Abstract
Interleukin-1 (IL-1) receptor-associated kinase (IRAK1) is phosphorylated, ubiquitinated, and degraded upon IL-1 stimulation. IRAK1 can be ubiquitinated through both K48- and K63-linked polyubiquitin chains upon IL-1 stimulation. While the Pellino proteins have been shown to meditate K63-linked polyubiquitination on IRAK1, the E3 ligase for K48-linked ubiquitination of IRAK1 has not been identified. In this study, we report that the SCF (Skp1–Cullin1–F-box)–β-TrCP complex functions as the K48-linked ubiquitination E3 ligase for IRAK1. IL-1 stimulation induced the interaction of IRAK1 with Cullin1 and β-TrCP. Knockdown of β-TrCP1 and β-TrCP2 attenuated the K48-linked ubiquitination and degradation of IRAK1. Importantly, β-TrCP deficiency abolished the translocation TAK1-TRAF6 complex from the membrane to the cytosol, resulting in a diminishment of the IL-1-induced TAK1-dependent pathway. Taken together, these results implicate a positive role of β-TrCP-mediated IRAK1 degradation in IL-1-induced TAK1 activation.
INTRODUCTION
Interleukin-1 (IL-1), a major proinflammatory cytokine, has a wide range of pathophysiological functions in autoimmune and inflammatory responses. Genetic and biochemical studies revealed that IL-1 receptor (IL-1R)-mediated signaling involves a cascade of kinases organized by multiple adapter molecules into signaling complexes, leading to activation of the transcription factor NF-κB. Based on studies by our group and others, we postulated a model for the IL-1 pathway (4, 6, 8–10, 14, 22). Upon IL-1 stimulation, the IL-1 receptor recruits the adaptor molecule MyD88 (1) and mediates the formation of complex I (IL-1R–MyD88–IRAK4–IRAK1/2–TRAF6), where IRAK4 (IL-1 receptor-associated kinase 4) (12) is activated, leading to hyperphosphorylation of IRAK1 (2). The phosphorylated IRAK1 interacts with Pellino proteins (7), which are E3 ubiquitin ligases, to mediate K63-linked IRAK1 polyubiquitination. The receptor-proximal components are then released from the receptor to form complex II (IRAK-TRAF6-TAK1-TAB2-TAB3) on the membrane, leading to phosphorylation of TAK1 (tumor growth factor beta [TGF-β]-activated kinase, a mitogen-activated protein kinase kinase kinase [MAP3K]), TAB2 (TAK1 binding protein 2), and TAB3 on the membrane (4, 6, 8–10, 14, 22). While the membrane-associated, modified IRAK1 is ubiquitinated and degraded, complex III (TRAF6-TAK1-TAB2-TAB3) is then dissociated from complex II and translocated from the membrane to the cytosol, where TAK1 is activated, followed by the activation of IKK (IκB kinase) and NF-κB (8).
One hallmark in IL-1 signaling is ligand-induced IRAK1 phosphorylation, ubiquitination, and degradation (13, 24, 25). In addition, other groups have found that IRAK1 could also undergo sumoylation besides phosphorylation and ubiquitination in Toll-like receptor (TLR) signaling (21). A point mutation changing lysine 134 to arginine (K134R) in IRAK1 abolished IL-1-induced IRAK ubiquitination and degradation (26). The IRAK1 ubiquitination mutant is no longer degraded upon IL-1 stimulation and loses the ability to mediate TAK1-dependent NF-κB activation. Furthermore, the proteasome inhibitor MG132 blocked IL-1-induced, TAK1-dependent signaling, suggesting that IL-1-induced IRAK1 degradation is a necessary step in the activation of the TAK1-dependent pathway. IRAK1 is ubiquitinated through both K48- and K63-linked polyubiquitin chains upon IL-1 induction. While Pellino proteins have been shown to meditate K63-linked polyubiquitination on IRAK1, the E3 ligase for K48-linked ubiquitination of IRAK1 has not been identified.
In this study, we found that the F-box protein β-transducin repeat-containing protein (β-TrCP), which functions as a substrate recognition subunit of the SCF-β-TrCP E3 ubiquitin (Ub) ligase, mediates K48-linked polyubiquitination on IRAK1 and subsequent IRAK1 degradation. Knockdown of endogenous β-TrCP1 and β-TrCP2 reduced K48-linked ubiquitination (but not non-K48-linked ubiquitination) on IRAK1 and attenuated degradation of IRAK1 in response to IL-1 stimulation. It is important to note that the modified IRAK1 is always membrane associated and detected in membrane-bound receptor complex I and II (IRAK-TRAF6-TAK1-TAB2-TAB3). One key step in IL-1 signaling is that while the membrane-associated, modified IRAK1 is ubiquitinated and degraded, complex III (TRAF6-TAK1-TAB2-TAB3) is translocated from the membrane to the cytosol, where TAK1 and IKK are activated, resulting in NF-κB activation (8). Importantly, we found that β-TrCP deficiency inhibited the IL-1-induced translocation of TAK1 and TRAF6 from the membrane to the cytosol, which correlated with the attenuated IRAK1 degradation. Consistent with this, IL-1-induced, TAK1-dependent NF-κB activation was substantially diminished in β-TrCP knockdown cells. Taking these findings together, we propose that SCF-β-TrCP-mediated, K48-linked IRAK1 ubiquitination and degradation are required for the release of TRAF6-TAK1 from complex II (IRAK-TRAF6-TAK1-TAB2-TAB3), resulting in the translocation of complex III (TAK1-TRAF6) from the membrane to the cytosol, leading to TAK1 activation followed by activation of IKK and NF-κB.
MATERIALS AND METHODS
Cell lines and reagents.
HEK 293-IL-1RI cells, HEK 293-IL-1R/IRAK-deficient (I1A) cells, and HeLa cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum. The short hairpin RNA (shRNA) lentiviral plasmids (pLKO.1-puro) targeting human β-TrCP were purchased from Sigma. The sequences targeting human β-TrCP1 are 5′-CCGGGCACATAAACTCGTATCTTAACTCGAGTTAAGATACGAGTTTATGTGCTTTTT-3′ and 5′-CCGGGCGTTGTATTCGATTTGATAACTCGAGTTATCAAATCGAATACAACGCTTTTT-3′; the sequences targeting human β-TrCP2 are 5′-CCGGAGAAGACTTGGCCTCTAATTTCTCGAGAAATTAGAGGCCAAGTCTTCTTTTTTG-3′ and 5′-CCGGTATCAGTGGCCTACGAGATAACTCGAGTTATCTCGTAGGCCACTGATATTTTTG-3′. HEK 293T cells were transfected with the β-TrCP1-targeting lentiviral vectors and packaging vectors to ensure proper viral packaging. Forty-eight h after transfection, virus in the medium was collected. The virus then was used to infect target cells in the presence of Polybrene (10 mg/ml; Sigma). Two days after infection, puromycin (1 g/ml) containing Dulbecco's modified Eagle's medium was added to the cells to select puromycin-resistant clones. After obtaining the β-TrCP1 knockdown cells, the packaged β-TrCP2-targeting virus was used to infect the β-TrcP1 knockdown cells. Finally, the β-TrCP1/2 knockdown cells were generated by sequential infection. The knockdown efficiency was confirmed by real-time PCR with the following primers: 5′-CAGTACAGGGACAGGCTGGT-3′ and 5′-TACAACGCACCAATTCCTCA-3′ for human β-TrCP1 and 5′-AAACCAGCCTGGAATGTTTG-3′ and 5′-CGTGTTCACATCCCACACTC-3′ for human β-TrCP2. IRAK (H-273; sc-7883), TRAF6 (H-274; sc-7221), IKKα/β (H-470; sc-7607), IκBα (FL; sc-847), c-Jun N-terminal kinase (JNK) (FL; sc-571), ubiquitin (FL-76; sc-9133; and P4D1; sc-8017), and actin (C-11; sc-1615) were purchased from Santa Cruz Biotechnology, Inc. Anti-K48-linked polyubiquitin antibody (Apu2.07) and anti-K63-linked polyubiquitin antibody (Apu3.A8) were a kind gift from Vishva M. Dixit (Department of Physiological Chemistry Genentech, Inc.). Antihemagglutinin (anti-HA) antibodies (H9658; mouse), anti-α-tubulin antibodies, puromycin (P9620), and MG132 (C2211) were from Sigma. Anti-β-catenin antibodies (70569) were purchased from BD Biosciences. Phospho-IKKα (Ser-176)/β(Ser-180) (catalog number 2694), phospho-stress-activated protein kinase/JNK (catalog number 9251), phospho-IκBα (Ser32) (catalog number 9241), and anti-β-TrCP antibodies (D13F10) were purchased from Cell Signaling Technology, Inc. Rabbit anti-Cullin1 (71-8700) was purchased from Invitrogen. Anti-TAK1 antibodies from rabbit were made by Lilly. Recombinant interleukin-1 was from R&D Systems.
