Abstract
The current understanding of dissimilatory metal reduction is based primarily on isolates from the proteobacterial genera Geobacter and Shewanella. However, environments undergoing active Fe(III) reduction often harbor less-well-studied phyla that are equally abundant. In this work, electrochemical techniques were used to analyze respiratory electron transfer by the only known Fe(III)-reducing representative of the Acidobacteria, Geothrix fermentans. In contrast to previously characterized metal-reducing bacteria, which typically reach maximal rates of respiration at electron acceptor potentials of 0 V versus standard hydrogen electrode (SHE), G. fermentans required potentials as high as 0.55 V to respire at its maximum rate. In addition, G. fermentans secreted two different soluble redox-active electron shuttles with separate redox potentials (−0.2 V and 0.3 V). The compound with the lower midpoint potential, responsible for 20 to 30% of electron transfer activity, was riboflavin. The behavior of the higher-potential compound was consistent with hydrophilic UV-fluorescent molecules previously found in G. fermentans supernatants. Both electron shuttles were also produced when cultures were grown with Fe(III), but not when fumarate was the electron acceptor. This study reveals that Geothrix is able to take advantage of higher-redox-potential environments, demonstrates that secretion of flavin-based shuttles is not confined to Shewanella, and points to the existence of high-potential-redox-active compounds involved in extracellular electron transfer. Based on differences between the respiratory strategies of Geothrix and Geobacter, these two groups of bacteria could exist in distinctive environmental niches defined by redox potential.
INTRODUCTION
While most of our understanding regarding electron transfer to metals is derived from a small number of Shewanella and Geobacter species, a diversity of Bacteria and Archaea are known to use insoluble Fe(III) oxides as terminal electron acceptors (19, 29, 43, 50). In particular, sequences similar to those for an Fe(III)-reducing isolate from the Acidobacteria phylum (sometimes referred to as group 8 Acidobacteria) are consistently detected in 16S rRNA surveys of aquifers undergoing Fe(III) reduction, as well as in Fe(III)-rich sediments undergoing uranium bioreduction (2, 8, 59), petroleum oxidation (1), technetium reduction (40), and arsenic reduction (21, 22). In many of these environments, such acidobacterial sequences are more abundant than Geobacter sequences, yet the basis for their competiveness with Geobacter is not known.
In general, members of the Acidobacteria are ubiquitous in both 16S and metagenomic surveys of soils, often representing over 50% of detected bacterial sequences (15, 23, 25, 45, 49) which are classified into 26 acidobacterial subdivisions (2, 7, 14, 17, 18, 34, 58). Cultivated Acidobacteria are slow-growing, nutritionally versatile oligotrophs able to use a variety of carbon sources and that typically prefer microaerophilic conditions. The exception is the Fe(III)-reducing anaerobic isolate Geothrix fermentans, originally obtained from the Fe(III)-reducing zone of a petroleum-contaminated aquifer (10).
To transfer electrons to extracellular compounds, bacteria must make contact with electron acceptors and/or secrete redox-active mediators. Both strategies have their advantages: flavin mononucleotide (FMN) actively secreted by Shewanella accelerates metal reduction (12, 37, 47, 54), and phenazines secreted by Pseudomonas facilitate movement of electrons to aerobic edges of a biofilm (13, 20, 56, 57). Direct mechanisms, such as those used by Geobacter (29, 41, 52), benefit only cells in contact with the acceptor and may also electrically link daughter cells to the metal, often allowing electron transfer over longer distances than soluble shuttles (52). Each strategy has potential tradeoffs; a contact-based strategy may represent a metabolic burden in terms of multiple structural and extracellular redox proteins to interface with unpredictable metal surfaces in the environment, while secreted molecules could be expensive to produce and be limited by diffusion.
G. fermentans reduces Fe(III) trapped within porous beads (42), and supernatants from G. fermentans cultures accelerate Fe(III) reduction by other bacteria (4), providing evidence that this bacterium may secrete a soluble compound as part of its metal reduction strategy. Both quinone-like compounds and metal chelators have been reported to occur in G. fermentans supernatants. However, the identity of any soluble agents, their redox potentials, and their relative importance to electron transfer under conditions relevant to subsurface growth remain unknown (42).
Within the last 5 years, a new set of electrochemical tools has emerged that allows monitoring of electron transfer from living cells. Compared to growth with insoluble metals, electrode potentials can be precisely controlled, allowing rates of electron transfer from cells to be measured without accumulation of reduced end products confounding results (36, 38). Such analyses show that Geobacter strains transfer electrons beyond the cell membrane at potentials as low as −0.2 V versus the standard hydrogen electrode (SHE) and reach their maximal rate of respiration at −0.1 V. These low values help explain the competitiveness of Geobacter in permanently anoxic environments. In Shewanella strains, direct electron transfer to electrodes can be measured, but the ability to easily remove the medium surrounding attached cells reveals that an indirect redox shuttling mechanism based on secreted FMN is responsible for nearly ∼80% of respiratory activity by biofilms under these conditions (37).
In this work, the electron transfer strategy of G. fermentans was studied for the first time using poised electrodes and was found to be different from that of any previously described organism. Specifically, G. fermentans electron transfer reached less than 30% of maximum rates between the commonly studied redox potential window of −0.2 and 0 V and instead required potentials above 0.55 V to support maximal rates. Removal of supernatants from electrode-grown biofilms reduced the rate of electron transfer, and cell-free supernatants from both Fe(III)-grown and electrode-grown G. fermentans cultures showed evidence of two independent soluble electron shuttles. Neither of these activities appeared to be due to a metal chelator. One of the two redox shuttles, active in the low-potential window of −0.2 V, was identified as riboflavin, while the higher-potential compound remains unidentified. This dual redox potential strategy reveals that flavin-based electron shuttles are used by Fe(III)-reducing bacteria outside the Proteobacteria and suggests that anaerobic Acidobacteria may be more competitive in higher-potential environments, such as near oxic-anoxic interfaces.
MATERIALS AND METHODS
Bacterial strains and culture media.
Geothrix fermentans (ATCC 700665) was grown anaerobically at 20°C unless otherwise indicated in minimal medium containing the following (per liter): 0.38 g KCl, 0.2 g NH4Cl, 0.069 g NaH2PO4 · H2O, 0.04 g CaCl2 · 2H2O, and 0.2 g MgSO4 · 7H2O. Ten milliliters of mineral mix containing 0.1 g MnCl2 · 4H2O, 0.3 g FeSO4 · 7H2O, 0.17 g CoCl2 · 6H2O, 0.1 g ZnCl2, 0.04 g CuSO4 · 5H2O, 0.005 g AlK(SO4)2 · 12H2O, 0.005 g H3BO3, 0.09 g Na2MoO4, 0.12 g NiCl2, 0.02 g NaWO4 · 2H2O, and 0.10 g Na2SeO4 per liter was added before autoclaving. Lactate at 20 mM was supplied as the electron donor and 40 mM fumarate as the soluble electron acceptor for routine growth, and 100 mM ferrihydrite (FeOOH) was added for metal-reducing conditions. Lactate at 20 mM as an electron donor and a poised electrode as an electron acceptor were used for all electrochemical experiments. All media were adjusted to pH 6.8, buffered with 2 g/liter NaHCO3, made anaerobic by being flushed with oxygen-free N2/CO2 (80:20, vol/vol), and sealed with butyl rubber stoppers prior to autoclaving. One milliliter of a filter-sterilized anaerobic vitamin mixture was added to all cultures before inoculation. The vitamin mixture contained 0.002 g biotin, 0.002 g folic acid, 0.01 g pyridoxine HCl, 0.005 g thiamine, 0.005 g nicotinic acid, 0.005 g pantothenic acid, 0.0001 g vitamin B12, 0.005 g p-aminobenzoic acid, and 0.005 g thioctic acid per 1 liter.
Electrode preparation and assembly.
