Abstract
Dishevelled (Dvl) is a key component in the canonical Wnt signaling pathway and becomes hyperphosphorylated upon Wnt stimulation. Dvl is required for LRP6 phosphorylation, which is essential for subsequent steps of signal transduction, such as Axin recruitment and cytosolic β-catenin stabilization. Here, we identify the HECT-containing Nedd4-like ubiquitin E3 ligase ITCH as a new Dvl-binding protein. ITCH ubiquitinates the phosphorylated form of Dvl and promotes its degradation via the proteasome pathway, thereby inhibiting canonical Wnt signaling. Knockdown of ITCH by RNA interference increased the stability of phosphorylated Dvl and upregulated Wnt reporter gene activity as well as endogenous Wnt target gene expression induced by Wnt stimulation. In addition, we found that both the PPXY motif and the DEP domain of Dvl are critical for its interaction with ITCH, as mutation in the PPXY motif (Dvl2-Y568F) or deletion of the DEP domain led to reduced affinity for ITCH. Consistently, overexpression of ITCH inhibited wild-type Dvl2-induced, but not Dvl2-Y568F mutant-induced, Wnt reporter activity. Moreover, the Y568F mutant, but not wild-type Dvl2, can reverse the ITCH-mediated inhibition of Wnt-induced reporter activity. Collectively, these results indicate that ITCH plays a negative regulatory role in modulating canonical Wnt signaling by targeting the phosphorylated form of Dvl.
INTRODUCTION
The Wnt signaling pathway plays pivotal roles during embryogenesis and is also linked to tumorigenesis (6, 22). Dvl is a central mediator of Wnt signaling in both canonical and noncanonical pathways. In the Wnt/β-catenin pathway, which is also termed the canonical Wnt pathway, it is generally believed that Dvl functions as a scaffold protein bridging the receptors and downstream signaling components (6, 11). It has been shown that LRP5/6 phosphorylation is an important event of Wnt signaling to promote Axin recruitment to membrane and cytosolic β-catenin stabilization, while Wnt-induced coclustering of receptors and Dvl in LRP signalosomes is required for this process (3). Moreover, previous studies have revealed that Dvl acts through regulating the production of Ptdlns(4,5)P2 to mediate LRP6 phosphorylation (29, 31). Although the signal transduction process from Frizzled/Dvl complex formation to stabilization of cytosolic β-catenin is complicated and remains incompletely understood, it is believed that Dvl-mediated LRP5/6 phosphorylation is clearly involved. On the other hand, recent findings indicated that Dvl also exists in the nucleus (9, 14, 41) and participates in β-catenin-T-cell factor (TCF) transcriptional complex formation by interacting with β-catenin and c-Jun, thus promoting canonical Wnt signaling (9). In addition, Dvl was also reported to interact with p65 in the nucleus to inhibit p65-mediated transcription (8).
As Dvl is a key component of the canonical Wnt pathway, regulation of its stability and activity is very important for proper signal transduction. During the last 10 years, several E3 ubiquitin (Ub) ligases for Dvl have been identified, including KLHL12–Cullin-3 ubiquitin ligase (1); pVHL, a component of an SCF (Skp1–Cdc53–F-box)-like ubiquitin ligase complex (10); NEDL1, a neuronal homologous to E6-AP carboxyl terminus (HECT)-type E3 ligase (26); and Malin, a RING finger domain containing E3 ubiquitin ligase (37). These E3 ubiquitin ligases were reported to promote ubiquitination and degradation of Dvl under different physiological conditions. As to the activation of Dvl, that Wnt stimulation induces Dvl phosphorylation has been known for decades; Wnt1, Wnt3a, and Wnt5a were reported to promote hyperphosphorylation of Dvl proteins (5, 12, 18). It has been shown that the phosphorylation of Dvl plays a relevant role to canonical Wnt signaling in the early embryogenesis of Xenopus; Dvl is phosphorylated at the certain time and place when and where canonical Wnt signaling is also activated (35). Some kinases, such as casein kinase 1ε (CK1ε), casein kinase 2 (CK2), and PAR-1, which can directly phosphorylate Dvl, were reported to play positive roles in canonical Wnt signaling (7, 16, 38, 40). Furthermore, some negative regulators of the canonical Wnt signaling pathway were shown to inhibit the phosphorylation of Dvl (15, 17, 42). All of these results suggest that phosphorylation of Dvl is required for Wnt signaling; however, the regulation of the activity and stability of Dvl in Wnt signaling is still incompletely understood.
To further elucidate the molecular mechanisms regulating the activity and stability of Dvl in canonical Wnt signaling, we performed a yeast two-hybrid screening to search for novel Dvl-interacting proteins. Here we report the identification of ITCH as a new Dvl-binding protein. ITCH is a ubiquitin ligase (E3) belonging to the HECT-type E3 subfamily. Like other HECT E3s, ITCH contains an N-terminal Ca2+-dependent phospholipid-binding C2 domain, four protein-protein interaction WW domains, and a C-terminal HECT domain (36). ITCH was initially found to be involved in immune responses (25). During the past 10 years, an increasing number of ITCH's targets have been implicated in tumorigenesis and chemosensitivity, such as p63, p73, Notch1, and others (23, 33, 34), indicating that ITCH may play a relevant role in cancer biology. In this work, we show that ITCH is functionally connected with the canonical Wnt pathway, which plays pivotal roles in many kinds of cancers (6, 22). Our data show that ITCH could promote the ubiquitination and degradation of phosphorylated Dvl and therefore inhibit canonical Wnt signaling.
MATERIALS AND METHODS
Plasmids, siRNAs, and antibodies.
Full-length human ITCH was amplified from total RNA of HEK293T cells by reverse transcription (RT)-PCR, and the PCR product was cloned into vectors pCMV-HA and pGEX-4T2. Site-directed mutagenesis was used to produce the ligase-dead mutant (C830A in human ITCH) of hemagglutinin (HA)-tagged ITCH and the PPXY motif mutants (mutations Y528F in human Dvl1, Y568F in human Dvl2, and Y662F in human Dvl3) of FLAG-tagged Dvls. Other plasmids have been used previously (9). Two small interfering RNAs (siRNAs) targeting ITCH (ITCH siRNA-1 and ITCH siRNA-2) were synthesized according to the sequences 5′-CGGGCGAGUUUACUAUGUATT-3′ and 5′-AGAUUACAGUGACAAGAAATT-3′, respectively. Dvl2 and Dvl3 antibodies were purchased from Cell Signaling. ITCH and β-catenin antibodies were from BD Biosciences. Antiactin, antiubiquitin, and anti-glycogen synthase kinase 3α/ß (anti-GSK3α/β) antibodies were from Santa Cruz, and anti-β-tubulin antibody was from Sigma-Aldrich.
Yeast two-hybrid screen.
Yeast two-hybrid screen systems and a pPC86-based mouse embryonic E10.5 cDNA library were purchased from Invitrogen. Mouse Dvl1 C1 (residues 377 to 695) was subcloned into pDB plasmid, which was used as bait. A yeast two-hybrid assay was performed according to the manufacturer's manual.
Cell culture, transfection, and reporter gene assay.