IP.
Cells were transfected with plasmids with Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. Untreated cells or cells treated with IL-1 for various times as indicated were harvested by washing with cold phosphate-buffered saline (PBS) and then lysed with immunoprecipitation (IP) buffer (0.5% Triton X-100, 20 mM HEPES [pH 7.6], 150 mM NaCl, 12.5 mM β-glycerophosphate, 1.5 mM MgCl2, 2 mM EGTA, 10 mM NaF, 1 mM Na3VO4, 1 mM phenylmethylsulfonyl fluoride [PMSF], and protease inhibitor cocktail tablets). The lysates were incubated on ice for 60 min, and then they were centrifuged at 13,200 rpm for 10 min and insoluble debris was discarded. For coimmunoprecipitation, the supernatants were incubated with 1 μg the indicated antibody and protein A-Sepharose beads. The protein A-Sepharose beads were pelleted and washed three times with immunoprecipitation buffer after overnight incubation. The precipitates were resolved by SDS-PAGE and subjected to Western blotting with the indicated antibodies.
Ubiquitination assays.
Cells were harvested by washing with cold PBS and then were lysed with a 1% SDS solution. The lysates were then sonicated for 15 s on ice to disrupt the DNA. The lysates were boiled at 100°C for 10 min to dissociate the protein interaction. The boiled samples were diluted with co-IP buffer to 0.1% SDS and then centrifuged at 12,000 rpm for 5 min, after which the pellet was discarded. The supernatants were then combined with protein A-Sepharose and antibodies against IRAK1, and they were rotated at 4°C overnight. The protein A-Sepharose beads were then pelleted and washed three times with co-IP buffer. The precipitates were resolved by SDS-PAGE and subjected to Western blotting with antibodies against HA, IRAK1, and K48- or K63-specific ubiquitin antibodies.
Immunoblotting.
Whole-cell lysates or immunoprecipitated proteins were dissolved in Laemmli buffer and resolved by 8 or 10% SDS-PAGE. For detecting ubiquitination, 6% SDS-PAGE was used. After electrophoresis, separated proteins were transferred onto polyvinylidene difluoride membranes (Millipore). For ubiquitination detection, proteins were transferred onto nitrocellulose membranes (Whatman). For immunoblotting, the polyvinylidene difluoride membrane was blocked with Tris-buffered saline (TBS) containing 0.1% Tween 20 and 5% nonfat dried milk for 1 h at room temperature. Following incubation with specific primary antibody, horseradish peroxidase-conjugated secondary antibody was applied. The positive immune-reactive signal was detected by ECL (Amersham Biosciences).
Subcellular fractionation.
Confluent cells in 15-cm plates, left untreated or treated with IL-1 (1 ng/ml) for various times, were resuspended in 1 ml of ice-cold hypotonic buffer (10 mM HEPES, pH 7.4, 1.5 mM MgCl2, 10 mM KCl, 0.2 mM phenylmethylsulfonyl fluoride, 0.5 mM dithiothreitol) and homogenized on ice with 45 strokes of a Dounce homogenizer. Unlysed cells, nuclei, and cell debris were pelleted by five cycles of centrifugation at 1,000 × g for 5 min. Soluble (supernatant; S100) and particulate (pellet; P100) fractions were generated by centrifugation at 100,000 × g for 1 h.
RESULTS
β-TrCP1 and β-TrCP2 promoted K48-linked IRAK1 ubiquitination.
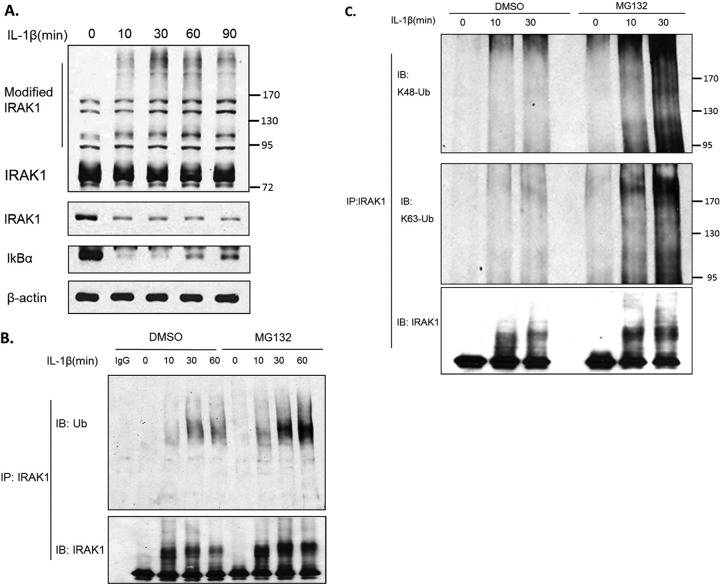
Upon IL-1 stimulation, IRAK1 is phosphorylated, polyubiquitinated, and eventually degraded (Fig. 1A and B). While the K48-linked polyubiquitin chain leads to protein degradation, the K63-linked polyubiquitin chain promotes protein-protein association (3). To examine whether IL-1-induced polyubiquitination of IRAK1 is linked via Lys 63 or Lys 48, cell lysates prepared from untreated or IL-1-treated 293-IL-1R cells were immunoprecipitated with anti-IRAK1, followed by Western analysis with antibodies specific for the K63- and K48-linked polyubiquitin chains. IL-1 stimulation induced both K63- and K48-linked ubiquitination of IRAK1, both of which were accumulated when IRAK1 degradation was blocked by pretreatment with proteasome inhibitor MG132 (Fig. 1C).
Fig 1.
