Abstract
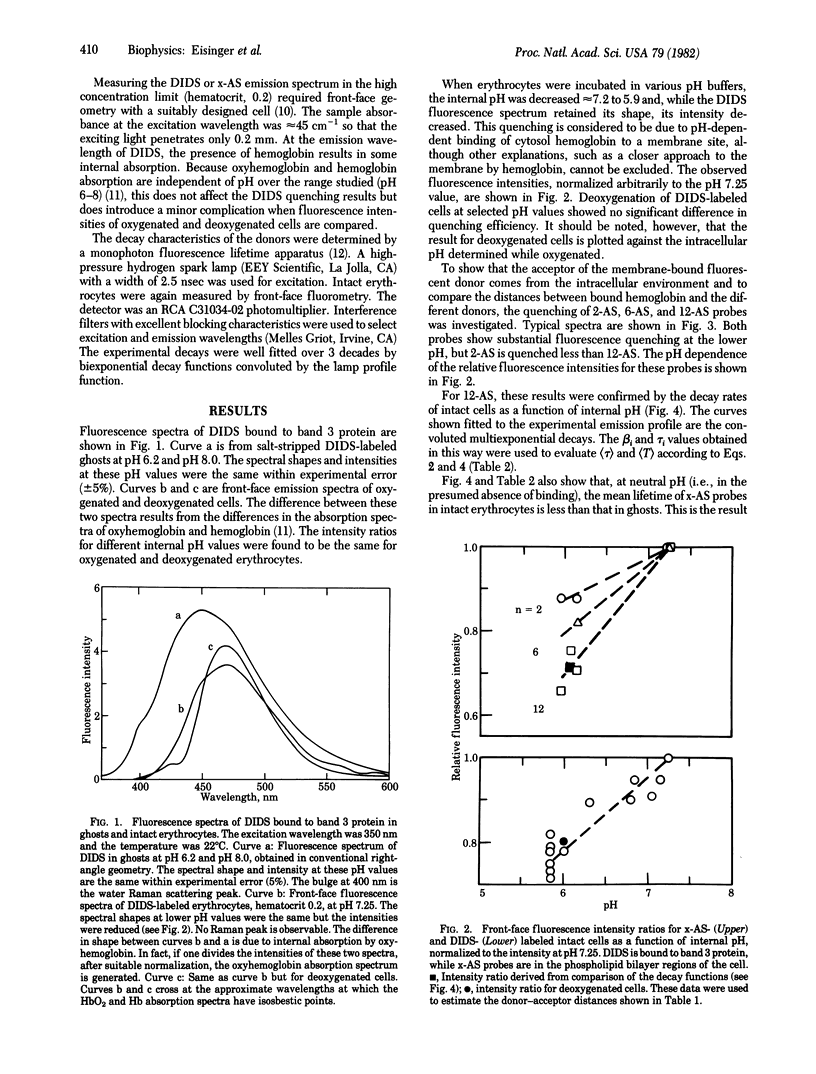
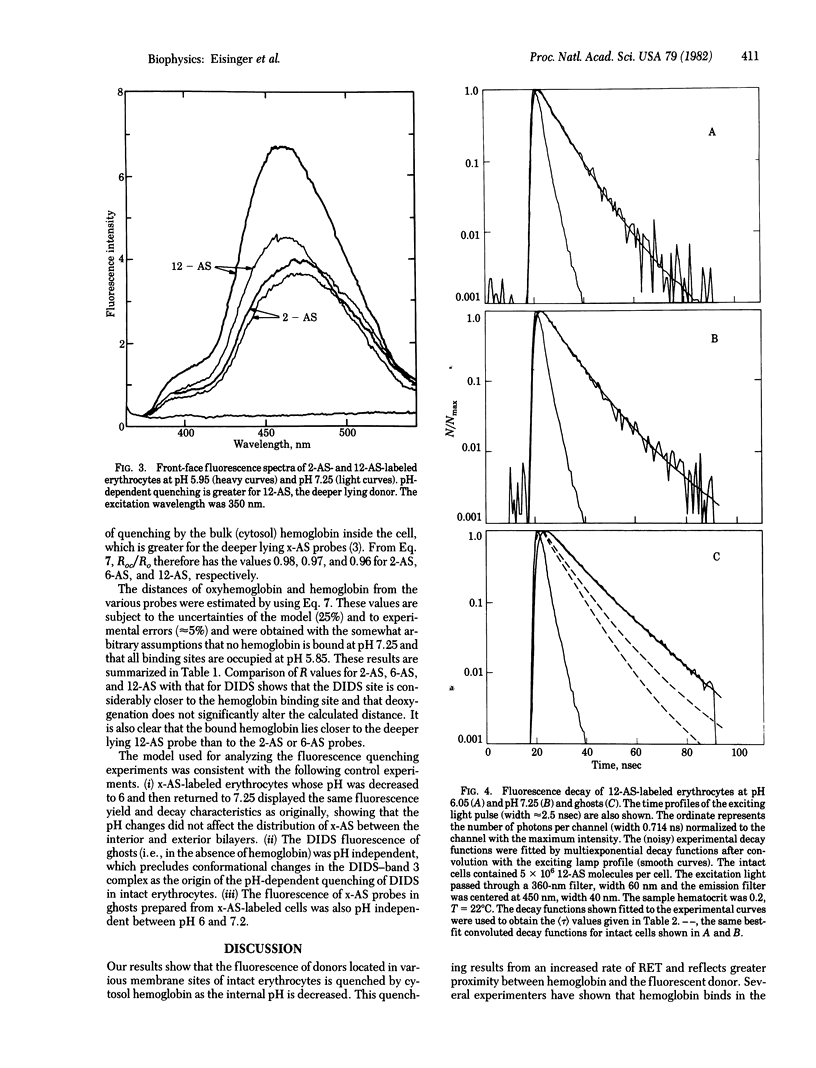
The problem of demonstrating hemoglobin binding to the erythrocyte membrane in intact cells was approached by observing the quenching of fluorescent membrane probes by hemoglobin as a function of pH. This quenching was studied by measuring fluorescence intensities and decay rates of membrane-bound donors by both right-angle and front-face fluorometry. The donors included 4,4'-bis(isothiocyano)-2,2'-stilbene disulfonate (DIDS) bound to the band 3 protein and 2-, 6-, and 12-(9-anthroyloxy(stearic acid (2-AS, 6-AS, and 12-AS) in the lipid portion of the membrane. The probe fluorescence is quenched progressively as the intracellular pH is decreased from 7.2 to 5.9 and does not depend on the oxygenation state. Since the fluorescence characteristic of the DIDS and x-AS probes in ghosts are independent of pH over this range, this quenching is due to the greater proximity of the donors and hemoglobin. Because hemoglobin binding to the band 3 protein in ghosts has previously been shown under conditions of low ionic strength and hemoglobin concentration, the present results were analyzed by a model in which a hemoglobin molecule is bound or in close proximity to a band 3 protein at pH 6. By using resonance energy transfer theory, we found the distance from DIDS to the bound hemoglobin to be approximately 4 A, which is within the range of distances measured between the DIDS binding site and the band 3 protein cytoplasmic sulfhydryl groups. Furthermore, the pH-dependent fluorescence quenching of 12-AS was stronger than that of 2-AS, showing that a cytoplasmic acceptor is involved and that the average distance between hemoglobin and these probes was greater than that for DIDS.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cass A., Dalmark M. Equilibrium dialysis of ions in nystatin-treated red cells. Nat New Biol. 1973 Jul 11;244(132):47–49. doi: 10.1038/newbio244047a0. [DOI] [PubMed] [Google Scholar]