Cells of HEK293T, NIH 3T3, MDA-MB-231, and SW480 were maintained in Dulbecco's modified Eagle's medium (Gibco) plus 10% fetal bovine serum (Gibco). HEK293 cells stably transfected with Dvl2 (Dvl2-293 stable cell line) were maintained in Essential medium (Gibco) plus 10% fetal bovine serum (Gibco). Cells were seeded in plates 24 h before transfection, and plasmids were transfected using Lipofectamine/Plus reagent (Invitrogen) according to the manufacturer's instructions. For the siRNA assay, Lipofectamine RNAiMAX (Invitrogen) was used. For the reporter gene assay, HEK293T cells in a 24-well plate were transfected with 250 ng of plasmids in total for each well, including 5 ng of reporter plasmid TOPflash and 10 ng of green fluorescent protein (GFP) plasmid that was cotransfected as the transfection control. After 18 h of transfection, cells were treated with Wnt3a conditioned medium (Wnt3a-CM) or control medium (Ctrl-CM) for an additional 6 h. Cells were then lysed, and luciferase assays were performed. The luciferase activities presented were normalized against the levels of GFP expression as described previously (20). Wnt3a-CM and Ctrl-CM were described previously (24).
Coimmunoprecipitation (co-IP) and Western blotting assays.
At 24 h posttransfection, cells were harvested and lysed in lysis buffer (20 mM Tris-HCl [pH 8.0], 1% NP-40, 10% glycerol, 135 mM NaCl with protease and phosphatase inhibitors). The lysates were centrifuged for 15 min at 13,000 rpm at 4°C. The supernatants were incubated with antibodies and protein A/G Plus-Agarose (Santa Cruz Biotechnology, Inc.) for 3 h at 4°C. Then, samples were washed three times with lysis buffer and denatured in SDS sample buffer for 10 min at 95°C. Proteins were separated by SDS-PAGE and blotted onto polyvinylidene difluoride (PVDF) membranes (Millipore). Membranes were blocked with 5% nonfat dry milk for 1 h and then incubated with primary antibodies for 1 h at room temperature or overnight at 4°C. After being washed membranes were incubated for 1 h at room temperature by using the appropriate IRDye 800 (Rockland) or horseradish peroxidase-conjugated secondary antibodies (Thermo Scientific) for 0.5 to 1 h at room temperature. Results were visualized by the Odyssey 9120 infrared imaging system (LI-COR) or FujiFilm Las 4000 (FujiFilm).
Cytosolic β-catenin assay.
Free β-catenin assays were performed as described previously (20).
RT-PCR and quantitative real-time PCR.
Total RNAs were extracted from cultured cells with TRIzol, and reverse transcription of purified RNA was performed using the Superscript III reverse transcription kit according to the manufacturer's instructions (Invitrogen). The quantification of gene transcripts was measured by quantitative real-time PCR using the Quantitative SYBR green PCR kit (TaKaRa SYBR premix Ex Taq) and the ABI 7500 Fast real-time PCR system (Applied Biosystems). The primer pairs used for human AXIN2 were 5′-AGTGTGAGGTCCACGGAAAC-3′ and 5′-CTTCACACTGCGATGCATTT-3′; for human NKD1, 5′-GTCAACCACTCCCCAACATC-3′ and 5′-AATGGTGGTAGCAGCCAGAC-3′; and for human GAPDH, 5′-GCACCACCAACTGCTTA-3′ and 5′-AGTAGAGGCAGGGATGAT-3′.
Ubiquitination analysis.
To detect exogenous Dvl2 ubiquitination, cells were transiently transfected with Ub-HA, Dvl2-FLAG, and the indicated combination of plasmids. At 24 h after transfection, cells were harvested and lysed in lysis buffer (20 mM Tris-HCl [pH 8.0], 1% NP-40, 10% glycerol, 135 mM NaCl with protease and phosphatase inhibitors), the insoluble fraction was removed by a high-speed spin, and the supernatants were subjected to immunoprecipitation using anti-FLAG antibody and protein A/G Plus-agarose. Ubiquitin conjugates were detected by immunoblotting. To detect the ubiquitination of stably expressed Dvl2 in Dvl2-293 stable cells, we transiently transfected cells with Ub-His and the indicated combination of plasmids. At 18 h after transfection, cells were treated with 25 μM MG132 for 5 h. The following steps were performed as described previously (10). In vitro ubiquitination assays were performed using the ubiquitin conjugation reaction buffer kit according to the manufacturer's instructions (Boston Biochem). The preparation of the substrate was performed as follows. HEK293T cells in 35-mm dishes were transfected with 1 μg of Dvl2-FLAG. At 24 h after transfection, cells were harvested in lysis buffer, the insoluble fraction was removed, and the supernatant was subjected to immunoprecipitation as described above. The beads, with the immunoprecipitated Dvl2-FLAG, were washed three times and then incubated with 5 μg of Ub-HA, 100 nM E1, 200 nM E2 (UbcH7), and 500 ng of the purified GST-ITCH or GST-ITCH-C830A in a reaction buffer with ATP-Mg2+ at 30°C for 90 min. After adding the stop buffer, the beads were washed three times with lysis buffer, followed by immunoblotting with anti-HA antibody. To identify the type of isopeptide linkage catalyzed by ITCH, Ub-WT-His and its mutants (Ub-K0-His, Ub-K29R-His, Ub-K48R-His, and Ub-K63R-His) were used. To detect the ubiquitination of unphosphorylated Dvl2, immunoprecipitated Dvl2-FLAG from 293T cell lysates were treated with alkaline phosphatase at 30°C for 5 or 30 min before being subjected to the in vitro ubiquitination reaction.
RESULTS AND DISCUSSION
ITCH is a Dvl-binding protein.
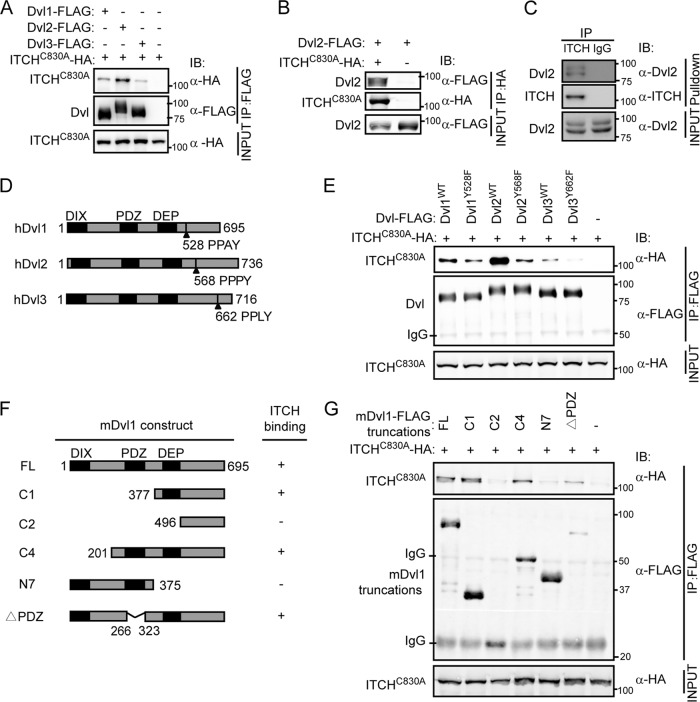
To search for novel Dvl-interacting proteins, we screened a mouse embryonic cDNA library using the yeast two-hybrid system with a truncated form of mouse Dvl1, mDvl1 C1 (residues 377 to 695), as the bait. We identified ITCH as one of the positive clones. We used co-IP to confirm the interaction. To avoid the influence of substrate degradation, we used a ligase-dead mutant of ITCH, ITCH-C830A, in which an active-site cysteine residue was mutated to alanine in the HECT domain (32), in the co-IP experiment. As shown in Fig. 1A, in HEK293T cells transiently cotransfected with HA-tagged ITCH-C830A and FLAG-tagged Dvl1, Dvl2, or Dvl3, ITCH-C830A could be coimmunoprecipitated by all three Dvl isoforms, with a preference for Dvl2. On the other hand, Dvl2 could also be coimmunoprecipitated with ITCH-C830A (Fig. 1B). Next, we examined the Dvl-ITCH interaction at endogenous levels. We immunoprecipitated endogenous ITCH from 293T cell lysates using anti-ITCH antibody and detected the endogenous Dvl2 in the immunoprecipitates (Fig. 1C).