IL-1 induces polyubiquitination of IRAK1. (A) 293-IL-1R cells were stimulated with 1 ng/ml IL-1β for the indicated times. Cell lysates were analyzed by Western blotting with rabbit anti-IRAK1 (that recognizes the modified forms; upper panel), mouse anti-IRAK1 (that does not recognize the modified forms; lower panel), anti-IkBα, and anti-β-actin antibodies. (B) 293-IL-1R cells were pretreated with dimethylsulfoxide (DMSO) or the proteasome inhibitor MG132 (20 nmol/ml) for 2 h, respectively. The cells then were treated with IL-1β for the indicated times. Cells were collected and then denatured with 1% SDS. The lysates were diluted with coimmunoprecipitation buffer to 0.1% SDS. The diluted lysates were subjected to immunoprecipitation (IP) with rabbit anti-IRAK1 antibody, followed by immune blotting with antiubiquitin antibody. The lower bands show the immunoprecipitated IRAK1. (C) Cells receiving the indicated treatments were subjected to immunoprecipitation as described for panel B. The pulled down IRAK1 was analyzed by Western blotting with anti-K48 and anti-K63 specific ubiquitin antibodies.
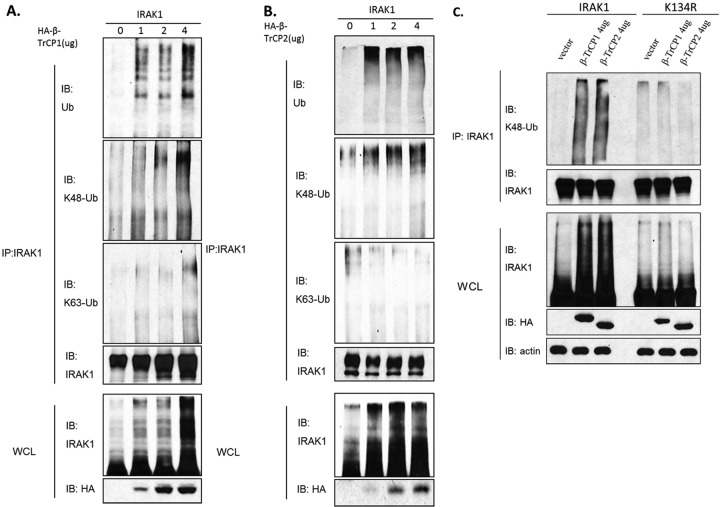
While Pellino proteins have been shown to mediate K63-linked IRAK1 ubiquitination (15, 16, 24), one important question is what E3 protein ligase is responsible for IL-1-induced, K48-linked IRAK1 ubiquitination. We screened several E3 ligases that are capable of mediating K48-linked polyubiquitination (including A20, cIAP1, cIAP2, β-TrCP1, and β-TrCP2) by cotransfecting them with IRAK1 into IRAK1-deficient cells derived from 293-IL-1R cells (293-I1A cells), following by analysis for IRAK1 ubiquitination. IRAK1 was immunoprecipitated from the 293-I1A cells transfected with increasing amounts of HA-tagged E3 ligases, followed by Western analyses with antibodies against HA tag, IRAK1, ubiquitin, and K63- and K48-linked polyubiquitin. We found that overexpression of β-TrCP1 or β-TrCP2 (but not the other E3s) promoted IRAK1 ubiquitination in a dose-dependent manner (Fig. 2A and B). Furthermore, the β-TrCP1- and β-TrCP2-mediated IRAK1 polyubiquitination chain was indeed K48 but not K63 linked (Fig. 2A and B).
Fig 2.
β-TrCP overexpression induced K48-linked IRAK1 ubiquitination. (A and B) IRAK1-deficent 293 (I1A) cells were transfected with the indicated amounts of expression vectors encoding HA-tagged β-TrCP1 and IRAK1 or HA-tagged β-TrCP2 and IRAK1. Lysates of the transfected cells prepared under denatured conditions were subjected to immunoprecipitation with anti-IRAK1 antibody. The samples then were analyzed by Western blotting with antiubiquitin antibody, K48-specific ubiquitin antibody, and K63-specific ubiquitin antibody. Whole-cell lysates (WCL) were probed with anti-IRAK1 and anti-HA antibody. (C) IRAK1-deficient (I1A) cells were transfected with the indicated amounts of expression vectors encoding HA-tagged β-TrCP and IRAK1 or IRAK1 mutant K134R. After 24 h, cells were harvested and denatured lysates were subjected to immunoprecipitation with anti-IRAK1 antibody. The samples then were analyzed by Western blotting with K48-specific ubiquitin antibody.
We previously reported that a point mutation changing lysine 134 to arginine (K134R) in IRAK1 abolished IL-1-induced IRAK1 ubiquitination and degradation (26). These results suggest that K134 is the site for both K48-linked and K63-linked polyubiquitin chains on IRAK1. We indeed found that β-TrCP1 and β-TrCP2 failed to induce ubiquitination of the K134R mutant, indicating that K134 is required for β-TrCP1- and β-TrCP2-mediated, K48-linked polyubiquitination (Fig. 2C).
IL-1 stimulation induced the interaction of β-TrCP1 and β-TrCP2 with modified IRAK1.
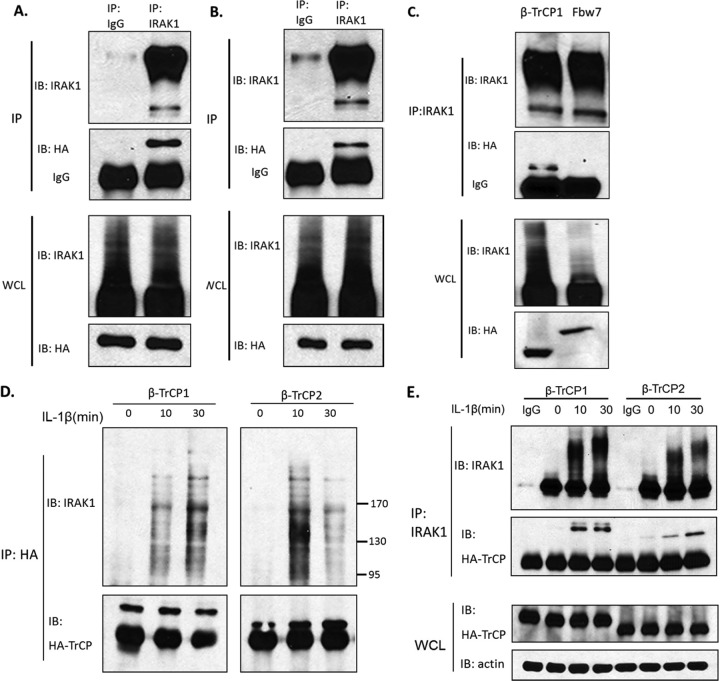
To investigate the ability of β-TrCP to interact with IRAK1, we coexpressed HA-tagged β-TrCP1 or β-TrCP2 with IRAK1 in IRAK1-deficient 293-I1A cells. Overexpressed IRAK1 was immunoprecipitated from the transfected cells, followed by Western analyses with antibodies against IRAK1 and HA tag. While the majority of IRAK1 protein was modified (autophosphorylated) upon its overexpression, β-TrCP1 and β-TrCP2 specifically interacted with IRAK1 (Fig. 3A and B). WD40 domain-containing protein Fbw7 was used as a negative control to show the specific interaction of β-TrCP with IRAK1 (Fig. 3C). We also examined the interaction of β-TrCP1 and β-TrCP2 with endogenous IRAK1 in response to IL-1 stimulation. HA-tagged β-TrCP1 and β-TrCP2 were transfected into 293-IL-1R cells. Whole-cell lysates from untreated or IL-1-treated, transfected cells were immunoprecipitated with anti-HA, followed by Western blotting with antibodies against IRAK1 and HA tag. We found that both β-TrCP1 and β-TrCP2 interacted with the modified IRAK1 in response to IL-1 stimulation (Fig. 3D). We also performed reverse immunoprecipitation. Whole-cell lysates from the untreated and treated 293-IL-1R cells transfected with HA-tagged β-TrCP1 or β-TrCP2 were immunoprecipitated with anti-IRAK1, followed by Western blotting with antibodies against IRAK1 and HA tag. The results showed that both β-TrCP1 and β-TrCP2 can interact with IRAK1, and the association was enhanced in response to IL-1 stimulation (Fig. 3E). The interaction of endogenous IRAK1 and β-TrCP was also detected in IL-1-treated 293-IL-1R cells (Fig. 3F). Taken together, these results indicate that both β-TrCP1 and β-TrCP2 interact with IRAK1 upon IL-1 stimulation and then promote K48-linked ubiquitination on IRAK1.