- Dale R. E., Eisinger J., Blumberg W. E. The orientational freedom of molecular probes. The orientation factor in intramolecular energy transfer. Biophys J. 1979 May;26(2):161–193. doi: 10.1016/S0006-3495(79)85243-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalmark M. Chloride and water distribution in human red cells. J Physiol. 1975 Aug;250(1):65–84. doi: 10.1113/jphysiol.1975.sp011043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisinger J., Flores J. Front-face fluorometry of liquid samples. Anal Biochem. 1979 Apr 1;94(1):15–21. doi: 10.1016/0003-2697(79)90783-8. [DOI] [PubMed] [Google Scholar]
- Gauduchon P., Wahl P. Pulsefluorimetry of tyrosyl peptides. Biophys Chem. 1978 Mar;8(1):87–104. doi: 10.1016/0301-4622(78)85026-1. [DOI] [PubMed] [Google Scholar]
- Rao A., Martin P., Reithmeier R. A., Cantley L. C. Location of the stilbenedisulfonate binding site of the human erythrocyte anion-exchange system by resonance energy transfer. Biochemistry. 1979 Oct 16;18(21):4505–4516. doi: 10.1021/bi00588a008. [DOI] [PubMed] [Google Scholar]
- Salhany J. M., Cordes K. A., Gaines E. D. Light-scattering measurements of hemoglobin binding to the erythrocyte membrane. Evidence for transmembrane effects related to a disulfonic stilbene binding to band 3. Biochemistry. 1980 Apr 1;19(7):1447–1454. doi: 10.1021/bi00548a028. [DOI] [PubMed] [Google Scholar]
- Scarpa A., Cecchetto A., Azzone G. F. The mechanism of anion translocation and pH equilibration in erythrocytes. Biochim Biophys Acta. 1970;219(1):179–188. doi: 10.1016/0005-2736(70)90073-8. [DOI] [PubMed] [Google Scholar]
- Shaklai N., Abrahami H. The interaction of deoxyhemoglobin with the red cell membrane. Biochem Biophys Res Commun. 1980 Aug 14;95(3):1105–1112. doi: 10.1016/0006-291x(80)91586-7. [DOI] [PubMed] [Google Scholar]