Carbon cloth (B-1/B WO W000179 E-TER; PEMEAS Fuel Cell Technologies/BASF, Somerset, NJ) was cut into 2- by 2-cm electrodes (geometric surface area, 8 cm2). Carbon cloth electrodes were soaked sequentially in deionized water, 0.1 N NaOH, deionized water, 0.1 N HCl, and ethanol to remove contaminants. This treatment was repeated, and electrodes were stored in deionized water. Electrodes were attached to 0.1-mm platinum wire. A bioreactor, containing a carbon cloth working electrode, a platinum counterelectrode, and a saturated calomel reference electrode, was prepared as described previously (36). Sterile anaerobic minimal medium (5 ml) lacking electron donors or acceptors was added to autoclaved bioreactors, and anaerobic conditions were maintained by a constant flow of humidified oxygen-free N2/CO2 (80:20, vol/vol) at 20°C and connected to a potentiostat (VMP; Bio-Logic, Knoxville, TN) (36). After equilibration, 5 ml of a culture entering stationary phase (optical density at 600 nm [OD600], 0.5) was added to the electrochemical cell (a 50% inoculum).
Electrochemical analysis.
The parameters for cyclic voltammetry of attached biofilms were as follows: equilibrium time = 5 s, scan rate = 1 mV/s, Einitial (Ei) = −0.645 V versus SHE, and Efinal (Ef) = 0.745 V versus SHE; the current was averaged over the last 80% of each poised voltage as described previously by Marsili et al. (36). Two sweeps were performed, with data from the second sweep reported. By changing the potential slowly (1 mV/s), data were collected across a range of applied redox potentials, reflecting steady-state electron transfer from cells to electrodes.
Analytical electrochemistry was also performed on supernatants in duplicate between an Ei of −0.645 V versus SHE and an Ef of 0.745 V versus SHE at a scan rate of 100 mV/s. This higher scan rate was chosen to rapidly oxidize or reduce redox active compounds near the electrode and enhance the sensitivity of detection. Data collected from the second sweeps were analyzed and processed using SOAS (16).
Biomass measurements.
Biomass was determined using the bicinchoninic acid protein assay (Thermo Scientific, Rockford, IL). After electrochemical analysis, electrodes were harvested and gently washed in minimal medium to remove loosely attached planktonic cells. The electrodes were disassembled and incubated in 1 ml of 0.2 M NaOH solution at 96°C for 20 min to remove attached biomass. Electrodes were also harvested, fixed in 3% glutaraldehyde, and dehydrated in increasing concentrations of ethanol (25%, 50%, 75%, 95%, and 100%) for 10 min. The electrodes were critical-point dried, sputter coated, and imaged using a S3500N scanning electron microscope (Hitachi, Japan).
Collection of culture supernatants.
Electrode-grown supernatants were harvested by carefully removing the surrounding medium into a sterile anaerobic tube with an 80% N2–20% CO2 headspace, centrifuged anaerobically to remove planktonic cells, and stored at −20°C after the headspace was flushed with 80% N2–20% CO2. Supernatants collected from FeOOH-grown cultures were centrifuged anaerobically at 1,500 rpm to remove insoluble FeOOH. The supernatant was anaerobically mixed with 0.5 g of Chelex 100 resin (Bio-Rad, Hercules, CA) per 10 ml of supernatant in a sterile anaerobic tube overnight at 4°C on a shaker. The supernatant was centrifuged to remove resin and stored in a sterile anaerobic tube or passed through an octadecyl-functionalized silica gel (C18) column anaerobically in a glove box. The effluent was stored at −20°C in a sterile anaerobic tube. Hydrophobic compounds bound to the C18 column were collected by adding 100% methanol and stored at −20°C for further analysis.
HPLC.
Cell-free electrode- and FeOOH-grown supernatants were analyzed by high-performance liquid chromatography (HPLC) using a protocol previously described (37). An Eclipse XDB-C18 column (4.6 mm by 525 mm and 5-μm particle size) from Agilent Technologies, Santa Clara, CA, was used. A fluorescence detector with an excitation wavelength of 440 nm and an emission wavelength of 525 nm was used to detect fluorescent compounds.
RESULTS
Conditions to minimize cell lysis.
G. fermentans culture supernatants have previously been reported to contain soluble factors that accelerate reduction of ferrihydrite (42). While some bacteria actively excrete redox shuttles (12, 37, 54, 57), an alternative explanation is always the possibility of cell lysis releasing intracellular compounds. When G. fermentans cultures were grown at 30°C, over 50% of cells lysed within 2 days of reaching stationary phase. Reducing the growth temperature to 25°C slowed this process, and when cells were grown at 20°C, the OD600 remained stable after more than 200 h of incubation in stationary phase (see Fig. S1 in the supplemental material). While cell turnover during prolonged incubations is a complex process, growth at 20°C was chosen for all further electrochemical studies to minimize the possibility of compounds being rapidly released that could create significant artifacts (27).
Colonization of electrodes by G. fermentans requires higher potentials.
Experiments were initially conducted using carbon fiber electrodes poised at 0.2 V versus SHE, as these redox potentials are typically used to study metal-reducing Geobacter and Shewanella (3, 36, 37, 38). After 2 weeks of incubation, a small increase in electron transfer was detected, but it rarely exceeded ∼5 μA/cm2 under these conditions. Voltammetry of these partially colonized electrodes revealed that the rate of electron transfer increased significantly when the electrode was raised to higher potentials. Based on these data, new growth experiments were initiated with electrodes poised at 0.55 V versus SHE.
When the electrode was poised at a higher potential, a 50% inoculum of cells demonstrated a rapid initial rise in electron transfer rate (Fig. 1A), followed by a slow increase over the next 300 to 400 h. Inoculated bioreactors required >2 weeks to reach stable rates of electron transfer to electrodes. For this study, 15 independent biofilms were cultivated at higher potential (0.55 V), analyzed electrochemically, and used to produce supernatants for further analysis.
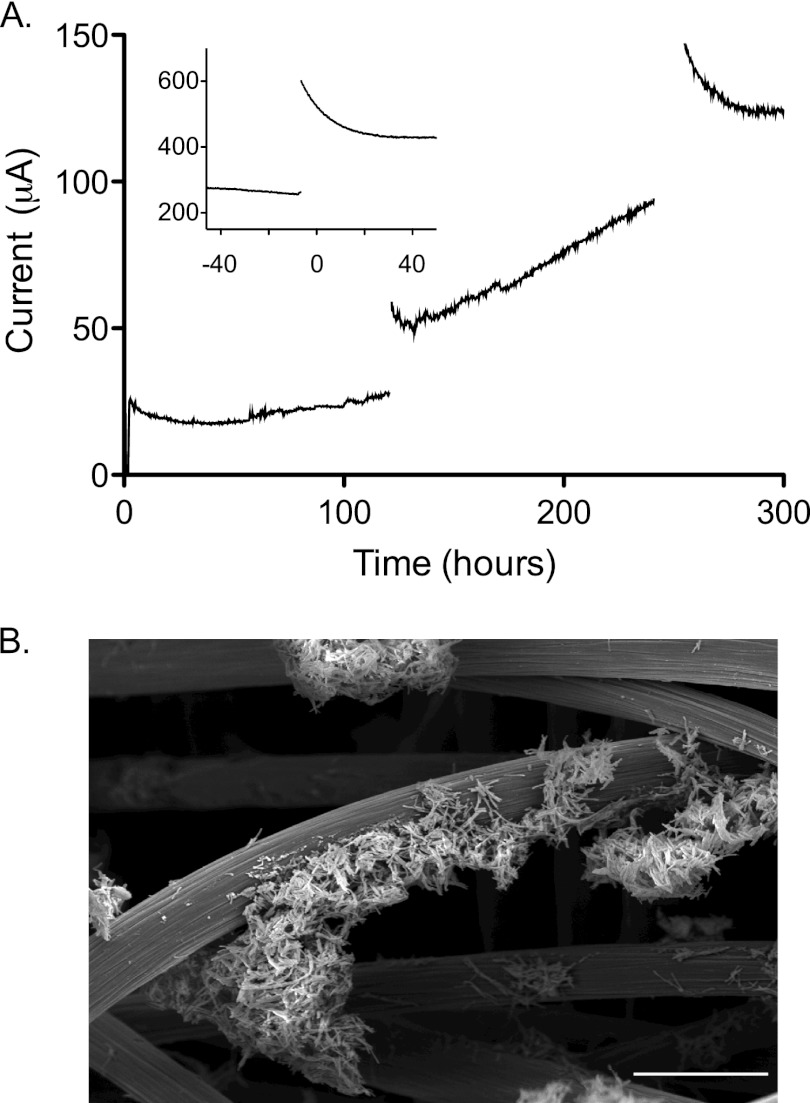
Fig 1.