Fig 3.
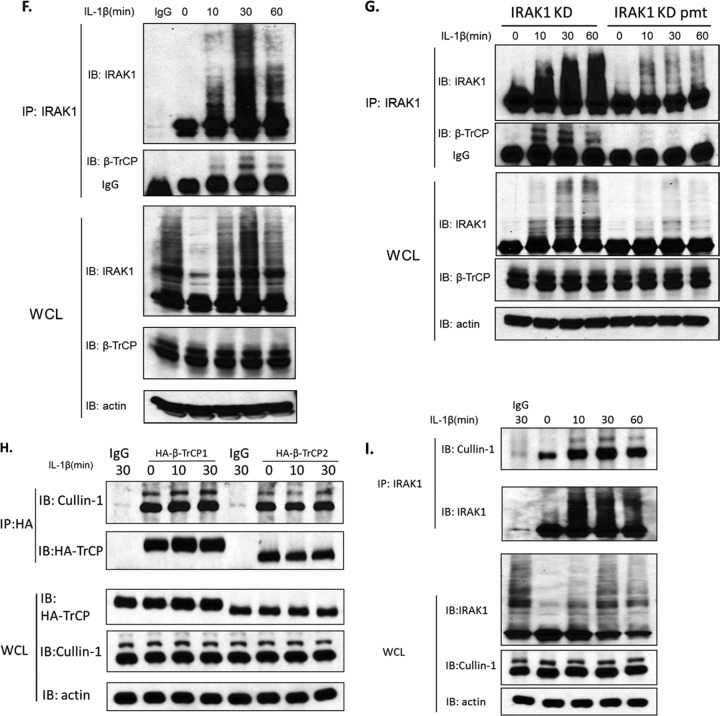
IL-1-induced interaction of IRAK1 with β-TrCP1/2 and Cullin1. (A to C) IRAK1-deficient (I1A) cells were transfected with expression constructs of IRAK1 and HA-tagged β-TrCP1 (A), IRAK1 and HA-tagged β-TrCP2 (B), and IRAK1 and HA-tagged β-TrCP1 or Fbw7 (C). The cell lysates were subjected to immunoprecipitation with anti-IRAK1 and then analyzed by Western blotting with anti-HA antibody. Whole-cell lysates (WCL) were also analyzed by Western blotting for the indicated proteins. All results are representative of three independent experiments. (D and E) 293-IL-1R cells were transiently transfected with HA-tagged β-TrCP1 or HA-tagged β-TrCP2. Lysates of transfected cells left untreated or treated with IL-1 were subjected to immunoprecipitation with anti-HA and then analyzed by Western blotting with anti-IRAK1 or immunoprecipitation with anti-IRAK1 (D) and then analyzed by Western blotting with anti-HA (E). All results are representative of three independent experiments. (F) Lysates of 293-IL-1R cells left untreated or treated with IL-1 were subjected to immunoprecipitation with anti-IRAK1 and then analyzed by Western blotting with anti-β-TrCP antibody. (G) I1A cells stably transfected with IRAK1 KD or IRAK1 KD pmt were treated with IL-1 for the indicated times. The whole-cell lysates were subjected to immunoprecipitation with anti-IRAK1 and then analyzed by Western blotting with anti-β-TrCP antibody. (H) 293-IL-1R cells were transiently transfected with HA-tagged β-TrCP1 or HA-tagged β-TrCP2. Lysates of transfected cells left untreated or treated with IL-1 were subjected to immunoprecipitation with anti-HA and then analyzed by Western blotting with anti-Cullin1. (I) Lysates of 293-IL-1R cells left untreated or treated with IL-1 were subjected to immunoprecipitation with anti-IRAK1 and then analyzed by Western blotting with anti-Cullin1 antibody.
β-TrCP is well known to recruit phosphorylated substrates to the SCF (Skp1–Cullin1–F-box) ubiquitin ligase complex (3, 5, 11, 20). For example, β-TrCP recruits phosphorylated IκBα to the SCF complex to mediate K48-linked ubiquitination of IκBα, followed by proteasome-dependent degradation of IκBα, releasing NF-κB to the nucleus. Since IL-1 induces hyperphosphorylation of IRAK1 (2, 25), it is logical to examine whether β-TrCP binding to IRAK1 requires the phosphorylation of IRAK1. We have previously shown that while a point mutation changing lysine 134 to arginine (K134R) in IRAK1 abolished IL-1-induced IRAK ubiquitination and degradation, mutations of serines and threonines adjacent to lysine 134 to alanines [designated (S/T)A(131-144)] reduced IL-1-induced IRAK1 phosphorylation/ubiquitination and impaired IRAK1 degradation (26). To avoid autophosphorylation, these mutations were generated in an IRAK1 kinase-inactive form for transfection experiments. To test the importance of IRAK1's phosphorylation, whole-cell lysates from the untreated and treated I1A (IRAK1-deficient) cells stably transfected with IRAK1 KD (the full-length, kinase-inactive IRAK1) or IRAK1 KD pmt (kinase-inactive full-length IRAK1 with phosphorylation mutant) [(S/T)A(131-144)] were immunoprecipitated with anti-IRAK1, followed by Western blotting with antibodies against β-TrCP (Fig. 3G). The results showed that IRAK1 KD pmt lost the ability to interact with β-TrCP in response to IL-1 stimulation, indicating that the recruitment of IRAK1 to β-TrCP is probably phosphorylation dependent.
β-TrCP1 and β-TrCP2 (by forming a homodimer or heterodimer) function as the substrate recognition subunit in the SCF ubiquitin ligase complex (5, 11), which mediates ubiquitination-dependent protein degradation. While the F-box domain of β-TrCP associates with Skp1-Cullin1, the WD40 domain of β-TrCP serves to recognize the substrates (20, 23). We indeed detected constitutive interaction of Cullin1 with β-TrCP and IRAK1 (Fig. 3H and I), while IL-1 stimulation further promoted the interaction of IRAK1 with Cullin1 (Fig. 3I), demonstrating the ligand-dependent recruitment of IRAK1 to the SCF (Skp1-Cullin1-F-box) ubiquitin ligase complex, probably through its interaction with β-TrCP.
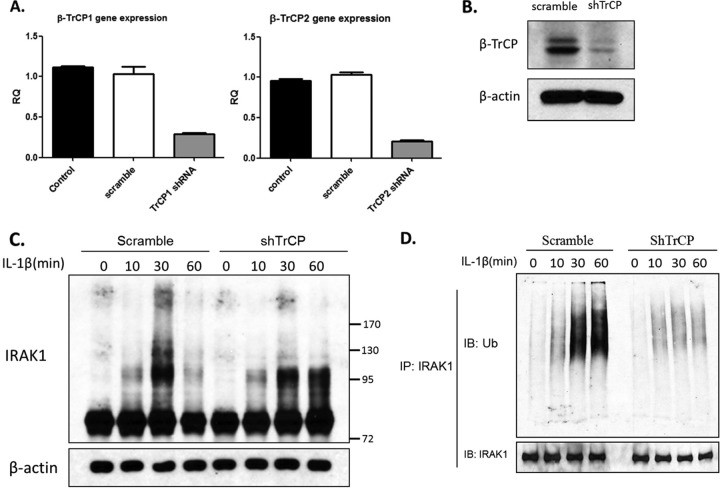
Knockdown of β-TrCP diminished IL-1-induced, K48-linked ubiquitination.