Fig 1.
Dvl interacts with ITCH. (A) ITCH was coimmunoprecipitated with all three Dvl isoforms. HEK293T cells were transiently transfected with different combinations of expression vectors for HA-tagged ITCH-C830A and FLAG-tagged Dvls as indicated. Cell lysates were incubated with anti-FLAG antibody and subsequently analyzed by Western blotting. (B) Dvl2 was coimmunoprecipitated with ITCH. HEK293T cells were transiently transfected with HA-tagged ITCH-C830A and FLAG-tagged Dvl2. Cell lysates were incubated with anti-HA antibody and subsequently analyzed by Western blotting. (C) Dvl2 could be immunoprecipitated by ITCH in vivo. Cell extracts of 293T cells were immunoprecipitated with monoclonal antibody against ITCH, and endogenous Dvl2 was detected by anti-Dvl2 antibody. IgG was used as a negative control. (D) Schematic representation of the modular structures of human Dvls. (E) The YF mutants with mutations within the PPXY motif of Dvls showed much lower affinity for ITCH. HEK293T cells were transiently transfected with different combinations of expression vectors for HA-tagged ITCH-C830A, FLAG-tagged Dvls, and their YF mutants Dvl1-Y528F, Dvl2-Y568F, and Dvl3-Y662F. Cell lysates were incubated with anti-FLAG antibody and subsequently analyzed by Western blotting. WT, wild type. (F, G) Interactions of various mouse Dvl1 truncation mutants with ITCH are shown. A schematic map of various mDvl1 mutants shows amino acid numbers and their interactions with ITCH (F).
To understand how Dvl interacts with ITCH, we tried to identify the specific site in Dvl that is important for ITCH binding. As previously reported, the WW domains of HECT E3s show a preference for the PPXY consensus sequence in target proteins (2). Each of the three isoforms of human Dvl contains a PPXY motif in the C terminus (Fig. 1D). As expected, the YF mutants, Dvl1-Y528F, Dvl2-Y568F, and Dvl3-Y662F, showed markedly reduced affinity for ITCH compared to the corresponding wild-type form in the co-IP assay (Fig. 1E). We also examined the binding affinity of various mouse Dvl1 truncation mutants for ITCH and found that the C2 mutant, which lacks the DEP domain, almost lost the ability to bind ITCH, while C1 showed a binding affinity for ITCH similar to that of the full-length Dvl, suggesting that the DEP domain is also required for Dvl-ITCH interaction (Fig. 1F and G). These results together indicate that ITCH interacts with Dvl and that both the PPXY motif and the DEP domain of Dvl are required for the interaction.
Steady-state levels of phosphorylated Dvl are affected by ITCH.
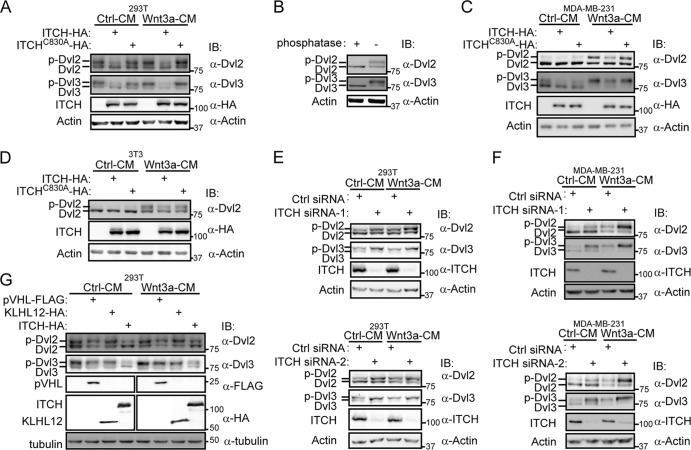
As ITCH is an E3 ubiquitin ligase that often regulates the stability of its substrates, we next examined whether ITCH could reduce the stability of Dvl. In HEK293T cells, we used anti-Dvl2 or anti-Dvl3 antibody to monitor endogenous Dvl2/3 levels and found that there were two bands for each Dvl isoform. Strikingly, transient expression of ITCH reduced the intensity of the upper band of Dvl2/3 (Fig. 2A), whereas the ligase-dead mutant ITCH-C830A had no such effect. Given that Dvl could be phosphorylated by several kinases, such as CK1ε, CK2, and PAR-1 (7, 16, 38, 40), and that activation of Wnt signaling could induce the hyperphosphorylation of Dvl (5, 12, 18), we hypothesized that the upper bands may represent the phosphorylated form of Dvl2/3. To test this possibility, we treated cell extracts with alkaline phosphatase. As shown in Fig. 2B, the upper bands shifted down to the same location as the lower bands after the phosphatase treatment, suggesting that the upper bands were phosphorylated Dvl2/3. These results suggest that ITCH reduced the steady-state level of phosphorylated Dvl but not the unphosphorylated form.
Fig 2.
ITCH reduces the level of phosphorylated Dvl. (A) Overexpression of ITCH, but not ITCH-C830A, decreased the upper bands of Dvl in HEK293T cells. HEK293T cells were transfected with ITCH or ITCH-C830A, and 24 h later, cells were treated with control conditioned medium (Ctrl-CM) or Wnt3a conditioned medium (Wnt3a-CM) for an additional 3 h. Dvl2 and Dvl3 were detected using anti-Dvl2 or anti-Dvl3 antibody, respectively, and actin was used as a control. (B) With alkaline phosphatase treatment, the upper bands of Dvl shifted down. HEK293T cells lysates were treated with alkaline phosphatase for 30 min at 30°C and denatured in SDS sample buffer, followed by immunoblotting with anti-Dvl2 and anti-Dvl3 antibodies. (C, D) Overexpression of ITCH reduced the level of the phosphorylated Dvl caused by Wnt treatment in MDA-MB-231 cells (C) and NIH 3T3 cells (D). Cells were transfected with ITCH or ITCH-C830A. Twenty-four hours later, cells were treated with Ctrl-CM or Wnt-CM for an additional 3 h and then lysed for Western blotting. (E, F) Knockdown of ITCH enhanced the level of phosphorylated Dvl in HEK293T cells (E) and MDA-MB-231 cells (F). Cells were transfected with Ctrl siRNA or ITCH siRNA (ITCH siRNA-1 or siRNA-2). Forty-eight hours later, cells were treated with Ctrl-CM or Wnt-CM for 3 h and then lysed for Western blotting. (G) ITCH acted differently from pVHL and KLHL12 when targeting Dvl. 293T cells were transfected with VHL-FLAG, KLHL12-HA, or ITCH-HA separately and, 24 h later, treated with Ctrl-CM or Wnt-CM for an additional 3 h.
It has been reported that Wnt ligands could induce the hyperphosphorylation of Dvl, which subsequently activates Wnt signaling (6, 28). It is intriguing that in HEK293T cells, phosphorylated Dvl existed at a high level even without Wnt stimulation; nevertheless, Wnt3a stimulation further slightly enhanced the phosphorylation of Dvl (Fig. 2A). To assess whether ITCH could affect Wnt3a-induced phosphorylation of Dvl, we used MDA-MB-231 and NIH 3T3 cells in which there was little basal phosphorylation of Dvl. As shown in Fig. 2C and D, Wnt3a stimulated the phosphorylation of Dvl in these two cell types and transient expression of ITCH effectively reduced the level of the phosphorylated Dvl2/3 induced by Wnt3a, while it had little effect on the unphosphorylated Dvl regardless of the presence or absence of Wnt3a. Thus, we conclude that ITCH could reduce the level of phosphorylated Dvl induced by Wnt3a.