Typical increase in electron transfer rate by electrode-attached G. fermentans. (A) Carbon fiber electrodes (8-cm2 geometric surface area) were poised at 0.55 V versus SHE and paused every 120 h for voltammetry analysis. The inset shows the increase in capacitance behavior over time after pausing current collection of a 500-h biofilm. (B) SEM image of a G. fermentans biofilm on a carbon fiber electrode, 1 month after inoculation, showing sparse coverage and microcolony formation. The scale bar represents 25 μm.
During colonization, data collection was paused at regular intervals for electrochemical analysis. For example, sweeping slowly between low and high potentials at 1 mV/s allowed measurement of electron transfer rates from bacteria to the electrode across a wide range of electron acceptor redox potentials. After electrodes were returned to the standard potential for growth (0.55 V), temporary surges of electron transfer from the biofilm were typically observed. In younger cultures, this surge decayed within 5 h, but up to 30 h was needed to fully discharge older cultures. Representative data from early and late stages of growth, illustrating this “capacitance” phenomenon where excess electrons were stored during pauses in current collection, are shown in Fig. 1A.
Routine G. fermentans biofilms allowed to grow over 640 h at 0.55 V achieved a density of 50 to 100 μA/cm2, a value 10-fold lower than what is observed for Geobacter strains, even when electrode roughness is considered. When attached biomass was measured, rates of electron transfer averaged 0.6 mA/mg protein (range = 0.3 to 1.1 mA/mg; higher values from older cultures), a range also an order of magnitude lower than that of Geobacter sulfurreducens but similar to rates achieved by Shewanella oneidensis (3, 38). Scanning electron microscopy (SEM) of fully colonized electrodes revealed clusters of microcolonies distributed between fibers, with exposed electrode still visible even after 1 month of growth (Fig. 1B).
Glassy carbon, polished graphite, gold, indium tin oxide (ITO), and graphitic paper electrodes were also screened in triplicate reactors for attachment and colonization of G. fermentans using the higher-potential conditions. None of these materials significantly improved colonization or exceeded current densities of 5 μA/cm2. None of these materials altered the potential required for growth or the two different potential windows observed during voltammetry (such as those described in Fig. 2). Standard high-temperature cleaning treatments also did not significantly alter colonization or growth rates on carbon fiber electrodes (55).
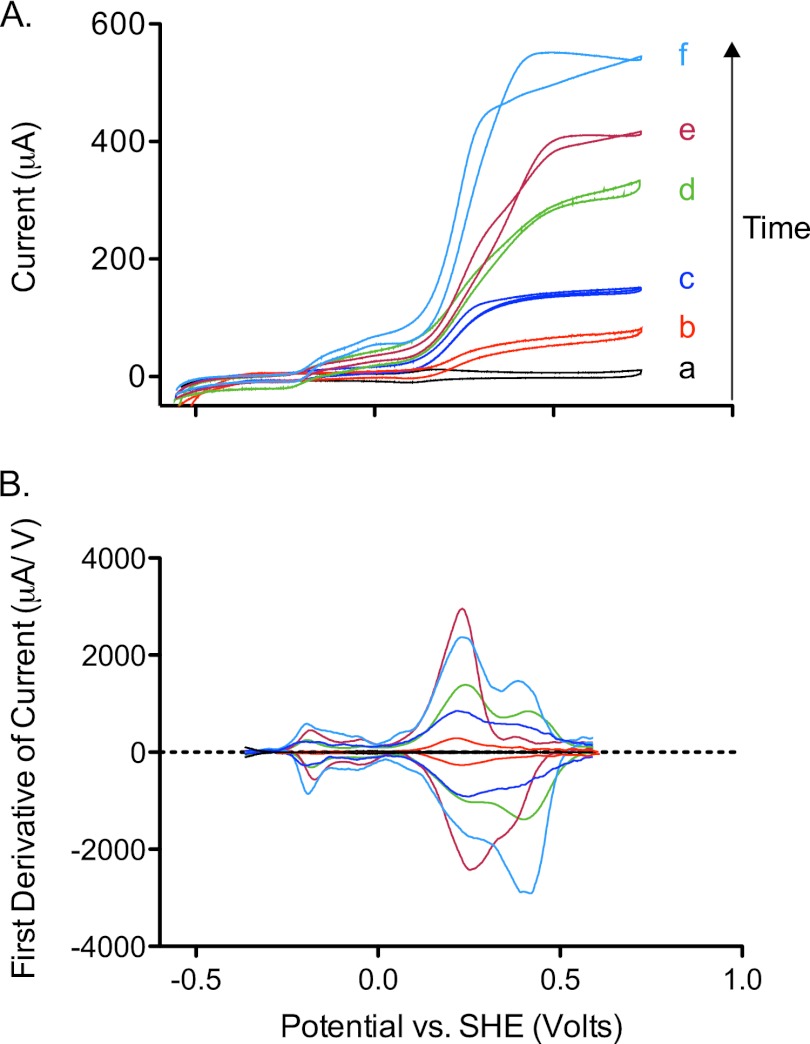
Fig 2.
(A) Cyclic voltammetry (1 mV/s) of G. fermentans biofilms collected over time at 100 (b), 240 (c), 360 (d), 480 (e), and 640 (f) h of growth on carbon fiber electrodes. A voltammogram of the uninoculated carbon fiber electrode is shown in black (a). (B) First derivatives of cyclic voltammograms from panel A, facilitating visualization of midpoint potentials in catalytic waves.
Cyclic voltammetry reveals two different potentials that stimulate electron transfer.
Low-scan-rate cyclic voltammetry performed on biofilms of electrode-reducing bacteria such as Geobacter (36, 38, 48), Rhodopseudomonas palustris DX-1 (60), and Thermincola ferriacetica (35) always produces a sigmoidal response that increases steeply above voltages of −0.2 V versus SHE and reaches a maximum by 0 V versus SHE. The response of G. fermentans differed from those of previously studied isolates and microbial fuel cell enrichments (9, 44, 51, 53). Most notable was how electron transfer from G. fermentans responded at two separate redox potentials. In addition, the majority of the increase in electron transfer rate occurred well above potentials of 0.2 V versus SHE. A representative time series is shown in Fig. 2A.
At all time points, these two characteristic windows were observed; one centered at approximately −0.2 V, and the second centered at 0.3 V. First-derivative analysis of data (Fig. 2B) often revealed a third inflection point at 0.4 V, which increased in intensity in older biofilms. Together, this series of G. fermentans electrode-grown biofilms showed that while G. fermentans could initiate electron transfer at potentials as low as −0.2 V, cells could not reach their maximum rates of sustained electron transfer unless provided with an electron acceptor near potentials of 0.55 V versus SHE, over half a volt higher than with any previously characterized organism. These data also revealed an unusual pattern of multiple independent features within this wide potential window.
Soluble mediators are involved at both low and high potentials.