While we have shown that overexpression of β-TrCP1 or β-TrCP2 promoted K48-linked IRAK1 ubiquitination, it is important to determine whether endogenous β-TrCP1 and β-TrCP2 are indeed critical for IL-1-induced, K48-linked IRAK1 ubiquitination. We infected 293-IL-1R cells with lentiviral β-TrCP1 and β-TrCP2 shRNA or control (scramble) shRNA, followed by selection with puromycin to obtain stable knockdown cells. By real-time PCR and Western analysis, we showed that endogenous β-TrCP1 and β-TrCP2 were effectively knocked down (Fig. 4A and B). We first examined the impact of β-TrCP1 and β-TrCP2 knockdown on IL-1-induced IRAK1 modification and degradation. IRAK1 was immunoprecipitated from untreated and IL-1-treated 293-IL-1R cells stably infected with lentiviral β-TrCP1 and β-TrCP2 shRNA or control (nontargeting) shRNA, followed by Western analyses with antibodies against IRAK1 and ubiquitin. We found that knockdown of β-TrCP1 and β-TrCP2 attenuated IL-1-induced IRAK1 ubiquitination and degradation (Fig. 4C and D). Interestingly, we observed the accumulation of lower-shifted, modified IRAK1 bands in the β-TrCP knockdown cells compared to IRAK1 in control cells (Fig. 4C). Furthermore, IL-1-induced, K48-linked but not K63-linked IRAK1 polyubiquitination was greatly reduced in the β-TrCP knockdown cells compared to that of IRAK1 in control cells (Fig. 4E to G). Therefore, the lower-shifted, modified IRAK1 bands could be due to K63-linked IRAK1 polyubiquitination or simply hyperphosphorylated IRAK1 in the absence of K48-linked polyubiquitination. Since IRAK1 KD pmt lost the ability to interact with β-TrCP, we also examined IL-1-induced K48- and K63-specific ubiquitination of this nondegradable mutant of IRAK1, IRAK1 KD pmt, in I1A cells. Interestingly, K48-specific ubiquitination of IRAK1 KD pmt was indeed abolished, whereas K63-linked ubiquitination was still detectable but reduced (Fig. 4H), supporting the role of β-TrCP in K48-linked IRAK1 ubiquitination. However, future studies are required to define the phosphorylation sites for IRAK1's interaction with Pellino proteins that are responsible for K63-linked IRAK1 ubiquitination. It is possible that since IRAK1 KD pmt had reduced K63-linked ubiquitination, the mutations in this construct also have partially affected the interaction of IRAK1 with Pellino proteins.
Fig 4.
Knockdown of β-TrCP attenuated K48-linked ubiquitination of IRAK1. (A and B) 293-IL-1R cells were infected with lentiviral β-TrCP1 shRNA or nontargeting shRNA and selected in puromycin. The puromycin-resistant clones were subjected to sequential infection with lentiviral β-TrCP2 shRNA. The knockdown efficiency was analyzed by human β-TrCP1- and β-TrCP2-specific quantitative real-time PCR (A) or Western blotting (B). (C) Lysates from β-TrCP knockdown and control cells left untreated or stimulated with IL-1 were analyzed by Western blotting with anti-IRAK1 antibody. (D) Lysates of the β-TrCP knockdown or control cells left untreated or treated with IL-1 were subjected to immunoprecipitation with anti-IRAK1 and analyzed by Western blotting with antiubiquitin antibody. (E and F) β-TrCP knockdown or control cells transfected with plasmids encoding the HA-tagged K48 only or the K48R ubiquitin mutants were treated with IL-1 for the indicated times. Cell lysates were subjected to immunoprecipitation with anti-IRAK1 and analyzed by Western blotting with anti-HA. Data are representative of at least three experiments. (G) Lysates of the β-TrCP knockdown or control cells left untreated or treated with IL-1 were subjected to immunoprecipitation with anti-IRAK1 and analyzed by Western blotting with anti-K63-specific ubiquitin chain antibody. (H) Cell lysates of I1A cells transfected with IRAK1 KD or IRAK1 KD pmt, which were treated for the indicated times, were subjected to immunoprecipitation with anti-IRAK1 antibody. The pulled down IRAK1 was analyzed by Western blotting with anti-K48- and anti-K63-specific ubiquitin antibodies.
Knockdown of β-TrCP attenuated IL-1-induced IKKα/β and JNK phosphorylation.
We have previously shown that TAK1 deficiency resulted in complete loss of IL-1-induced JNK activation, but NF-κB activation was only partially impaired (26). This phenomenon indicated that there was a parallel NF-κB activation pathway in IL-1 signaling. We indeed identified two parallel IL-1-mediated NF-κB activation pathways, one TAK1 dependent and one MEKK3 dependent (26). The TAK1-dependent pathway causes IKKα/β phosphorylation and IKKβ activation, leading to classical NF-κB activation through IκBα phosphorylation and degradation. The TAK1-independent, MEKK3-dependent pathway induces IKKγ phosphorylation and IKKα activation, resulting in NF-κB activation through IκBα phosphorylation (but without IκBα degradation) and subsequent dissociation from NF-κB. These two pathways are regulated at the level of IRAK1 modification (26).
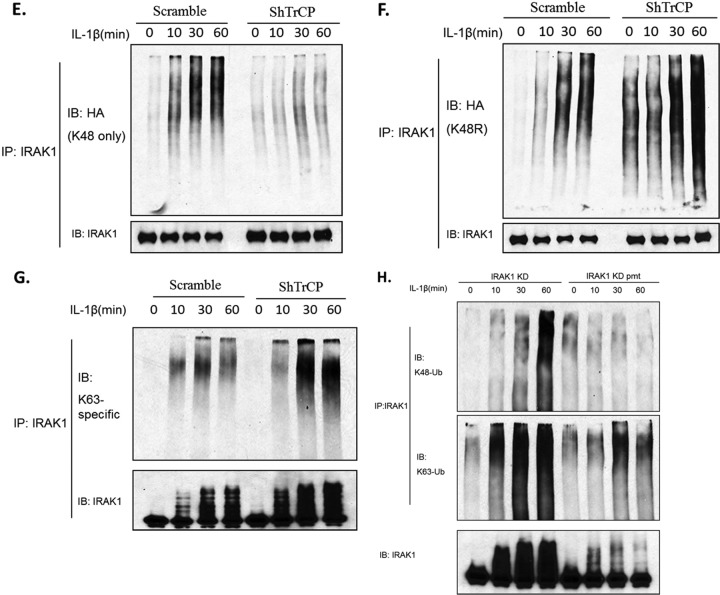
We examined the impact of β-TrCP1 and β-TrCP2 knockdown on IL-1-mediated signaling downstream of IRAK1. Importantly, knockdown of β-TrCP attenuated IL-1-induced IKKα/β phosphorylation with attenuated IκBα degradation, implicating a critical role of β-TrCP in the TAK1-dependent NF-κB activation pathway (Fig. 5A). It is important to note that whereas TAK1 deficiency resulted in partially impaired NF-κB activation, IL-1-induced JNK activation was completely abolished (19, 26). Consistent with this, we found that the knockdown of β-TrCP also substantially reduced IL-1-induced JNK phosphorylation.
