17. Hilgenboecker K, Hammerstein P, Schlattmann P, Telschow A, Werren JH. 2008. How many species are infected with Wolbachia? A statistical analysis of current data. FEMS Microbiol. Lett. 281:215–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hoffmann AA, Clancy D, Duncan J. 1996. Naturally-occurring Wolbachia infection in Drosophila simulans that does not cause cytoplasmic incompatibility. Heredity 76:1–8 [DOI] [PubMed] [Google Scholar]
- 19. Hoffmann AA, et al. 2011. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature 476:454–457 [DOI] [PubMed] [Google Scholar]
- 20. Hoffmann AA, Turelli M. 1997. Cytoplasmic incompatibility in insects, p 42–80. In O'Neill SL, Hoffmann AA, Werren JH. (ed), Influential passengers: inherited microorganisms and arthropod reproduction. Oxford University Press, Oxford, United Kingdom [Google Scholar]
- 21. Hoffmann AA, Turelli M, Simmons GM. 1986. Unidirectional incompatibility between populations of Drosophila simulans. Evolution 40:692–701 [DOI] [PubMed] [Google Scholar]
- 22. Hughes GL, Koga R, Xue P, Fukatsu T, Rasgon JL. 2011. Wolbachia infections are virulent and inhibit the human malaria parasite Plasmodium falciparum in Anopheles gambiae. PLoS Pathog. 7:e1002043 doi:10.1371/journal.ppat.1002043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Iturbe-Ormaetxe I, Walker T, O'Neill SL. 2011. Wolbachia and the biological control of mosquito-borne disease. EMBO Rep. 12:508–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jeyaprakash A, Hoy MA. 2000. Long PCR improves Wolbachia DNA amplification: wsp sequences found in 76% of sixty-three arthropod species. Insect Mol. Biol. 9:393–405 [DOI] [PubMed] [Google Scholar]
- 25. Johnson KN, Christian PD. 1998. The novel genome organization of the insect picorna-like virus Drosophila C virus suggests this virus belongs to a previously undescribed virus family. J. Gen. Virol. 79:191–203 [DOI] [PubMed] [Google Scholar]
- 26. Kambris Z, et al. 2010. Wolbachia stimulates immune gene expression and inhibits plasmodium development in Anopheles gambiae. PLoS Pathog. 6:e1001143 doi:10.1371/journal.ppat.1001143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kambris Z, Cook PE, Phuc HK, Sinkins SP. 2009. Immune activation by life-shortening Wolbachia and reduced filarial competence in mosquitoes. Science 326:134–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kondo NI, et al. 2011. Wolbachia infections in world populations of bean beetles (Coleoptera: Chrysomelidae: Bruchinae) infesting cultivated and wild legumes. Zoolog. Sci. 28:501–508 [DOI] [PubMed] [Google Scholar]
- 29. Longdon B, Fabian DK, Hurst GD, Jiggins FM. 2012. Male-killing Wolbachia do not protect Drosophila bifasciata against viral infection. BMC Microbiol. 12(Suppl 1):S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McGraw EA, O'Neill SL. 2004. Wolbachia pipientis: intracellular infection and pathogenesis in Drosophila. Curr. Opin. Microbiol. 7:67–70 [DOI] [PubMed] [Google Scholar]
- 31. Merçot H, Poinsot D. 1998. Wolbachia transmission in a naturally bi-infected Drosophila simulans strain from New-Caledonia. Entomol. Exp. Appl. 86:97–103 [Google Scholar]
- 32. Miller WJ, Riegler M. 2006. Evolutionary dynamics of wAu-like Wolbachia variants in neotropical Drosophila spp. Appl. Environ. Microbiol. 72:826–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Min KT, Benzer S. 1997. Wolbachia, normally a symbiont of Drosophila, can be virulent, causing degeneration and early death. Proc. Natl. Acad. Sci. U. S. A. 94:10792–10796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Moreira LA, et al. 2009. A Wolbachia symbiont in Aedes aegypti limits infection with Dengue, Chikungunya, and Plasmodium. Cell 139:1268–1278 [DOI] [PubMed] [Google Scholar]
- 35. O'Neill SL, Karr TL. 1990. Bidirectional incompatibility between conspecific populations of Drosophila simulans. Nature 348:178–180 [DOI] [PubMed] [Google Scholar]
- 36. Osborne SE, Leong YS, O'Neill SL, Johnson KN. 2009. Variation in antiviral protection mediated by different Wolbachia strains in Drosophila simulans. PLoS Pathog. 5:e1000656 doi:10.1371/journal.ppat.1000656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Poinsot D, Bourtzis K, Markakis G, Savakis C, Mercot H. 1998. Wolbachia transfer from Drosophila melanogaster into D. simulans: host effect and cytoplasmic incompatibility relationships. Genetics 150:227–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rasgon JL. 2011. Dengue fever: mosquitoes attacked from within. Nature 476:407–408 [DOI] [PubMed] [Google Scholar]
- 39. Teixeira L, Ferreira A, Ashburner M. 2008. The bacterial symbiont Wolbachia induces resistance to RNA viral infections in Drosophila melanogaster. PLoS Biol. 6:e1000002 doi:10.1371/journal.pbio.1000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Turelli M, Hoffmann AA. 1991. Rapid spread of an inherited incompatibility factor in California Drosophila. Nature 353:440–442 [DOI] [PubMed] [Google Scholar]
- 41. Veneti Z, Clark ME, Karr TL, Savakis C, Bourtzis K. 2004. Heads or tails: host-parasite interactions in the Drosophila-Wolbachia system. Appl. Environ. Microbiol. 70:5366–5372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Walker T, et al. 2011. The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature 476:450–453 [DOI] [PubMed] [Google Scholar]
- 43. Walker T, Moreira LA. 2011. Can Wolbachia be used to control malaria? Mem. Inst. Oswaldo Cruz 106(Suppl 1):212–217 [DOI] [PubMed] [Google Scholar]
- 44. Werren JH. 1997. Biology of Wolbachia. Annu. Rev. Entomol. 42:587–609 [DOI] [PubMed] [Google Scholar]
- 45. Werren JH, Windsor DM. 2000. Wolbachia infection frequencies in insects: evidence of a global equilibrium? Proc. R. Soc. Lond. B Biol. Sci. 267:1277–1285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wiwatanaratanabutr I, Kittayapong P. 2009. Effects of crowding and temperature on Wolbachia infection density among life cycle stages of Aedes albopictus. J. Invertebr. Pathol. 102:220–224 [DOI] [PubMed] [Google Scholar]
- 47. Wong ZS, Hedges LM, Brownlie JC, Johnson KN. 2011. Wolbachia-mediated antibacterial protection and immune gene regulation in Drosophila. PLoS One 6:e25430 doi:10.1371/journal.pone.0025430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhou W, Rousset F, O'Neill S. 1998. Phylogeny and PCR-based classification of Wolbachia strains using wsp gene sequences. Proc. R. Soc. Lond. B Biol. Sci. 265:509–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.