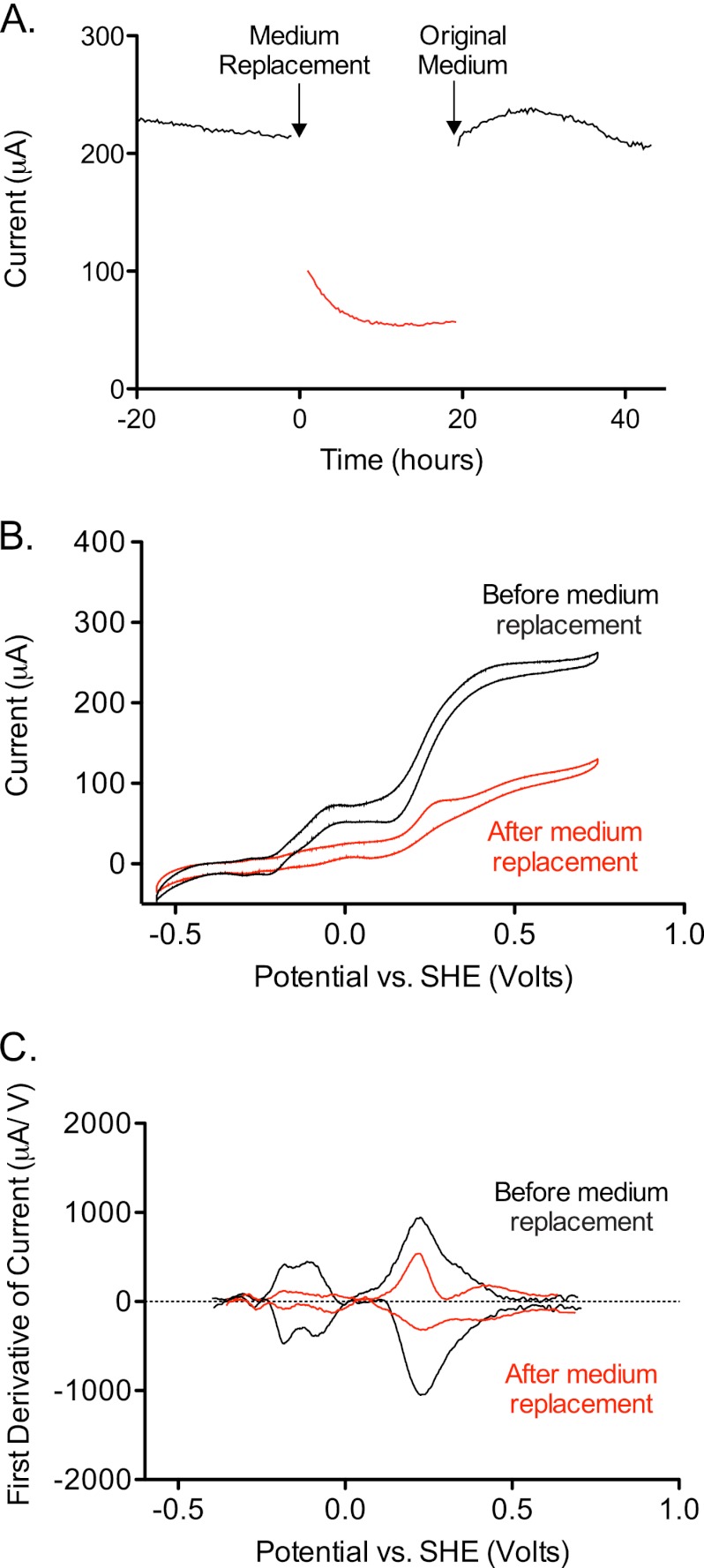
Earlier work with indirect assays based on acceleration of Fe(III) reduction implied that G. fermentans releases at least one soluble compound (4, 42). To test for the presence of soluble electron shuttles, the medium surrounding electrode-attached biofilms was removed and replaced with sterile anaerobic medium containing only the electron donor. In all cases, an immediate drop in current production was detected. Electron transfer could always be recovered by readdition of the original medium, even after anaerobic centrifugation or filtration to remove planktonic cells.
The absolute magnitude of the current drop after fresh medium was added increased with the age of the culture, varying from as low as 50% to as high as 75%. Data from a 3-week-old biofilm grown at 0.55 V are shown in Fig. 3A. These experiments verified that soluble compounds facilitate electron transfer between the cell surface and the electron acceptor, accounting for the majority of electron transfer. Surprisingly, these voltammetry experiments revealed changes in both redox potential windows in all experiments. This indicated that soluble compounds were involved in both low- and high-potential electron transfer mechanisms (Fig. 3B and C).
Fig 3.
Evidence for two soluble mediators. (A) Rate of electron transfer by an established G. fermentans biofilm (black trace), subsequent drop in electron transfer upon medium replacement (red trace), and recovery of electron transfer to the original level after readdition of original medium following centrifugation (black trace). (B) Cyclic voltammograms performed before addition (black) and after addition of fresh medium (red) show changes in the catalytic waves in both low- and high-potential regions. (C) First-derivative analysis of voltammograms from panel B used to identify potential windows affected by medium replacement.
Evidence for two independent redox-active compounds.
As medium replacement always decreased electron transfer by G. fermentans in both potential windows, this raised two possibilities. Either a single soluble compound was produced, with two distinct redox potentials, or two independent compounds were accumulating. As the relative amount of electron transfer in each window appeared to vary independently with culture age, growth data supported the hypothesis of two independent compounds.
An electrochemical assay was used to detect electrochemically active compounds in filtered supernatants. Voltammetry of sterile supernatants obtained from biofilms of G. fermentans always revealed two distinct redox peaks, at the two potential windows observed for electron transfer by whole cells (Fig. 4A). These assays verified the presence of redox mediators in culture supernatants.
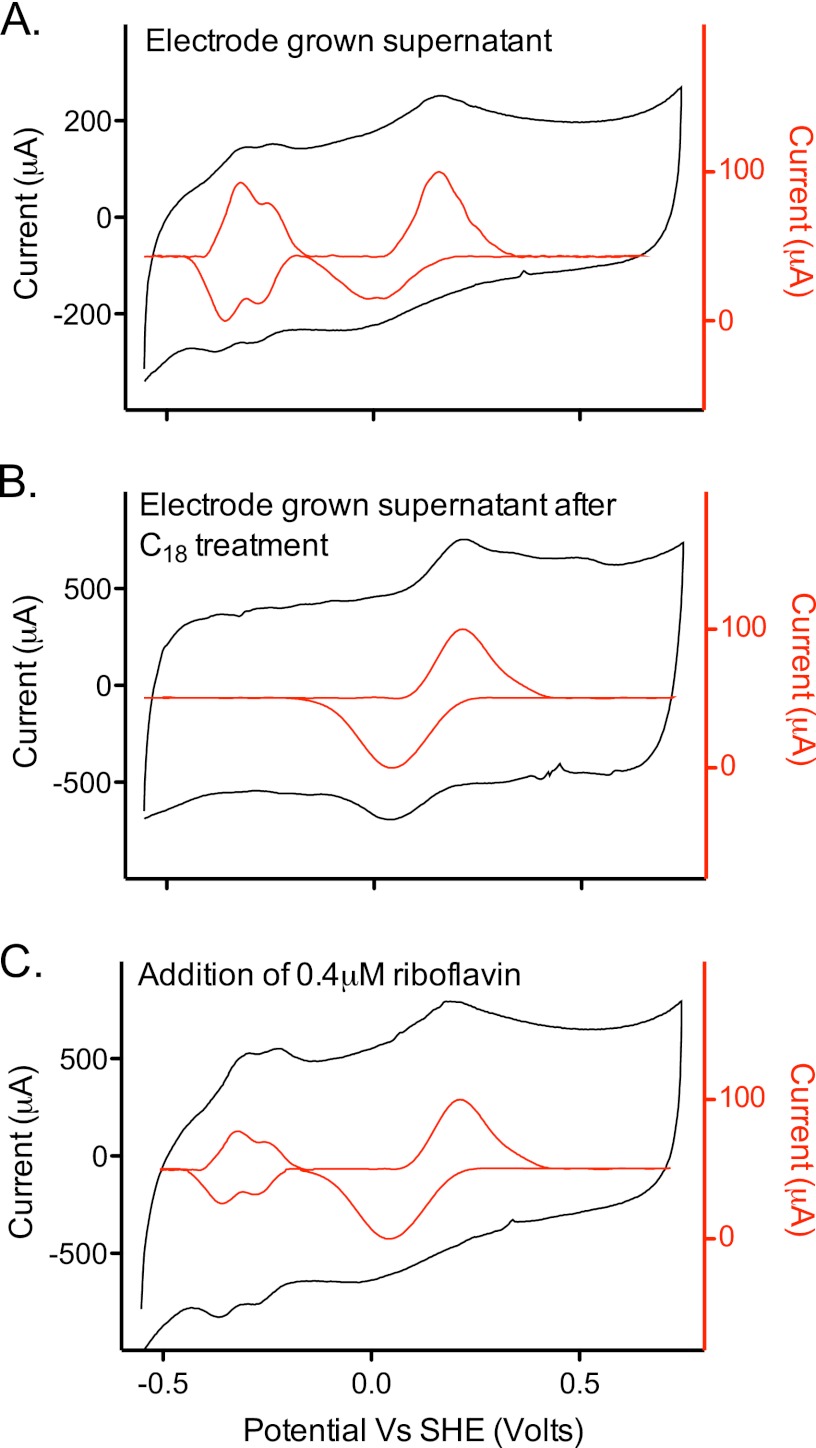
Fig 4.