Fig 5.
Knockdown of β-TrCP attenuated the IL-1 signaling pathway. (A) β-TrCP knockdown cells or control cells were stimulated with IL-1 (1 ng/ml) for the indicated times. Cell lysates were analyzed by Western blotting for the indicated proteins. (B) 293-IL-1R cells were pretreated with DMSO or proteasome inhibitor MG132 (20 nmol/ml) for 2 h. Lysates of untreated or IL-1-treated cells were analyzed by Western blotting with the indicated antibodies. All results are representative of three independent experiments. (C) I1A cells transfected with IRAK1 KD or IRAK1 KD pmt were stimulated with IL-1 (1 ng/ml) for the indicated times. Cell lysates were analyzed by Western blotting for the indicated proteins.
The important question then became whether the impact of β-TrCP knockdown on IL-1-induced IKKα/β and JNK phosphorylation resulted from the diminished IL-1-induced, K48-linked IRAK1 polyubiquitination or IRAK1 degradation. We have previously shown that the proteasome inhibitor MG132 blocked IL-1-induced, TAK1-dependent signaling, suggesting that IL-1-induced IRAK1 degradation is a necessary step in the activation of the TAK1-dependent pathway. We indeed found that pretreatment of 293-IL-1R cells with MG132 abolished IL-1-induced IRAK1 degradation as well as IKKα/β and JNK phosphorylation with attenuated IκBα degradation (Fig. 5B). Taken together, these results strongly suggest that abolished IL-1-induced IRAK1 degradation is the cause of the diminished IL-1-induced TAK1-dependent signaling events, including IKKα/β and JNK phosphorylation and IκBα degradation. In support of this, the TAK1-dependent signaling events were also attenuated in I1A cells stably transfected with the nondegradable IRAK1 mutant (IRAK1 KD pmt) (Fig. 5C).
Knockdown of β-TrCP blocked the IL-1-induced translocation of TAK1-TRAF6 complex.
The next question is how β-TrCP-mediated IRAK1 degradation controls the IL-1-induced, TAK1-dependent NF-κB activation. It is important to note that the modified IRAK1 is always membrane associated and detected in membrane-bound receptor complex I (IL-1R–IRAK1–TRAF6) and complex II (IRAK1-TRAF6-TAK1). One critical step in IL-1 signaling is that complex III (TRAF6-TAK1) needs to be released from the membrane-bound complex II and translocates from the membrane to the cytosol, where TAK1 and IKK are activated, resulting in NF-κB activation (8). We therefore hypothesize that β-TrCP-mediated, K48-linked IRAK1 ubiquitination and subsequent degradation plays a key role in the release of complex III (TRAF6-TAK1) from the membrane to the cytosol, leading to the activation of the TAK1-dependent NF-κB pathway.
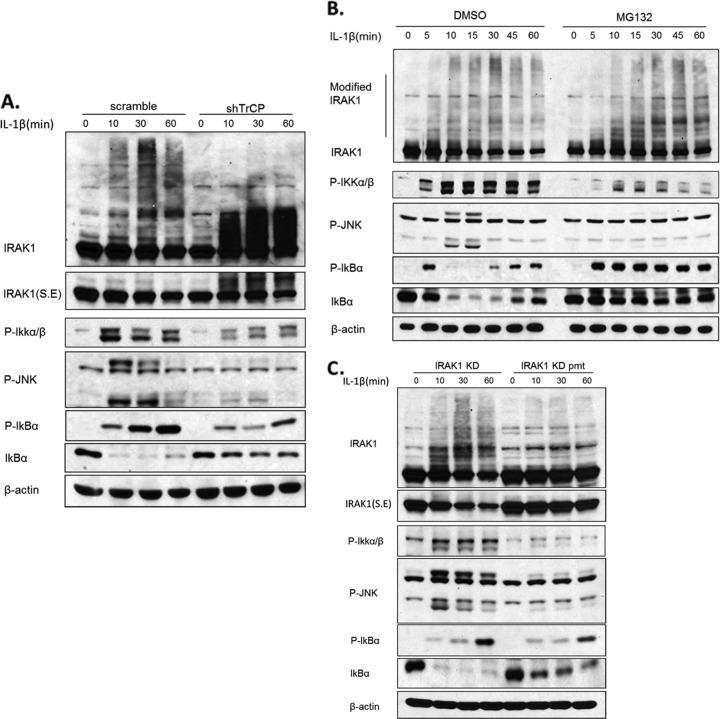
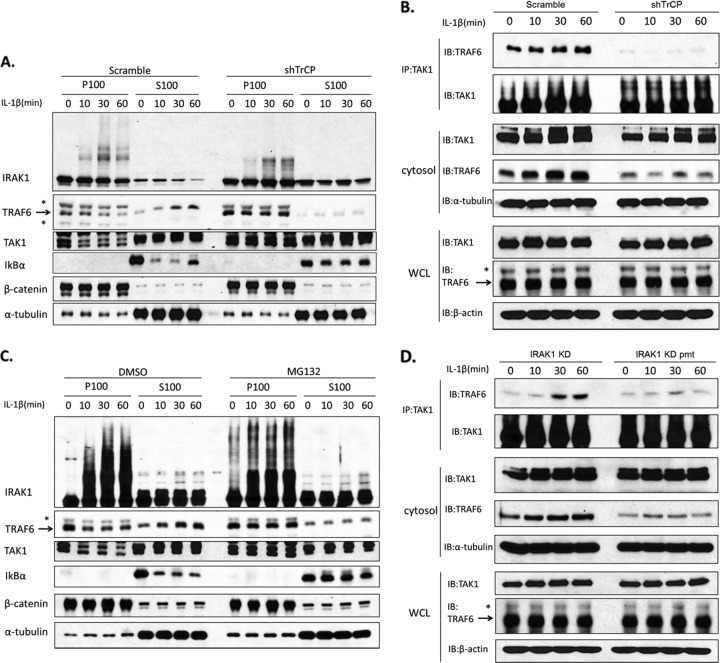
To test this hypothesis, we examined the impact of TrCP knockdown on IL-1-induced TAK1-TRAF6 translocation. Wild-type 293-IL-1R cells, left untreated or treated with IL-1, were fractionated into membrane and cytosol, followed by Western analyses with antibodies against IRAK1, TRAF6, and TAK1 (Fig. 6A). β-Catenin and α-tubulin were used as markers for the membrane and cytosol fractions, respectively. Whereas unmodified IRAK1 was found in both membrane and cytosol fractions, the phosphorylated and ubiquitinated IRAK1 induced by stimulation with IL-1 was found only in the membrane fraction. TRAF6, which was mostly in the membrane fraction in untreated cells, translocated to the cytosol upon stimulation with IL-1. On the other hand, TAK1 was found in both membrane and cytosol before and after stimulation. Although it was difficult to detect the translocated TAK1 in the cytosol, we did observe the disappearance of TAK1 from the membrane after stimulation. Importantly, β-TrCP knockdown greatly attenuated IL-1-induced IRAK1 degradation, which correlated with the loss of IL-1-induced TRAF6 translocation from the membrane to the cytosol. In support of this, the IL-1-induced disappearance of TAK1 from the membrane was also blocked in β-TrCP knockdown cells. These results indicate that β-TrCP-mediated, IL-1-induced IRAK1 degradation is required for the transition of TRAF6 and TAK1 from complex II to complex III.