We also investigated the effect of ITCH knockdown on the level of endogenous phosphorylated Dvl. As shown in Fig. 2E and F, knockdown of ITCH increased the level of phosphorylated Dvl2 in both HEK293T cells and MDA-MB-231 cells. In MDA-MB-231 cells, the level of phosphorylated Dvl induced by Wnt3a was further enhanced by transfection of the ITCH siRNAs. These observations further support that ITCH is involved in the regulation of phosphorylated Dvl. We noticed that knockdown of endogenous ITCH expression could also enhance the level of phosphorylated Dvl in MDA-MB-231 cells without Wnt stimulation (Fig. 2F), which suggests that ITCH may play a role in keeping phosphorylated Dvl at a low level in the absence of Wnt signaling activation.
Next, we compared ITCH with pVHL and KLHL12, two previously identified E3 ligases for Dvl (1, 10). As shown in Fig. 2G, overexpression of ITCH reduced the level of phosphorylated Dvl only, while pVHL and KLHL12 tend to reduce both phosphorylated and unphosphorylated Dvl, although KLHL12 seemed to prefer targeting Dvl3 over Dvl2. This result showed that ITCH acts differently from the other two previously identified E3s for Dvl.
ITCH ubiquitinates and degrades phosphorylated Dvl.
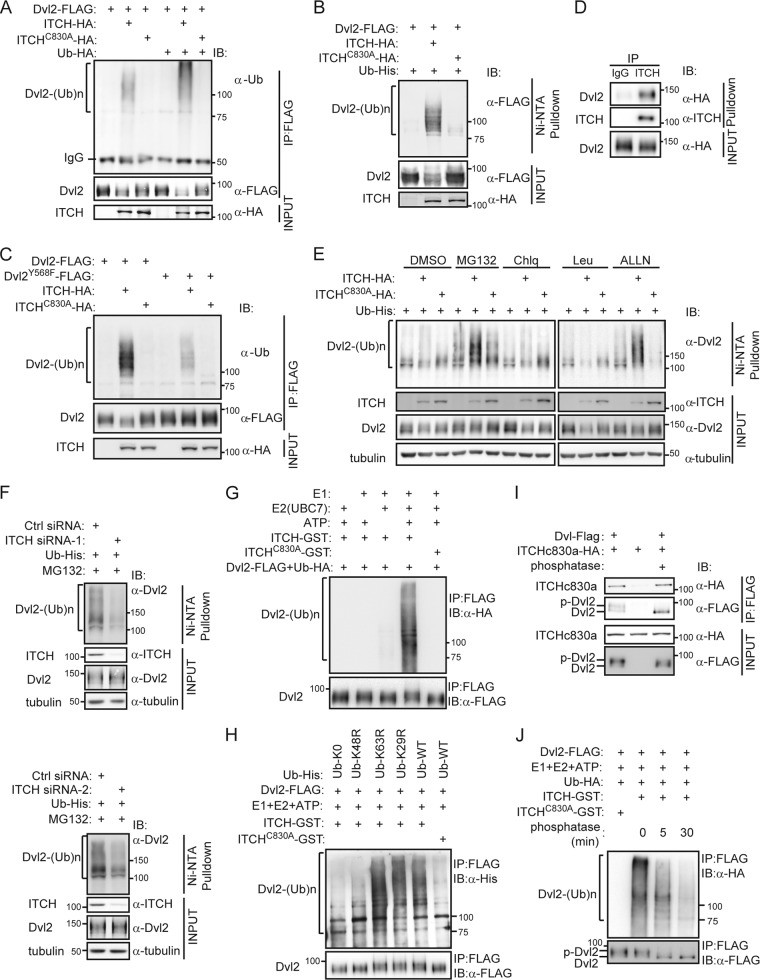
Having demonstrated that ITCH interacts with Dvl and reduces the stability of phosphorylated Dvl, we next performed ubiquitination assays to examine if Dvl is a substrate of ITCH. First, we tested whether ectopic expression of ITCH could enhance the polyubiquitination of Dvl. We overexpressed FLAG-tagged Dvl2 together with either HA-tagged ITCH or ITCH-C830A in HEK293T cells and used antiubiquitin Western blotting to analyze the appearance of ubiquitinated Dvl2 in the immunoprecipitates pulled down by the anti-FLAG antibody. As shown in Fig. 3A, cotransfection of ITCH, but not ITCH-C830A, enhanced Dvl2 ubiquitination. To exclude the possibility that the polyubiquitination signal from the above-described experiment originated from Dvl2-associated proteins, we also detected the ubiquitination of Dvl2 in the immunoprecipitates pulled down by ubiquitin. We cotransfected cells with constructs expressing Flag-tagged Dvl2, HA-tagged ITCH, or ITCH-C830A and His-tagged ubiquitin (Ub-His) and then lysed cells in 6 M guanidinium-HCl-containing buffer and purified His-ubiquitinated proteins with Ni2+-agarose beads, after which we used anti-FLAG Western blotting to detect ubiquitinated forms of Dvl. Denaturing conditions were used in order to prevent deubiquitination of protein-ubiquitin conjugates as well as any noncovalent protein-protein interactions (10). Figure 3B shows that a ladder of the ubiquitinated forms of Dvl2 appeared only when ITCH was cotransfected, which strongly suggests that ITCH ubiquitinates Dvl2. As ubiquitination was much reduced for the Dvl2-Y568F mutant (Fig. 3C), which has a lower affinity for ITCH (Fig. 1E), the interaction between Dvl2 and ITCH is likely important for ITCH-mediated polyubiquitination of Dvl2.
Fig 3.
ITCH ubiquitinates and promotes degradation of phosphorylated Dvl in a proteasome-dependent way. (A and B) ITCH was found to ubiquitinate exogenous Dvl2. HEK293T cells were transfected with the indicated plasmids. Cell lysates were subjected to immunoprecipitation with anti-FLAG antibody, and ubiquitin conjugates were then detected using antiubiquitin antibody (A) or subjected to Ni2+-agarose beads for pulling down His-ubiquitinated proteins; Ub-His-conjugated Dvl2-FLAG was then detected using anti-FLAG antibody (B). (C) The Dvl2-Y568F mutant showed less ubiquitination induced by ITCH than did wild-type Dvl2. The experiment was performed as described for panel A. (D) Stably expressed HA-Dvl2 could be coimmunoprecipitated by ITCH in Dvl2-293 cells. Cell extracts were immunoprecipitated with ITCH antibody. Dvl2-HA was detected by anti-HA antibody. (E) Dvl2 was ubiquitinated by ITCH and degraded in a proteasome-dependent pathway. Dvl2-293 stable cells were transfected with the indicated plasmids for 16 h and then treated with 25 μM MG132, 100 μM chloroquine (Chlq), 100 μM ALLN, or 50 μM leupeptin (Leu) separately, for an additional 5 h. His-ubiquitinated proteins were pulled down using Ni2+-agarose beads and subjected to Western blotting. DMSO, dimethyl sulfoxide. (F) Knockdown of ITCH reduced the ubiquitination of Dvl2. Dvl2-293 stable cells were transfected with Ctrl siRNA or ITCH siRNA (ITCH siRNA-1 or siRNA-2). Twenty-four hours later, Ub-His was transfected, and after another 24 h, cells were treated with 25 μM MG132 for an additional 5 h. His-ubiquitinated proteins were pulled down using Ni2+-agarose beads. (G) In vitro assay of Dvl2 ubiquitination. Using the immunoprecipitated Dvl2-FLAG from the transfected HEK293T cells as the substrate, in vitro experiments were carried out as described in Materials and Methods. (H) ITCH ubiquitinated Dvl by K48-linked chains. Ub-WT-His and several Ub mutants (Ub-K29R-His, Ub-K48R-His, Ub-K63R-His, and Ub-K0-His) were used as indicated for an in vitro ubiquitination assay. (I) Phosphorylation of Dvl is not required for its interaction with ITCH. Dvl2-FLAG and ITCH-C830A-HA were overexpressed separately in different pools of HEK293T cells, and then the Dvl2-FLAG-containing cell lysate was treated with or without alkaline phosphatase and subsequently mixed with the ITCH-C830A-HA-containing cell lysate, followed by co-IP assay. (J) ITCH prefers to ubiquitinate phosphorylated Dvl2. Immunoprecipitated Dvl2-FLAG from 293T cell lysates were treated with alkaline phosphatase for 5 or 30 min at 30°C before the in vitro ubiquitination reaction.