Analytical electrochemistry evidence for two independent mediators. Supernatants collected from electrode-grown biofilms were centrifuged anaerobically to remove planktonic cells and analyzed using graphite electrodes. (A) Supernatant containing both the low- and high-potential-redox-active compounds. (B) Same supernatant after elution from a C18 column, showing the presence of only the higher-potential-redox-active compound. (C) Supernatant from panel B, after addition of 0.4 μM riboflavin. Both compounds were detected in electrode-grown and FeOOH-grown cultures but not in fumarate-grown cultures. All scan rates were 100 mV/s. Insets (red traces) are baseline subtracted.
Differential chromotographic separation was able to selectively remove one of these compounds, proving that two different mediators were present. Hydrophobic affinity columns, such as octadecyl-functionalized silica gel (C18), removed the compound associated with the lower potential window (−0.2 V versus SHE) without altering the higher potential peak (Fig. 4B). When analyzed by HPLC, a single compound was detected in all supernatants that eluted at exactly the same time as authentic riboflavin standards, and this compound was not present in C18-treated samples.
Unlike for Shewanella, which first secretes flavin adenine dinucleotide (FAD) and enzymatically processes it to FMN, no FMN or FAD could be detected in cell-free supernatants of G. fermentans cultures. Elution of C18 columns with methanol detected only a compound that eluted in HPLC as riboflavin, and the fluorescence emission profile of this eluted compound also was identical to that of riboflavin. When pure riboflavin was added back to C18-treated supernatants, the low-potential redox peak returned in electrochemical assays (Fig. 4C).
Throughout these studies, the higher-redox-potential compound showed no affinity for binding to graphite electrodes, hydrophobic matrices (e.g., compare high potential peaks in Fig. 4A and 4B), or filters used to sterilize supernatants. Treatment with high concentrations of commercial proteases had no effect on the size or location of this peak, suggesting that this mediator was not protein based. Treatment with metal-binding resins also had no effect on the size or location of this residual redox activity, suggesting that this redox peak was not due to a chelator or metal.
When riboflavin was depleted from these samples by C18 treatment, the supernatant containing the remaining high potential compound demonstrated a characteristic UV fluorescence with an excitation maximum of 229 nm. Emission by this fluorescent compound shifted to lower wavelengths after chemical reduction of samples. This redox activity and fluorescence were not detected in supernatants from fumarate-grown cultures. These observations were consistent with a hydrophilic fluorescent compound reported previously for G. fermentans supernatants (42).
Both redox shuttles are also produced in Fe(III)-grown G. fermentans cultures.
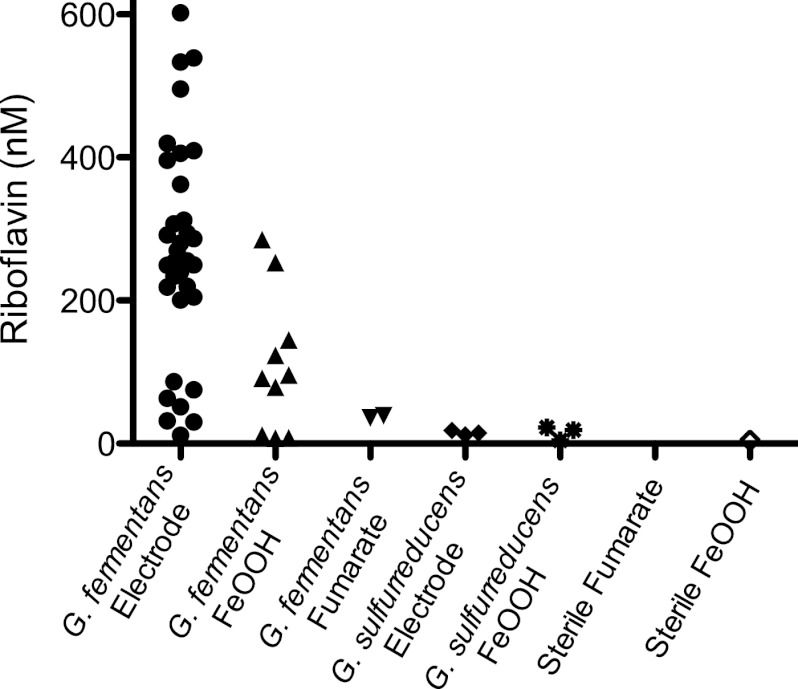
All electrode-grown and FeOOH-grown supernatants were reanalyzed using HPLC for the presence of riboflavin. All electrode-grown cultures contained riboflavin, although the concentration varied widely, from ∼200 nM (after 1 to 2 weeks of incubation) to ∼600 nM (after 3 to 4 weeks of incubation) (Fig. 5). Supernatants from FeOOH-grown cultures also contained a range of riboflavin concentrations, with levels between 75 nM and 300 nM detected. Since riboflavin is known to bind FeOOH and to spontaneously degrade upon exposure to light (37), these values represent a lower boundary of riboflavin concentrations. In contrast, G. fermentans cultures grown using soluble electron acceptors always had less than 20 nM riboflavin, a level at the limit of detection and similar to trace levels found in G. sulfurreducens supernatants from fumarate-, FeOOH-, and electrode-grown cultures. FeOOH-grown supernatants collected from four recently isolated Geothrix spp. (all within 95% 16S rRNA similar to G. fermentans; kindly provided by Z. Shelobolina) also contained riboflavin in the range of 100 to 150 nM, providing preliminary evidence that secretion of riboflavin may be conserved in related strains.
Fig 5.
Ranges of riboflavin concentrations in cultures as detected by HPLC. Electrode-grown G. fermentans cultures always accumulated riboflavin, with the levels generally increasing with length of incubation. Riboflavin was always detected in FeOOH-grown cultures, but recovery was typically lower. Fumarate-grown G. fermentans cultures always contained less than 20 nM riboflavin, similar to Geobacter supernatants collected from electrode- and FeOOH-grown cultures.
Sterile supernatants from FeOOH-grown G. fermentans cultures were also analyzed to determine if the high-redox-potential compound was also secreted under these conditions. As previous studies detected significant levels of soluble Fe(III) in G. fermentans cultures reducing FeOOH (42), it was possible that chelators maintaining trace minerals in a soluble state could create shuttling-like activity in our assays. To limit this possibility, all supernatants analyzed in this study were treated with metal-binding resins to remove soluble metals. Electrochemistry of sterile supernatants from FeOOH-grown cultures revealed that FeOOH-grown cultures always produced both riboflavin and the fluorescent high-potential compound. When these sterile, metal-depleted supernatants from FeOOH-grown cultures were added back to washed biofilms of G. fermentans, electron transfer to electrodes was stimulated in the same two potential windows as electrode-grown supernatants (data not shown). This confirmed that redox-active compounds produced during growth with electrodes were also produced during more environmentally relevant conditions, such as growth with FeOOH.
Stimulation of one pathway is at the expense of the other.
The presence of two redox-active compounds raised the question of whether the two mediators competed for electrons from the same supply or were part of independent additive pathways. The experiments where riboflavin was depleted from supernatants and added back to growing biofilms required medium exchanges, elution of medium through columns, and extensive reactor manipulation to execute. At the very least, switching respiration on and off introduced the artifact of the biofilm capacitance described in Fig. 1A, and the requirement for discharging the biofilm before additional electrochemical measurements could be obtained. These manipulations limited the sensitivity of the assay.