Fig 6.
β-TrCP was required for the translocation of TAK1-TRAF6 complex. (A) The β-TrCP knockdown cells or control cells were left untreated or were treated with IL-1 (1 ng/ml) as indicated and then fractionated into membrane and cytosol. The fractions were analyzed by Western blot analysis with antibodies against IRAK, TRAF6, TAK1, IκBα, α-tubulin, and β-catenin. (B) IL-1-treated β-TrCP knockdown cells or control cells were fractionated. The cytosol fractionation was subjected to immunoprecipitation with anti-TAK1 and analyzed by Western blotting with anti-TRAF6. (C) 293-IL-1R cells were pretreated with DMSO or proteasome inhibitor MG132 (20 nmol/ml) for 2 h. The cells then were fractionated into membrane and cytosol. The fractions were analyzed by Western blotting as described for panel A. (D) IL-1-treated IRAK1 KD or IRAK1 KD pmt-transfected I1A cells were fractionated. The cytosol fractionation was subjected to immunoprecipitation with anti-TAK1 and analyzed by Western blotting with anti-TRAF6. Asterisks indicate nonspecific bands detected by anti-TRAF6 antibody.
To confirm the impact of β-TrCP on the IL-1-induced translocation of the TRAF6-TAK1 complex, we performed coimmunoprecipitation with cytosolic lysates from untreated and IL-1-treated β-TrCP knockdown and control cells. Importantly, we found that IL-1-induced cytosolic TRAF6-TAK1 complex was detected only in the control cells and not in the β-TrCP knockdown cells (Fig. 6B). These results clearly indicate that β-TrCP is indeed required for the IL-1-mediated translocation of TRAF6-TAK1 from the membrane to the cytosol. Consistent with this, the IL-1-induced cytosolic TRAF6-TAK1 complex was not detected in the IRAK1 KD pmt cells, indicating that phosphorylation and degradation of IRAK1 are required for the translocation of the complex (Fig. 6D). IL-1-induced, TAK1-dependent signaling events (including IKKα/β and JNK phosphorylation) were substantially diminished in β-TrCP knockdown cells (Fig. 5). Importantly, pretreatment of 293-IL-1R cells with MG132 also abolished the IL-1-induced translocation of TAK1 and TRAF6 from the membrane to the cytosol, which was probably due to its inhibition of IRAK1 degradation (Fig. 6C). Taking these results together, we propose that SCF–β-TrCP-mediated, K48-linked IRAK1 ubiquitination and degradation releases TRAF6-TAK1 from membrane-bound complex II (IRAK-TRAF6-TAK1) to complex III (TAK1-TRAF6) in the cytosol, leading to TAK1, IKK, and NF-κB activation.
DISCUSSION
In this work, we report that SCF (Skp1–Cullin1–F-box)–β-TrCP complex functions as the K48-linked ubiquitination E3 ligase for IRAK1. IL-1 stimulation induced the interaction of IRAK1 with Cullin1 and β-TrCP. By overexpressing and knocking down β-TrCP1 and β-TrCP2, we demonstrated that β-TrCP plays a key role in mediating K48-linked ubiquitination and degradation of IRAK1, which correlates with the translocation of TAK1-TRAF6 complex from the membrane to the cytosol and IL-1-induced, TAK1-dependent NF-κB activation. Taken together, these results suggest that β-TrCP-mediated IRAK1 degradation releases TAK1-TRAF6 to the cytosol, resulting in IL-1-induced TAK1, IKK, and NF-κB activation.
Although it was speculated that IRAK1 degradation is one of the mechanisms to shut down IL-1 signaling to control inflammatory responses, our studies suggest that IRAK1 degradation is required for the activation of the TAK1-dependent pathway. We have previously shown that TAK1-TABs are preassociated as a complex on the membrane before IL-1 stimulation (8). Upon IL-1 stimulation, the modified IRAK1-TRAF6 complex dissociates from the receptor complex (complex I) and forms complex II with the preassociated TAK1-TABs on the membrane. The membrane-bound, modified IRAK1 is eventually ubiquitinated and degraded, which is accompanied by the release of the TAK1 complex (TRAF6-TAK1-TABs) from the membrane to the cytosol, where TAK1 is activated (8). We have now shown that IL-1-induced, β-TrCP-mediated, K48-linked IRAK1 ubiquitination and subsequent degradation play a critical role in the release of TAK1 complex from the membrane to the cytosol. Future studies are required to define the molecular nature and subcellular localization of the membrane association of complex II.
It is important to note that IRAK1 can be ubiquitinated through both K48- and K63-linked polyubiquitin chains upon IL-1 induction. We and others have previously shown that Pellino proteins meditate K63-linked polyubiquitination on IRAK1 (7, 15, 16). We recently found that Pellino 2 is required for IL-1- and lipopolysaccharide (LPS)-induced, K63-linked IRAK1 polyubiquitination. In Pellino 2 knockdown cells, the K63-linked polyubiquitination of IRAK1 was greatly reduced, which correlated with reduced IRAK1-TAK1 complex formation, suggesting that Pellino 2-mediated, K63-linked polyubiquitination of IRAK1 positively affects TLR-IL-1R signaling (10a). Furthermore, when Pellino 2 was ablated, TLR–IL-1R-induced, TAK1-dependent NF-κB activation was compromised. Since Pellino 2 has been shown to play a positive role in IL-1-induced NF-κB activation, it is possible that Pellino 2-mediated, K63-linked IRAK1 polyubiquitination plays an important role in the interaction of IRAK1 with the TAK1-TAB2-TAB3 complex. It has been shown that TAB2 and TAB3 specifically bind to K63-linked polyubiquitin chains through the highly conserved C-terminal zinc finger domain (10).
We previously reported that a point mutation changing lysine 134 to arginine (K134R) in IRAK1 abolished IL-1-induced IRAK1 ubiquitination and degradation (24, 26). While Pellino proteins failed to mediate K63-linked polyubiquitination on the K134R mutant, β-TrCP-mediated, K48-linked IRAK1 polyubiquitination was also abolished by K134R mutation. These results suggest that K134 is the site for both K48-linked and K63-linked polyubiquitin chains on IRAK1. Therefore, K63-linked polyubiquitin chains on IRAK1 need to be removed by a deubiquitination enzyme followed by K48-linked IRAK1 ubiquitination to target IRAK1 degradation. It has been reported that the deubiquitinase A20 can inhibit the NF-κB signaling by A20 through disruption of ubiquitin enzyme complexes. However, A20 or TAX1BP1 deficiency had no effect on the ubiquitination or degradation of IRAK1 (17, 18). Thus, the deubiquitinase for IRAK1 still needs further investigation. We propose that Pellino 2-mediated, K63-linked IRAK1 polyubiquitination precedes β-TrCP-mediated, K48-linked IRAK1 polyubiquitination and degradation. To add more complexity to IRAK1 ubiquitination, in contrast to Pellino 2, we previously reported that Pellino 3b has a negative impact on IL-1-induced, TAK1-dependent NF-κB activation (24). Pellino 3b-mediated, K63-linked IRAK polyubiquitination competed with K48-linked IRAK1 polyubiquitination for the same ubiquitination site, Lys 134 of IRAK, leading to the inhibition of IL-1-induced IRAK1 degradation. Interestingly, IL-1 stimulation upregulated the expression of endogenous Pellino 3b, suggesting a negative feedback on IRAK1-mediated signaling. It is important to note that since both K63- and K48-linked ubiquitination of IRAK1 were accumulated when IRAK1 degradation was blocked by pretreatment with proteasome inhibitor MG132. It is possible that K63-linked polyubiquitination takes place on other lysines besides K134. Future studies are required to clarify the multiple steps of IRAK1 polyubiquitination and their specific roles in IRAK1-mediated signaling.