We also examined the ubiquitination of Dvl2 in Dvl2-293 stable cell lines, in which the stably expressed HA-Dvl2 could be coimmunoprecipitated by endogenous ITCH (Fig. 3D). We cotransfected Dvl2-293 cells with constructs expressing HA-tagged ITCH or ITCH-C830A and Ub-His, His-ubiquitinated proteins were then purified with Ni2+-agarose beads, and the samples were analyzed by Western blotting using anti-Dvl2 antibody. As shown in Fig. 3E, ectopic expression of ITCH enhanced the ubiquitination of Dvl2. Moreover, we found that proteasome inhibitors (MG132 and ALLN), but not lysosome inhibitors (chloroquine and leupeptin), could further enhance ITCH-mediated ubiquitination of Dvl2 (Fig. 3E), which suggests that the ubiquitinated Dvl is subjected to degradation by proteasomes. Finally, knockdown of ITCH in Dvl2-293 cells resulted in a decrease of Dvl2 ubiquitination, further confirming that endogenous ITCH is involved in ubiquitination of Dvl (Fig. 3F).
Next, in vitro ubiquitination assays were performed. We used FLAG-tagged Dvl2 proteins immunoprecipitated from transfected HEK293T cells, which contained both the phosphorylated and unphosphorylated forms of Dvl2, as the substrates for ITCH. The data in Fig. 3G show that Dvl2-FLAG could be ubiquitinated in the presence of the purified ITCH, but not ITCH-C830A, in an in vitro ubiquitination system containing Ub-HA, E1, E2 (UbcH7), and ATP-Mg2+, indicating that Dvl2 could be ubiquitinated by ITCH in vitro. Using this in vitro ubiquitination system, we tried to determine which type of ubiquitin linkage is involved in such ubiquitination. The K29R, K48R, and K63R ubiquitin mutants containing a single lysine-to-arginine mutation at positions 29, 48, and 63, respectively, and the K0 mutant, containing no lysine residues, with all lysines mutated to arginines, were used in the assays. As shown in Fig. 3H, ITCH-mediated ubiquitination of Dvl2 was largely abolished when the Ub-K48R-His mutant was used, while the ubiquitination was largely normal with Ub-K29R-His or Ub-K63R-His. Therefore, ITCH ubiquitinates Dvl by K48-linked chains, which is consistent with our data that ITCH-mediated Dvl ubiquitination led to its degradation by proteasomes (Fig. 3E).
Our aforementioned data strongly suggest that ITCH preferentially ubiquitinates phosphorylated Dvl. To further test this issue, we first asked whether the phosphorylation of Dvl is required for its interaction with ITCH. Given that the immunoprecipitated Dvl2 from transfected HEK293T cells contained both phosphorylated and unphosphorylated forms, we used alkaline phosphatase to decrease the level of phosphorylated Dvl2 and then examined whether the binding of ITCH was affected by the phosphatase treatment. The result showed that the phosphatase treatment did not affect the binding of ITCH to Dvl (Fig. 3I), suggesting that the phosphorylation state of Dvl has little effect on the interaction between Dvl and ITCH. Next, we applied the phosphatase treatment in the in vitro ubiquitination experiment. As shown in Fig. 3J, when we increased the time of phosphatase treatment, the phosphorylation of the immunoprecipitated Dvl2-FLAG was progressively decreased, and its ubiquitination by ITCH in vitro was also decreased accordingly. Therefore, while both phosphorylated and unphosphorylated Dvl can bind to ITCH, ITCH preferentially ubiquitinates phosphorylated Dvl. The mechanism for this preferential ubiquitination of phosphorylated Dvl by ITCH is not yet clear. It is possible that the phosphorylation of Dvl may induce a conformational change that exposes the cognate sites for subsequent ubiquitination by ITCH.
ITCH is a negative regulator of canonical Wnt signaling.
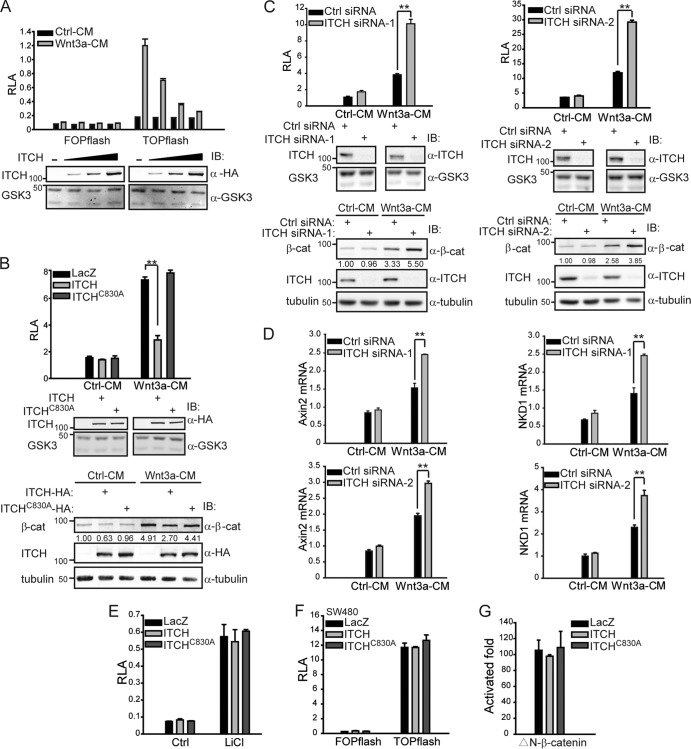
Given that ITCH can ubiquitinate phosphorylated Dvl to promote its degradation and that phosphorylation of Dvl is required for canonical Wnt signaling, we asked whether ITCH regulates the canonical Wnt pathway. We overexpressed ITCH in HEK293T cells and evaluated its effects using the TOPflash reporter system. As shown in Fig. 4A, overexpression of ITCH inhibited Wnt-induced TOPflash activity in a dose-dependent manner. In contrast, ITCH-C830A failed to inhibit Wnt-induced TOPflash activity (Fig. 4B, upper and middle panels). We also tested the free β-catenin level and found that overexpression of ITCH, but not ITCH-C830A, reduced the accumulation of free β-catenin induced by Wnt3a (Fig. 4B, lower panel). Furthermore, to confirm the function of ITCH in vivo, we performed knockdown experiments using ITCH siRNAs. As shown in Fig. 4C, knockdown of ITCH significantly increased Wnt-induced TOPflash activity (Fig. 4C, upper and middle panels), and meanwhile the accumulation of free β-catenin induced by Wnt3a was also enhanced (Fig. 4C, lower panel). We also investigated the effect of ITCH knockdown on the expression of endogenous Wnt target genes. As shown in Fig. 4D, in cells transfected with the ITCH siRNA, Wnt3a-mediated induction of Wnt target genes Axin2 and NDK1 was further potentiated. Collectively, these results suggest that ITCH negatively regulates the canonical Wnt pathway and that its E3 ligase activity is required for this regulation.