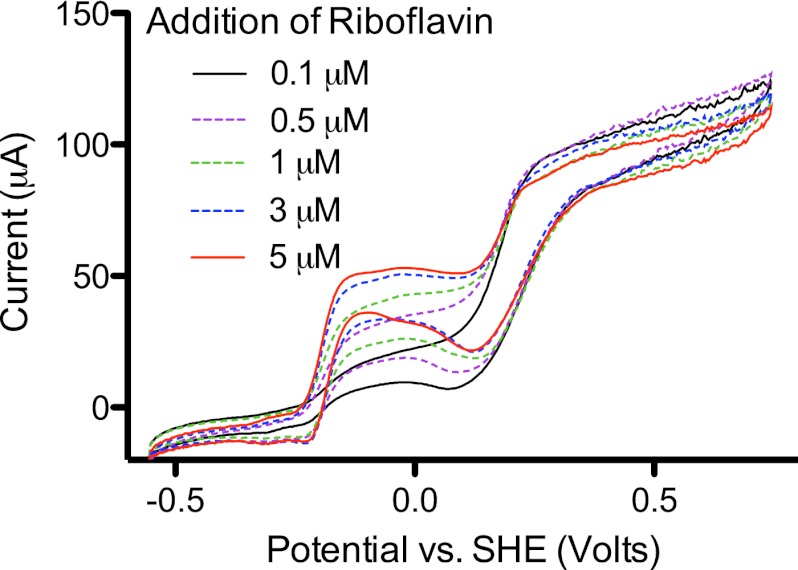
To alter redox shuttle concentrations under more controlled conditions, riboflavin was added stepwise to biofilms, allowing the concentration of the high-potential mediator and attached biomass to remain constant. Addition of riboflavin in this manner only increased the rate of electron transfer at the redox potential of riboflavin, without altering the total rate of electron transfer by the biofilm (Fig. 6). Thus, when riboflavin was present, electrons could be transferred at a lower potential, but at the expense of flux available to flow through the higher-potential pathway.
Fig 6.
Dose response of an electrode-grown biofilm of G. fermentans. Addition of riboflavin, up to concentrations of 5 μM, affected electron transfer only at the potential of −0.2 V versus SHE, without stimulating overall current production.
DISCUSSION
Acidobacterial sequences related to Geothrix are regularly detected in Fe(III)-reducing environments, particularly in zones recently stimulated to cause bioprecipitation of U(VI) (9, 59). For example, Geothrix-like Acidobacteria were present in unamended sediments from the Oak RidgeField Research Center and increased after lactate was injected into wells (5). Microcosm experiments from the same site also found Geothrix-like Acidobacteria comprising between 22 and 90% of 16S rRNA sequences after stimulation, depending on the electron donor (32). While such experiments also consistently detect Geobacter-like organisms, there is little information to address how these Fe(III)-reducing bacteria might coexist in the environment. These electrochemical data show that these organisms have fundamentally different approaches, in terms of respiration rates, need for direct contact, and the redox potentials at which they can operate.
A two-mediator, two-redox-potential window strategy.
To characterize the Fe(III) reduction strategy of G. fermentans, electrochemistry was used to control the redox potential of its electron acceptor during growth. These experiments revealed a dependence on higher redox potentials not previously observed in Fe(III)-reducing bacteria (Fig. 2 and 3). Sterile filtered supernatants from both electrode-grown and FeOOH-grown cultures confirmed that two separate redox-active compounds were secreted that stimulated electron transfer of G. fermentans (Fig. 4). The low-potential mediator demonstrated a high affinity for hydrophobic resins, facilitating separation and identification of the mediator as riboflavin. The other electron transfer mediator secreted by Geothrix had a much higher potential (∼0.3 V versus SHE) than natural phenazine or isoalloxazine ring-based flavin compounds and was much more hydrophilic. Unlike flavins that bind tightly to graphite electrodes (37), the high-potential compound was easily washed from electrodes. Nevin and Lovley (42) reported that G. fermentans supernatants contained a water-soluble compound which fluoresced under UV light and that migrated in thin-layer chromatography similarly to model quinones. We also observed UV fluorescence in fractions from both electrode biofilms and Fe(III)-grown cultures. Even after riboflavin was removed, a hydrophilic high-potential redox-active compound remained. This behavior is consistent with substituted hydrophilic quinones, but as over 1,500 naturally produced quinones exist, the exact identity of this compound remains speculative. Purification of this compound will aid in the study of its reactivity with Fe(III) or other possible terminal acceptors under environmental conditions.
Because the low- and high-potential compounds could be easily separated, it was also possible to alter the concentration of each mediator in supernatants and correlate their presence with electron transfer at their characteristic redox potential. These experiments indicated that the effects of the two mediators were not additive. When additional riboflavin was added to biofilms, electron transfer rates at lower potentials increased but at the expense of electron transfer at higher potentials, resulting in the same total flux.
Comparisons with other redox shuttles.
Some mediators appear to be secreted by bacteria to increase electron transfer to oxygen. Examples include phenazines (midpoint potential [E°′] = −0.040 to −0.170 versus SHE) secreted by Pseudomonas spp., which improve survival of biofilm cells in anaerobic zones (56). Strains of Bifidobacterium grow to higher yields in the presence of 2-amino-3-carboxy-1,4-naphthoquinone (E°′ = −0.071 V) (a substituted hydrophilic quinone secreted by Propionibacteria), by shuttling electrons to oxygen and increasing NADH oxidation (61).
In contrast, flavins have been shown to participate in reactions related to reduction of metals. FMN secreted by Shewanella spp. (E°′ = −0.21 V) enhances reduction of many external metals and azo dyes and accounts for approximately 80% of electron transfer to extracellular acceptors (11, 37, 54). Gradients of oxidized flavins allow motile Shewanella strains to locate insoluble acceptors in space via energy taxis (26). Flavins secreted by plant roots are hypothesized to increase microbial rhizosphere availability of Fe(III) (46), and flavins in both yeast extract and intestinal contents promote electron transfer to oxygen (24) and electrodes (39). The finding that members of this phylogenetically distant acidobacterial group secrete riboflavin at concentrations similar to those secreted by Shewanella, specifically under Fe(III)-reducing conditions, shows that use of this versatile electron shuttle may be more widely distributed throughout the bacterial domain than previously recognized.
What is the benefit of using higher-potential electron acceptors?
A final question is the ecological implication of electron transfer in the higher-potential domain. One benefit to higher-potential electron acceptors, in general, is the opportunity to capture more ATP. Based on the lowest potential at which Geobacter's respiratory chain operates (−0.22 V), and the redox potential of acetate (−0.28 V), the poor ΔGo′ of this redox couple (−46 kJ/mol acetate) explains why Geobacter has a growth yield consistent with only capturing <1 ATP per acetate (33, 38). However, this low yield also means that in low-redox-potential environments, such as permanently anoxic zones, Geobacter can still respire and compete even when environmental conditions lower the ΔG of acetate oxidation to values in this range (Fig. 7A and B).
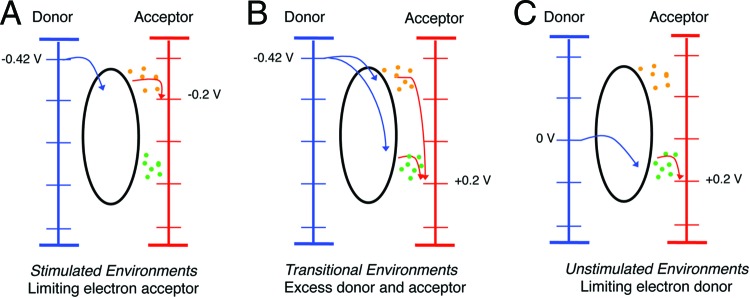
Fig 7.
Examples of environmental niches that could exist as a function of varying electron donor and acceptor concentrations if an organism can take advantage of multiple redox potentials. (A) Environments where donor is available, but acceptor concentrations (or metal redox state) limit the acceptor redox potential to −0.2 V. (B) Environments with excess electron donors and acceptors, such as during laboratory growth, shortly after stimulation of an oxidized habitat with electron donor, or with electrodes poised at high potentials. (C) Environments where donor concentrations are limiting but the environment is oxidized, such as in low-carbon sediments or near oxic-anoxic interfaces.
If Geothrix were to capture additional ATP via electron flow to higher-potential electron acceptors (such as above an E°′ of 0.2 V), a nearly 600% higher yield would be expected. In practical terms, Geothrix should reach OD600s of at least 0.3 (instead of the ∼0.05 observed) when respiring Fe(III)-citrate. On electrodes, instead of producing ∼100 μg protein/cm2 from oxidation of 10 mM acetate (standard for Geobacter), one would expect over 600 μg/cm2 from the equivalent amount of lactate. Yet, in all of our experiments, and all reported incubations with Geothrix and Fe(III) (10, 42), there is no evidence that Geothrix achieves significantly higher ATP yields from respiration.