ACKNOWLEDGMENTS
This work was supported by grants from the NIH (2PO1 HL 029582-26A1 and 2PO1CA062220-16A1). This research was partly supported by grants from National Basic Research Program, China (2010CB911801). This research was partly supported by the China Scholarship Council.
We thank Vishva M. Dixit (Department of Physiological Chemistry, Genentech, Inc., South San Francisco, CA) for generously providing the anti-K48-linked and anti-K63-linked polyubiquitin antibody. We thank Yinon Ben-Neriah (The Hebrew University—Hadassah Medical School, Jerusalem, Israel) for generously providing β-TrCP constructs.
Footnotes
Published ahead of print 30 July 2012
REFERENCES
- 1. Burns K, et al. 1998. MyD88, an adapter protein involved in interleukin-1 signaling. J. Biol. Chem. 273: 12203– 12209 [DOI] [PubMed] [Google Scholar]
- 2. Cao Z, Henzel WJ, Gao X. 1996. IRAK: a kinase associated with the interleukin-1 receptor. Science 271: 1128– 1131 [DOI] [PubMed] [Google Scholar]
- 3. Chen ZJ. 2005. Ubiquitin signalling in the NF-kappaB pathway. Nat. Cell Biol. 7: 758– 765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cheung PC, Nebreda AR, Cohen P. 2004. TAB3, a new binding partner of the protein kinase TAK1. Biochem. J. 378: 27– 34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fuchs SY, Chen A, Xiong Y, Pan ZQ, Ronai Z. 1999. HOS, a human homolog of Slimb, forms an SCF complex with Skp1 and Cullin1 and targets the phosphorylation-dependent degradation of IkappaB and beta-catenin. Oncogene 18: 2039– 2046 [DOI] [PubMed] [Google Scholar]
- 6. Ishitani T, et al. 2003. Role of the TAB2-related protein TAB3 in IL-1 and TNF signaling. EMBO J. 22: 6277– 6288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jiang Z, et al. 2003. Pellino 1 is required for interleukin-1 (IL-1)-mediated signaling through its interaction with the IL-1 receptor-associated kinase 4 (IRAK4)-IRAK-tumor necrosis factor receptor-associated factor 6 (TRAF6) complex. J. Biol. Chem. 278: 10952– 10956 [DOI] [PubMed] [Google Scholar]
- 8. Jiang Z, Ninomiya-Tsuji J, Qian Y, Matsumoto K, Li X. 2002. Interleukin-1 (IL-1) receptor-associated kinase-dependent IL-1-induced signaling complexes phosphorylate TAK1 and TAB2 at the plasma membrane and activate TAK1 in the cytosol. Mol. Cell. Biol. 22: 7158– 7167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jin G, et al. 2004. Identification of a human NF-kappaB-activating protein, TAB3. Proc. Natl. Acad. Sci. U. S. A. 101: 2028– 2033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kanayama A, et al. 2004. TAB2 and TAB3 activate the NF-kappaB pathway through binding to polyubiquitin chains. Mol. Cell 15: 535– 548 [DOI] [PubMed] [Google Scholar]
- 10a. Kim TW, et al. 2012. Pellino 2is critical for Toll-like receptor/interleukin-1 receptor (TLR/IL-1R)-mediated post-transcriptional control. J. Biol. Chem. 287: 25686– 25695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Latres E, Chiaur DS, Pagano M. 1999. The human F box protein beta-Trcp associates with the Cul1/Skp1 complex and regulates the stability of beta-catenin. Oncogene 18: 849– 854 [DOI] [PubMed] [Google Scholar]
- 12. Li S, Strelow A, Fontana EJ, Wesche H. 2002. IRAK-4: a novel member of the IRAK family with the properties of an IRAK-kinase. Proc. Natl. Acad. Sci. U. S. A. 99: 5567– 5572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Newton K, et al. 2008. Ubiquitin chain editing revealed by polyubiquitin linkage-specific antibodies. Cell 134: 668– 678 [DOI] [PubMed] [Google Scholar]
- 14. Ninomiya-Tsuji J, et al. 1999. The kinase TAK1 can activate the NIK-I kappaB as well as the MAP kinase cascade in the IL-1 signalling pathway. Nature 398: 252– 256 [DOI] [PubMed] [Google Scholar]
- 15. Ordureau A, et al. 2008. The IRAK-catalysed activation of the E3 ligase function of Pellino isoforms induces the Lys63-linked polyubiquitination of IRAK1. Biochem. J. 409: 43– 52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schauvliege R, Janssens S, Beyaert R. 2006. Pellino proteins are more than scaffold proteins in TLR/IL-1R signalling: a role as novel RING E3-ubiquitin-ligases. FEBS Lett. 580: 4697– 4702 [DOI] [PubMed] [Google Scholar]
- 17. Shembade N, Harhaj NS, Liebl DJ, Harhaj EW. 2007. Essential role for TAX1BP1 in the termination of TNF-alpha-, IL-1- and LPS-mediated NF-kappaB and JNK signaling. EMBO J. 26: 3910– 3922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shembade N, Ma A, Harhaj EW. 2010. Inhibition of NF-kappaB signaling by A20 through disruption of ubiquitin enzyme complexes. Science 327: 1135– 1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shim JH, et al. 2005. TAK1, but not TAB1 or TAB2, plays an essential role in multiple signaling pathways in vivo. Genes Dev. 19: 2668– 2681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Spencer E, Jiang J, Chen ZJ. 1999. Signal-induced ubiquitination of IκBα by the F-box protein Slimb/beta-TrCP. Genes Dev. 13: 284– 294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Su J, Richter K, Zhang C, Gu Q, Li L. 2007. Differential regulation of interleukin-1 receptor associated kinase 1 (IRAK1) splice variants. Mol. Immunol. 44: 900– 905 [DOI] [PubMed] [Google Scholar]
- 22. Takaesu G, et al. 2000. TAB2, a novel adaptor protein, mediates activation of TAK1 MAPKKK by linking TAK1 to TRAF6 in the IL-1 signal transduction pathway. Mol. Cell 5: 649– 658 [DOI] [PubMed] [Google Scholar]
- 23. Tan P, et al. 1999. Recruitment of a ROC1-CUL1 ubiquitin ligase by Skp1 and HOS to catalyze the ubiquitination of I kappa B alpha. Mol. Cell 3: 527– 533 [DOI] [PubMed] [Google Scholar]
- 24. Xiao H, et al. 2008. Pellino 3b negatively regulates interleukin-1-induced TAK1-dependent NF kappaB activation. J. Biol. Chem. 283: 14654– 14664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yamin TT, Miller DK. 1997. The interleukin-1 receptor-associated kinase is degraded by proteasomes following its phosphorylation. J. Biol. Chem. 272: 21540– 21547 [DOI] [PubMed] [Google Scholar]
- 26. Yao J, et al. 2007. Interleukin-1 (IL-1)-induced TAK1-dependent versus MEKK3-dependent NFkappaB activation pathways bifurcate at IL-1 receptor-associated kinase modification. J. Biol. Chem. 282: 6075– 6089 [DOI] [PubMed] [Google Scholar]