Fig 4.
ITCH inhibits canonical Wnt signaling upstream of β-catenin. (A) ITCH inhibited Wnt-induced TOPflash activity in a dose-dependent manner. TOPflash was cotransfected with ITCH into HEK293T cells. Eighteen hours later, cells were treated with Ctrl-CM or Wnt3a-CM for 6 h and then lysed for reporter assay. Plasmids of ITCH were transfected in three different doses to evaluate its inhibitory effects. RLA, relative luciferase activity. FOPflash served as a negative control. The expression level of ITCH is shown (lower panel); GSK3α/β (GSK3) was used as a control. (B) ITCH and its ligase-dead mutant, ITCH-C830A, exhibited different abilities to inhibit Wnt-induced TOPflash activity (upper and middle panels) and accumulation of free β-catenin (lower panel). TOPflash was cotransfected with ITCH or ITCH-C830A into HEK293T cells. Eighteen hours later, cells were treated with Ctrl-CM or Wnt3a-CM for 6 h and then lysed for reporter assay; the expression levels of ITCH and ITCH-C830A are shown in the middle panel. P values were calculated for differences between overexpression of LacZ and ITCH in the Wnt3a-CM treatment. To detect the free β-catenin level in the cytosol, 18 h after transfection, cells were treated with Wnt3a-CM for 3 h and examined by anti-β-catenin antibody. β-Tubulin was used as a cytoplasmic marker. (C) Knockdown of ITCH enhanced Wnt-induced TOPflash activity (upper and middle panels) and accumulation of free β-catenin (lower panel). Ctrl siRNA or ITCH siRNA (ITCH siRNA-1 or siRNA-2) was transfected into HEK293T cells, and TOPflash reporter plasmids were transfected 24 h later. After another 24 h, cells were treated with Ctrl-CM or Wnt3a-CM for an additional 6 h and then lysed for reporter assay. The efficiency of ITCH knockdown is shown in the middle panel. To detect the free β-catenin level in the cytosol, 48 h after transfection with siRNA, cells were stimulated with Wnt3a-CM for 3 h and examined by anti-β-catenin antibody. The efficiency of ITCH siRNAs was also examined using anti-ITCH antibody. (D) ITCH knockdown enhanced Wnt-induced activation of target genes AXIN2 and NKD1. Ctrl siRNA or ITCH siRNA (ITCH siRNA-1 or siRNA-2) was transfected into HEK293T cells. After 48 h, cells were treated with Ctrl-CM or Wnt3a-CM for 8 h. Total RNAs were then extracted from cultured cells with TRIzol. The expression of Wnt target genes was detected by quantitative real-time PCR and normalized by GAPDH expression. (E) ITCH could not inhibit the TOPflash activity induced by LiCl. HEK293T cells were transfected with reporter gene and ITCH-HA or ITCH-C830A-HA, treated with 20 mM LiCl for 6 h, and then lysed for reporter assay. (F) ITCH could not inhibit the constitutive TOPflash activity in SW480 cells. SW480 cells were transfected with TOPflash and ITCH-HA or ITCH-C830A-HA. (G) ITCH could not inhibit the TOPflash activity induced by ΔN-β-catenin. Data are representative results from at least two independent experiments, and error bars were calculated from triplicate samples. Student's t test was used to determine statistical significance based on at least three independent experiments. **, P < 0.01.
We have previously reported that, besides its cytoplasmic function upstream of β-catenin stability regulation, Dvl also plays an important role in the nucleus by stabilizing the β-catenin/TCF4 transcriptional complex in the canonical Wnt pathway (9, 14, 41). Therefore, we further examined whether ITCH could also regulate nuclear Dvl. First, we treated cells with LiCl, which can inhibit the kinase activity of GSK3β and stabilize β-catenin, thereby activating canonical Wnt signaling without Wnt ligands. We found ITCH could not inhibit LiCl-induced TOPflash activity (Fig. 4E). Second, we performed a reporter gene assay in SW480 cells, in which adenomatous polyposis coli (APC) is mutated to a truncated form and as a result, β-catenin is stabilized, leading to constitutive activation of Wnt signaling (13). Using TOPflash as a readout, we found that ITCH could not inhibit the constitutive activity of Wnt signaling in these cells either (Fig. 4F). Third, overexpression of ITCH did not inhibit TOPflash activity induced by ΔN-β-catenin, a constitutively active form of β-catenin (Fig. 4G). These results indicate that ITCH does not play a role downstream of β-catenin in Wnt signaling. In other words, ITCH does not appear to influence the function of nuclear Dvl in the Wnt signaling pathway.
ITCH inhibits canonical Wnt signaling by directly targeting Dvl.
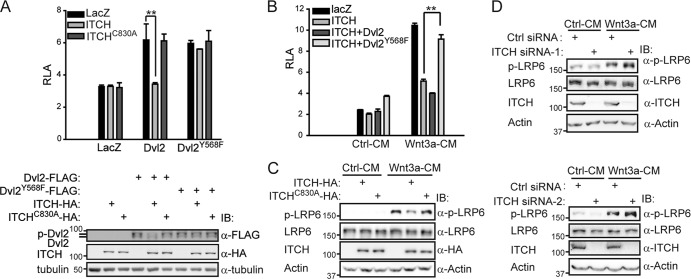
Our data so far indicate that ITCH could promote the ubiquitination and degradation of phosphorylated Dvl and that ITCH could inhibit the canonical Wnt signaling pathway. Finally, we wanted to determine whether ITCH regulates Wnt signaling through directly targeting phosphorylated Dvl. We used the Dvl2-Y568F mutant, which showed a lower affinity than wild-type Dvl2 for ITCH (Fig. 1E). As shown in Fig. 5A, overexpression of ITCH reduced the wild-type Dvl2-induced TOPflash activity and the level of phosphorylated Dvl2 but affected neither Dvl2-Y568F-induced TOPflash activity (Fig. 5A, upper panel) nor the level of phosphorylated Dvl2-Y568F (Fig. 5A, lower panel). Moreover, when the same amount of Dvl2 or Dvl2-Y568F was cotransfected with ITCH (data not shown), only Dvl2-Y568F could reverse the ITCH-mediated inhibition of Wnt3a-induced TOPflash activity (Fig. 5B). These data strongly suggest that the interaction between Dvl and ITCH is essential for ITCH's function in canonical Wnt signaling.
Fig 5.
Dvl is the direct target for ITCH to inhibit the canonical Wnt pathway. (A) The interaction of Dvl and ITCH is important for ITCH to inhibit Dvl-induced TOPflash activity. HEK293T cells were transfected with the indicated plasmids for 24 h. One half of the cell lysates were used for reporter assay (upper panel), and the other half were used for Western blotting (lower panel). P values were calculated for differences between overexpression of LacZ and ITCH in Dvl2-induced TOPflash activity. **, P < 0.01. (B) Dvl2-Y568F, but not wild-type Dvl2, was able to reverse ITCH-mediated inhibition of Wnt3a-induced TOPflash activity. P values were calculated for differences between overexpression of ITCH with and without Dvl2-Y568F. **, P < 0.01. (C) Overexpression of ITCH, but not ITCH-C830A, downregulated Wnt-induced LRP6 phosphorylation. HEK293T cells were transfected with ITCH or ITCH-C830A, and 24 h later, cells were treated with Ctrl-CM or Wnt3a-CM for an additional hour. Cell lysates were subsequently analyzed by Western blotting. Phosphorylation of endogenous LRP6 was detected using an anti-phospho-LRP6 (p1490) antibody; the anti-LRP6 antibody was used as an internal control. (D) Knockdown of ITCH upregulated Wnt3a-induced LRP6 phosphorylation. HEK293T cells were transfected with Ctrl siRNA or ITCH siRNA (ITCH siRNA-1 or siRNA-2), and 48 h later, cells were treated with Ctrl-CM or Wnt3a-CM for an additional hour. Cells were lysed, and phosphorylation of LRP6 was then detected by Western blotting.