In the absence of any evidence for increased ATP production, and based also on the fact that this slow-growing organism persists in unstimulated habitats where electron donor concentrations are low, another option is a strategy to enable survival under conditions where a low ΔG exists. Oligotrophic sediments lacking a constant input of electron donor are higher in potential and interspersed with oxygenated zones. An organism able to shuttle electrons to freshly precipitated Fe(III) or Mn(IV) oxides, or low levels of oxygen, could grow at very low donor concentrations, but only if that organism conserved a small amount of energy from the process. By keeping only a small amount of energy for itself, an organism like Geothrix could position its metabolic strategy to respire at very low electron donor concentrations, as shown in Fig. 7C.
It was coupling of hydrogen partial pressure measurements with thermodynamic calculations that led Lovley and Goodwin (31) to explain zones where respiration was dominated by the presence of a single electron acceptor. In the typical version of this model, electron donors such as H2 are released from fermentation of organic matter. Strong electron acceptors such as NO3 provide a sufficiently negative ΔGo′ to support growth at H2 levels below 1 nM, a concentration unfavorable for reduction of Fe(III) and SO4. This concept of “threshold” donor concentrations was confirmed by measurements of pure cultures, typically under conditions of excess electron acceptor (6, 28, 30).
The observation that G. fermentans can utilize higher-potential acceptors, and actively releases a compound which would be beneficial only at higher redox potentials, provides an example of an organism which may gain a competitive advantage under different conditions than typical permanently anoxic Fe(III)-reducing zones. Whether this high-potential window evolved for oxidized metals, shuttling to oxygen, or other acceptors remains to be discovered. Future enrichments targeting bacteria that have evolved to survive in such thermodynamic windows may need to control both redox potential and donor concentrations, to avoid organisms like Geobacter shifting conditions to where they can become dominant. In addition, work characterizing communities at oxic-anoxic interfaces, or in subsurface environments where this niche could exist, should consider the possibility that not all metal-reducing bacteria function best at low environmental redox potentials.
Supplementary Material
ACKNOWLEDGMENTS
D.R.B. is supported by the Office of Naval Research and the DOE Office of Science (BER). M.G.M.-K. was supported by a 3M Science and Technology Fellowship.
We thank Z. Shelobolina for kindly providing recently isolated Geothrix spp.
Footnotes
Published ahead of print 27 July 2012
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1. Abed RMM, et al. 2002. Microbial diversity of a heavily polluted microbial mat and its community changes following degradation of petroleum compounds. Appl. Environ. Microbiol. 68: 1674–1683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barns S, Cain E, Sommerville L. 2007. Acidobacteria phylum sequences in uranium contaminated subsurface sediments greatly expand the known diversity within the phylum. Appl. Environ. Microbiol. 73: 3113–3116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baron D, LaBelle E, Coursolle D, Gralnick JA, Bond DR. 2009. Electrochemical measurement of electron transfer kinetics by Shewanella oneidensis MR-1. J. Biol. Chem. 284: 28865–28873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bond DR, Lovley DR. 2005. Evidence for involvement of an electron shuttle in electricity generation by Geothrix fermentans. Appl. Environ. Microbiol. 71: 2186–2189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brodie EL, et al. 2006. Application of a high-density oligonucleotide microarray approach to study bacterial population dynamics during uranium reduction and reoxidation. Appl. Environ. Microbiol. 72: 6288–6298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brown D, Komlos J, Jaffe P. 2005. Simultaneous utilization of acetate and hydrogen by Geobacter sulfurreducens and implications for use of hydrogen as an indicator of redox conditions. Environ. Sci. Technol. 39: 3069–3076 [DOI] [PubMed] [Google Scholar]
- 7. Bryant DA, et al. 2007. Candidatus Chloracidobacterium thermophilum: an aerobic phototrophic acidobacterium. Science 317: 523–526 [DOI] [PubMed] [Google Scholar]
- 8. Cardenas E, et al. 2008. Microbial communities in contaminated sediments, associated with bioremediation of uranium to submicromolar levels. Appl. Environ. Microbiol. 74: 3718–3729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chae K-J, Choi M-J, Lee J-W, Kim K-Y, Kim IS. 2009. Effect of different substrates on the performance, bacterial diversity, and bacterial viability in microbial fuel cells. Bioresour. Technol. 100: 3518–3525 [DOI] [PubMed] [Google Scholar]
- 10. Coates JD, Ellis DJ, Gaw CV, Lovley DR. 1999. Geothrix fermentans gen. nov., sp. nov., a novel Fe(III)-reducing bacterium from a hydrocarbon-contaminated aquifer. Int. J. Syst. Bacteriol. 49: 1615–1622 [DOI] [PubMed] [Google Scholar]
- 11. Coursolle D, Baron DB, Bond DR, Gralnick JA. 2010. The Mtr respiratory pathway is essential for reducing flavins and electrodes in Shewanella oneidensis. J. Bacteriol. 192: 467–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Covington ED, Gelbmann CB, Kotloski NJ, Gralnick JA. 2010. An essential role for UshA in processing of extracellular flavin electron shuttles by Shewanella oneidensis. Mol. Microbiol. 78: 519–532 [DOI] [PubMed] [Google Scholar]
- 13. Dietrich LEP, Teal TK, Price-Whelan A, Newman DK. 2008. Redox-active antibiotics control gene expression and community behavior in divergent bacteria. Science 321: 1203–1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Eichorst SA, Breznak JA, Schmidt TM. 2007. Isolation and characterization of soil bacteria that define Terriglobus gen. nov., in the phylum Acidobacteria. Appl. Environ. Microbiol. 73: 2708–2717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fierer N, et al. 2012. Comparative metagenomic, phylogenetic, and physiological analyses of soil microbial communities across nitrogen gradients. ISME J. 6: 1007–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fourmond V, et al. 2009. SOAS: a free program to analyze electrochemical data and other one-dimensional signals. Bioelectrochemistry 76: 141–147 [DOI] [PubMed] [Google Scholar]
- 17. Garcia Costas AM, et al. 2012. Complete genome of Candidatus Chloracidobacterium thermophilum, a chlorophyll-based photoheterotroph belonging to the phylum Acidobacteria. Environ. Microbiol. 14: 177–190 [DOI] [PubMed] [Google Scholar]
- 18. George IF, Hartmann M, Liles MR, Agathos SN. 2011. Recovery of as-yet-uncultured soil acidobacteria on dilute solid media. Appl. Environ. Microbiol. 77: 8184–8188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gralnick JA, Newman DK. 2007. Extracellular respiration. Mol. Microbiol. 65: 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hernandez M, Kappler A, Newman D. 2004. Phenazines and other redox-active antibiotics promote microbial mineral reduction. Appl. Environ. Microbiol. 70: 921–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Islam F, Boothman C, Gault A, Polya D, Lloyd J. 2005. Potential role of the Fe(III)-reducing bacteria Geobacter and Geothrix in controlling arsenic solubility in Bengal delta sediments. Mineral. Mag. 69: 865–875 [Google Scholar]
- 22. Islam F, et al. 2004. Role of metal-reducing bacteria in arsenic release from Bengal delta sediments. Nature 430: 68–71 [DOI] [PubMed] [Google Scholar]
- 23. Kalyuzhnaya MG, Lidstrom ME, Chistoserdova L. 2008. Real-time detection of actively metabolizing microbes by redox sensing as applied to methylotroph populations in Lake Washington. ISME J. 2: 696–706 [DOI] [PubMed] [Google Scholar]
- 24. Khan MT, et al. 2012. The gut anaerobe Faecalibacterium prausnitzii uses an extracellular electron shuttle to grow at oxic-anoxic interphases. ISME J. 6: 1578–1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kielak AM, van Veen JA, Kowalchuk GA. 2010. Comparative analysis of acidobacterial genomic fragments from terrestrial and aquatic metagenomic libraries, with emphasis on Acidobacteria subdivision 6. Appl. Environ. Microbiol. 76: 6769–6777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li R, Tiedje JM, Chiu C, Worden RM. 2012. Soluble electron shuttles can mediate energy taxis toward insoluble electron acceptors. Environ. Sci. Technol. 46: 2813–2820 [DOI] [PubMed] [Google Scholar]
- 27. Lloyd J, Blunt-Harris E, Lovley D. 1999. The periplasmic 9.6-kilodalton c-type cytochrome of Geobacter sulfurreducens is not an electron shuttle to Fe(III). J. Bacteriol. 181: 7647–7649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Löffler F, Tiedje J, Sanford R. 1999. Fraction of electrons consumed in electron acceptor reduction and hydrogen thresholds as indicators of halorespiratory physiology. Appl. Environ. Microbiol. 65: 4049–4056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lovley DR, Holmes DE, Nevin KP. 2004. Dissimilatory Fe(III) and Mn(IV) reduction. Adv. Microb. Physiol. 49: 219–286 [DOI] [PubMed] [Google Scholar]
- 30. Lovley D. 1985. Minimum threshold for hydrogen metabolism in methanogenic bacteria. Appl. Environ. Microbiol. 49: 1530–1531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lovley D, Goodwin S. 1988. Hydrogen concentrations as an indicator of the predominant terminal electron-accepting reactions in aquatic sediments. Geochim. Cosmochim. Acta 52: 2993–3003 [Google Scholar]
- 32. Luo W, et al. 2007. Influence of bicarbonate, sulfate, and electron donors on biological reduction of uranium and microbial community composition. Appl. Microbiol. Biotechnol. 77: 713–721 [DOI] [PubMed] [Google Scholar]
- 33. Mahadevan R, et al. 2006. Characterization of metabolism in the Fe(III)-reducing organism Geobacter sulfurreducens by constraint-based modeling. Appl. Environ. Microbiol. 72: 1558–1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Männistö MK, Rawat S, Starovoytov V, Häggblom MM. 2011. Terriglobus saanensis sp. nov., an acidobacterium isolated from tundra soil. Int. J. Syst. Evol. Microbiol. 61: 1823–1828 [DOI] [PubMed] [Google Scholar]
- 35. Marshall CW, May HD. 2009. Electrochemical evidence of direct electrode reduction by a thermophilic Gram-positive bacterium, Thermincola ferriacetica. Energ. Environ. Sci. 2: 699–705 [Google Scholar]
- 36. Marsili E, Rollefson JB, Baron DB, Hozalski RM, Bond DR. 2008. Microbial biofilm voltammetry: direct electrochemical characterization of catalytic electrode-attached biofilms. Appl. Environ. Microbiol. 74: 7329–7337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Marsili E, et al. 2008. Shewanella secretes flavins that mediate extracellular electron transfer. Proc. Natl. Acad. Sci. U. S. A. 105: 3968–3973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Marsili E, Sun J, Bond DR. 2010. Voltammetry and growth physiology of Geobacter sulfurreducens biofilms as a function of growth stage and imposed electrode potential. Electroanalysis 22: 865–874 [Google Scholar]
- 39. Masuda M, Freguia S, Wang Y-F, Tsujimura S, Kano K. 2010. Flavins contained in yeast extract are exploited for anodic electron transfer by Lactococcus lactis. Bioelectrochemistry 78: 173–175 [DOI] [PubMed] [Google Scholar]
- 40. McBeth JM, et al. 2007. Technetium reduction and reoxidation in aquifer sediments. Geomicrobiol. J. 24: 189–197 [Google Scholar]
- 41. Nevin K, Lovley D. 2000. Lack of production of electron-shuttling compounds or solubilization of Fe(III) during reduction of insoluble Fe(III) oxide by Geobacter metallireducens. Appl. Environ. Microbiol. 66: 2248–2251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nevin K, Lovley D. 2002. Mechanisms for accessing insoluble Fe(III) oxide during dissimilatory Fe(III) reduction by Geothrix fermentans. Appl. Environ. Microbiol. 68: 2294–2299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Paquete CM, Louro RO. 2010. Molecular details of multielectron transfer: the case of multiheme cytochromes from metal respiring organisms. Dalton Trans. 39: 4259–4266 [DOI] [PubMed] [Google Scholar]
- 44. Parameswaran P, Zhang H, Torres CI, Rittmann BE, Krajmalnik-Brown R. 2010. Microbial community structure in a biofilm anode fed with a fermentable substrate: the significance of hydrogen scavengers. Biotechnol. Bioeng. 105: 69–78 [DOI] [PubMed] [Google Scholar]
- 45. Quaiser A, et al. 2003. Acidobacteria form a coherent but highly diverse group within the bacterial domain: evidence from environmental genomics. Mol. Microbiol. 50: 563–575 [DOI] [PubMed] [Google Scholar]
- 46. Rodríguez-Celma J, et al. 2011. Characterization of flavins in roots of Fe-deficient strategy I plants, with a focus on Medicago truncatula. Plant Cell Physiol. 52: 2173–2189 [DOI] [PubMed] [Google Scholar]
- 47. Ross DE, Brantley SL, Tien M. 2009. Kinetic characterization of OmcA and MtrC, terminal reductases involved in respiratory electron transfer for dissimilatory iron reduction in Shewanella oneidensis MR-1. Appl. Environ. Microbiol. 75: 5218–5226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ross DE, Flynn JM, Baron DB, Gralnick JA, Bond DR. 2011. Towards electrosynthesis in Shewanella: energetics of reversing the mtr pathway for reductive metabolism. PLoS One 6: e16649 doi:10.1371/journal.pone.0016649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sait M, Hugenholtz P, Janssen P. 2002. Cultivation of globally distributed soil bacteria from phylogenetic lineages previously only detected in cultivation-independent surveys. Environ. Microbiol. 4: 654–666 [DOI] [PubMed] [Google Scholar]
- 50. Shi L, Squier TC, Zachara JM, Fredrickson JK. 2007. Respiration of metal (hydr)oxides by Shewanella and Geobacter: a key role for multihaem c-type cytochromes. Mol. Microbiol. 65: 12–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Torres CI, et al. 2009. Selecting anode-respiring bacteria based on anode potential: phylogenetic, electrochemical, and microscopic characterization. Environ. Sci. Technol. 43: 9519–9524 [DOI] [PubMed] [Google Scholar]
- 52. Torres CI, et al. 2010. A kinetic perspective on extracellular electron transfer by anode-respiring bacteria. FEMS Microbiol. Rev. 34: 3–17 [DOI] [PubMed] [Google Scholar]
- 53. Torres CI, Marcus AK, Parameswaran P, Rittmann BE. 2008. Kinetic experiments for evaluating the Nernst-Monod model for anode-respiring bacteria (ARB) in a biofilm anode. Environ. Sci. Technol. 42: 6593–6597 [DOI] [PubMed] [Google Scholar]
- 54. von Canstein H, Ogawa J, Shimizu S, Lloyd JR. 2008. Secretion of flavins by Shewanella species and their role in extracellular electron transfer. Appl. Environ. Microbiol. 74: 615–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wang X, et al. 2009. Use of carbon mesh anodes and the effect of different pretreatment methods on power production in microbial fuel cells. Environ. Sci. Technol. 43: 6870–6874 [DOI] [PubMed] [Google Scholar]
- 56. Wang Y, Kern SE, Newman DK. 2010. Endogenous phenazine antibiotics promote anaerobic survival of Pseudomonas aeruginosa via extracellular electron transfer. J. Bacteriol. 192: 365–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wang Y, Newman DK. 2008. Redox reactions of phenazine antibiotics with ferric (hydr)oxides and molecular oxygen. Environ. Sci. Technol. 42: 2380–2386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ward NL, et al. 2009. Three genomes from the phylum Acidobacteria provide insight into the lifestyles of these microorganisms in soils. Appl. Environ. Microbiol. 75: 2046–2056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wu W-M, et al. 2007. In situ bioreduction of uranium (VI) to submicromolar levels and reoxidation by dissolved oxygen. Environ. Sci. Technol. 41: 5716–5723 [DOI] [PubMed] [Google Scholar]
- 60. Xing D, Zuo Y, Cheng S, Regan JM, Logan BE. 2008. Electricity generation by Rhodopseudomonas palustris DX-1. Environ. Sci. Technol. 42: 4146–4151 [DOI] [PubMed] [Google Scholar]
- 61. Yamazaki S, Kaneko T, Taketomo N, Kano K, Ikeda T. 2002. 2-Amino-3-carboxy-1,4-naphthoquinone affects the end-product profile of bifidobacteria through the mediated oxidation of NAD(P)H. Appl. Microbiol. Biotechnol. 59: 72–78 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.