It has been reported that phosphorylation of LRP5/6 is an essential event for the initiation of canonical Wnt signal transduction and that Dvl is required for this process (3, 29, 31). Therefore, we also examined the phosphorylation of LRP6 as another readout for the effect of ITCH on the function of Dvl in Wnt signaling. We overexpressed ITCH in HEK293T cells and examined the level of phosphorylated LRP6 using a phospho-LRP6 antibody that specifically detects Ser-1490 phosphorylation that could be induced by Wnt ligand. Results showed that overexpression of ITCH decreased the level of phosphorylated LRP6 upon Wnt3a stimulation (Fig. 5C). In addition, when ITCH was knocked down in HEK293T cells, phosphorylation of LRP6 induced by Wnt3a was further enhanced (Fig. 5D). Meanwhile, we noticed that knockdown of ITCH had little influence on the basal level of phosphorylated LRP6 upon Ctrl-CM treatment, similar to what is observed for the effect on basal β-catenin stability and TOPflash activity (Fig. 4C). Nevertheless, the level of phosphorylated Dvl was significantly increased when ITCH was knocked down, even upon Ctrl-CM treatment (Fig. 2E and F). These data are consistent with previous reports showing that phosphorylation of Dvl is required but not sufficient for canonical Wnt signaling activation (5, 16). Collectively, our above-described results indicate that Dvl is the direct target of ITCH for regulation of the canonical Wnt pathway.
This study clearly shows that ITCH is a negative regulator of the canonical Wnt signaling pathway. We show here that ITCH interacts with Dvl and promotes the ubiquitination and degradation of phosphorylated Dvl. It is not uncommon that phosphorylation serves as a signal for subsequent ubiquitination. For instance, phosphorylation-dependent ubiquitination has been previously reported in the Wnt pathway: the E3 ubiquitin ligase β-TrCP could recognize only the phosphorylated form of β-catenin and target it for ubiquitination and degradation (21). Similarly, ITCH ubiquitinates and promotes the degradation of only phosphorylated Dvl, which is different from the already-found E3 ubiquitin ligases for Dvl, such as the KLHL12-Cullin-3 complex, pVHL, NEDL1, and Malin (1, 10, 26, 37), whose ubiquitination of Dvl does not relate to Dvl's phosphorylation state. A previous study reported that Dsh (the Dvl homolog in flies) phosphorylation does not seem to have a major effect on canonical Wnt signaling in Drosophila (43). This may reflect intrinsic differences in the fine regulation of the Wnt signaling pathways between the vertebrate and invertebrate systems. Indeed, a number of studies showed that the phosphorylated form of Dvl is likely to be the active form of Dvl in canonical Wnt signaling in mammalian and Xenopus systems (4, 16, 35). Thus, ITCH seems to target only activated Dvl for degradation. Selective ubiquitination and degradation of the active form of their targets by E3s have been reported previously. For example, Thr-308 and Ser-473 phosphorylation could activate Akt, while the E3 ubiquitin ligase CHIP promotes the ubiquitination and degradation of such phosphorylated Akt only, therefore inhibiting Akt signaling (39). Both E3 ubiquitin ligases Fbw7 and MuRF1 could target activated phospho-c-Jun for ubiquitination and degradation, effectively inhibiting the Jun N-terminal protein kinase (JNK) signaling pathway (19, 27). The regulation of canonical Wnt signaling by ITCH provides another example whereby an E3 ubiquitin ligase targets the active form of a key component in a signaling pathway for degradation.
Although nuclear Dvl also plays an important role in canonical Wnt signaling (9), our data showed that ITCH, as an E3 ubiquitin ligase for Dvl, inhibits canonical Wnt signaling upstream of β-catenin, while it has no effect downstream of β-catenin (Fig. 4). The results suggest that ITCH inhibits the function of cytoplasmic Dvl only and has no effect on nuclear Dvl. It is possible that ITCH does not interact with nuclear Dvls due to their distinct cellular compartmentalization. Another possibility is that nuclear Dvl is not phosphorylated or the phosphorylation of Dvl is not important enough for nuclear Dvl to perform its function.
There is compelling evidence indicating that aberrant activation of the canonical Wnt pathway is involved in the development and progression of many cancers (6, 22). As for ITCH, it is a member of the HECT-type E3 family, targeting different substrates to regulate multiple signaling pathways and pathological conditions. ITCH knockout mice exhibited dysregulated immune responses (30), but phenotypes related to a potential abnormal activation of canonical Wnt signaling have not been reported. The dysregulation of immune responses in the ITCH-null mice is consistent with its well-known role in immune regulations (25). Given our new findings of this study, it would be of interest to further examine the ITCH-null animals for potential Wnt signaling-related phenotypes in the future. Although a direct correlation of ITCH with cancer biology has not yet been explored, an increasing number of ITCH targets have been implicated in tumorigenesis and chemosensitivity (23, 33, 34). Future studies are warranted to further investigate the physiological significance of ITCH-mediated inhibition of canonical Wnt signaling and to test whether the ITCH-Dvl interaction plays roles in events such as tumorigenesis.
ACKNOWLEDGMENTS
We greatly appreciate the gift of Ub-HA, Ub-His, VHL, and KLHL12 plasmids from B. Sun, L. Yu, and R. G. Hu and thank M. F. Liu for MDA-MB-231 cells. We thank D. Li for critical reading of the manuscript.
This work is supported by grants from the Ministry of Science and Technology of China (2010CB912100) and the National Natural Science Foundation of China (30930052).
Footnotes
Published ahead of print 23 July 2012
REFERENCES
- 1. Angers S, et al. 2006. The KLHL12-Cullin-3 ubiquitin ligase negatively regulates the Wnt-beta-catenin pathway by targeting Dishevelled for degradation. Nat. Cell Biol. 8:348–357 [DOI] [PubMed] [Google Scholar]
- 2. Bernassola F, Karin M, Ciechanover A, Melino G. 2008. The HECT family of E3 ubiquitin ligases: multiple players in cancer development. Cancer Cell 14:10–21 [DOI] [PubMed] [Google Scholar]
- 3. Bilic J, et al. 2007. Wnt induces LRP6 signalosomes and promotes dishevelled-dependent LRP6 phosphorylation. Science 316:1619–1622 [DOI] [PubMed] [Google Scholar]
- 4. Bryja V, Gradl D, Schambony A, Arenas E, Schulte G. 2007. Beta-arrestin is a necessary component of Wnt/beta-catenin signaling in vitro and in vivo. Proc. Natl. Acad. Sci. U. S. A. 104:6690–6695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bryja V, Schulte G, Rawal N, Grahn A, Arenas E. 2007. Wnt-5a induces Dishevelled phosphorylation and dopaminergic differentiation via a CK1-dependent mechanism. J. Cell Sci. 120:586–595 [DOI] [PubMed] [Google Scholar]
- 6. Clevers H. 2006. Wnt/beta-catenin signaling in development and disease. Cell 127:469–480 [DOI] [PubMed] [Google Scholar]
- 7. Cong F, Schweizer L, Varmus H. 2004. Casein kinase Iepsilon modulates the signaling specificities of Dishevelled. Mol. Cell. Biol. 24:2000–2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Deng N, Ye Y, Wang W, Li L. 2010. Dishevelled interacts with p65 and acts as a repressor of NF-kappaB-mediated transcription. Cell Res. 20:1117–1127 [DOI] [PubMed] [Google Scholar]
- 9. Gan XQ, et al. 2008. Nuclear Dvl, c-Jun, beta-catenin, and TCF form a complex leading to stabilization of beta-catenin-TCF interaction. J. Cell Biol. 180:1087–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gao C, et al. 2010. Autophagy negatively regulates Wnt signalling by promoting Dishevelled degradation. Nat. Cell Biol. 12:781–790 [DOI] [PubMed] [Google Scholar]
- 11. Gao C, Chen YG. 2010. Dishevelled: the hub of Wnt signaling. Cell. Signal. 22:717–727 [DOI] [PubMed] [Google Scholar]
- 12. Gonzalez-Sancho JM, Brennan KR, Castelo-Soccio LA, Brown AM. 2004. Wnt proteins induce Dishevelled phosphorylation via an LRP5/6-independent mechanism, irrespective of their ability to stabilize beta-catenin. Mol. Cell. Biol. 24:4757–4768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ilyas M, Tomlinson IP, Rowan A, Pignatelli M, Bodmer WF. 1997. Beta-catenin mutations in cell lines established from human colorectal cancers. Proc. Natl. Acad. Sci. U. S. A. 94:10330–10334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Itoh K, Brott BK, Bae GU, Ratcliffe MJ, Sokol SY. 2005. Nuclear localization is required for Dishevelled function in Wnt/beta-catenin signaling. J. Biol. 4:3 doi:10.1186/jbiol20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jacob LS, et al. 2011. Genome-wide RNAi screen reveals disease-associated genes that are common to Hedgehog and Wnt signaling. Sci. Signal. 4:ra4 doi:10.1126/scisignal.2001225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Klimowski LK, Garcia BA, Shabanowitz J, Hunt DF, Virshup DM. 2006. Site-specific casein kinase 1epsilon-dependent phosphorylation of Dishevelled modulates beta-catenin signaling. FEBS J. 273:4594–4602 [DOI] [PubMed] [Google Scholar]
- 17. Korswagen HC. 2006. Regulation of the Wnt/beta-catenin pathway by redox signaling. Dev. Cell 10:687–688 [DOI] [PubMed] [Google Scholar]
- 18. Lee JS, Ishimoto A, Yanagawa S. 1999. Characterization of mouse dishevelled (Dvl) proteins in Wnt/Wingless signaling pathway. J. Biol. Chem. 274:21464–21470 [DOI] [PubMed] [Google Scholar]
- 19. Li HH, et al. 2011. The ubiquitin ligase MuRF1 protects against cardiac ischemia/reperfusion injury by its proteasome-dependent degradation of phospho-c-Jun. Am. J. Pathol. 178:1043–1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li M, et al. 2010. TAB2 scaffolds TAK1 and NLK in repressing canonical Wnt signaling. J. Biol. Chem. 285:13397–13404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu C, et al. 1999. beta-Trcp couples beta-catenin phosphorylation-degradation and regulates Xenopus axis formation. Proc. Natl. Acad. Sci. U. S. A. 96:6273–6278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Logan CY, Nusse R. 2004. The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 20:781–810 [DOI] [PubMed] [Google Scholar]
- 23. Maillard I, Pear WS. 2003. Notch and cancer: best to avoid the ups and downs. Cancer Cell 3:203–205 [DOI] [PubMed] [Google Scholar]
- 24. Mao J, et al. 2001. Low-density lipoprotein receptor-related protein-5 binds to Axin and regulates the canonical Wnt signaling pathway. Mol. Cell 7:801–809 [DOI] [PubMed] [Google Scholar]
- 25. Melino G, et al. 2008. Itch: a HECT-type E3 ligase regulating immunity, skin and cancer. Cell Death Differ. 15:1103–1112 [DOI] [PubMed] [Google Scholar]
- 26. Miyazaki K, et al. 2004. NEDL1, a novel ubiquitin-protein isopeptide ligase for dishevelled-1, targets mutant superoxide dismutase-1. J. Biol. Chem. 279:11327–11335 [DOI] [PubMed] [Google Scholar]
- 27. Nateri AS, Riera-Sans L, Da Costa C, Behrens A. 2004. The ubiquitin ligase SCFFbw7 antagonizes apoptotic JNK signaling. Science 303:1374–1378 [DOI] [PubMed] [Google Scholar]
- 28. Nusse R. 2005. Wnt signaling in disease and in development. Cell Res. 15:28–32 [DOI] [PubMed] [Google Scholar]
- 29. Pan W, et al. 2008. Wnt3a-mediated formation of phosphatidylinositol 4,5-bisphosphate regulates LRP6 phosphorylation. Science 321:1350–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Perry WL, et al. 1998. The itchy locus encodes a novel ubiquitin protein ligase that is disrupted in a18H mice. Nat. Genet. 18:143–146 [DOI] [PubMed] [Google Scholar]
- 31. Qin Y, Li L, Pan W, Wu D. 2009. Regulation of phosphatidylinositol kinases and metabolism by Wnt3a and Dvl. J. Biol. Chem. 284:22544–22548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Qiu L, et al. 2000. Recognition and ubiquitination of Notch by Itch, a hect-type E3 ubiquitin ligase. J. Biol. Chem. 275:35734–35737 [DOI] [PubMed] [Google Scholar]
- 33. Rossi M, et al. 2006. The E3 ubiquitin ligase Itch controls the protein stability of p63. Proc. Natl. Acad. Sci. U. S. A. 103:12753–12758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rossi M, et al. 2005. The ubiquitin-protein ligase Itch regulates p73 stability. EMBO J. 24:836–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rothbacher U, et al. 2000. Dishevelled phosphorylation, subcellular localization and multimerization regulate its role in early embryogenesis. EMBO J. 19:1010–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schwarz SE, Rosa JL, Scheffner M. 1998. Characterization of human hect domain family members and their interaction with UbcH5 and UbcH7. J. Biol. Chem. 273:12148–12154 [DOI] [PubMed] [Google Scholar]
- 37. Sharma J, Mulherkar S, Mukherjee D, Jana NR. 2012. Malin regulates Wnt signalling pathway through degradation of dishevelled 2. J. Biol. Chem. doi:10.1074/jbc.M111.315135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Song DH, Sussman DJ, Seldin DC. 2000. Endogenous protein kinase CK2 participates in Wnt signaling in mammary epithelial cells. J. Biol. Chem. 275:23790–23797 [DOI] [PubMed] [Google Scholar]
- 39. Su CH, et al. 2011. Akt phosphorylation at Thr308 and Ser473 is required for CHIP-mediated ubiquitination of the kinase. Cell. Signal. 23:1824–1830 [DOI] [PubMed] [Google Scholar]
- 40. Sun TQ, et al. 2001. PAR-1 is a Dishevelled-associated kinase and a positive regulator of Wnt signalling. Nat. Cell Biol. 3:628–636 [DOI] [PubMed] [Google Scholar]
- 41. Torres MA, Nelson WJ. 2000. Colocalization and redistribution of dishevelled and actin during Wnt-induced mesenchymal morphogenesis. J. Cell Biol. 149:1433–1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Varelas X, et al. 2010. The Hippo pathway regulates Wnt/beta-catenin signaling. Dev. Cell 18:579–591 [DOI] [PubMed] [Google Scholar]
- 43. Yanfeng WA, et al. 2011. Functional dissection of phosphorylation of Disheveled in Drosophila. Dev. Biol. 360:132–142 [DOI] [PMC free article] [PubMed] [Google Scholar]