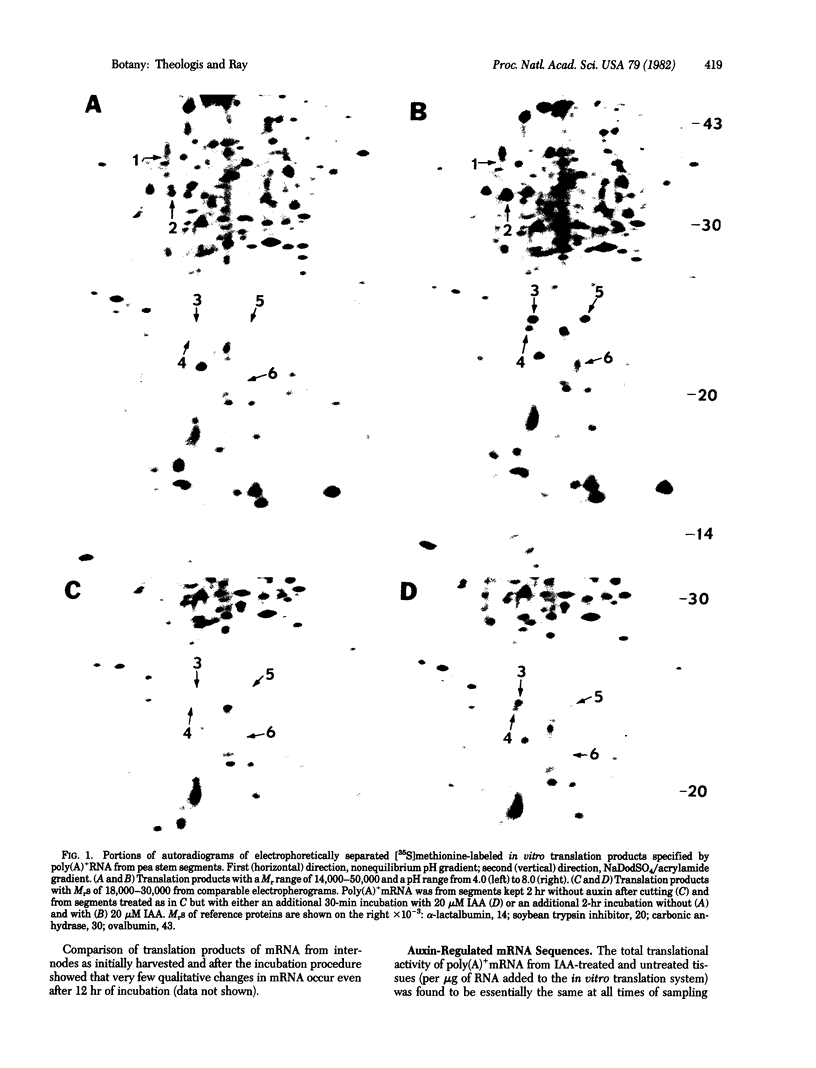
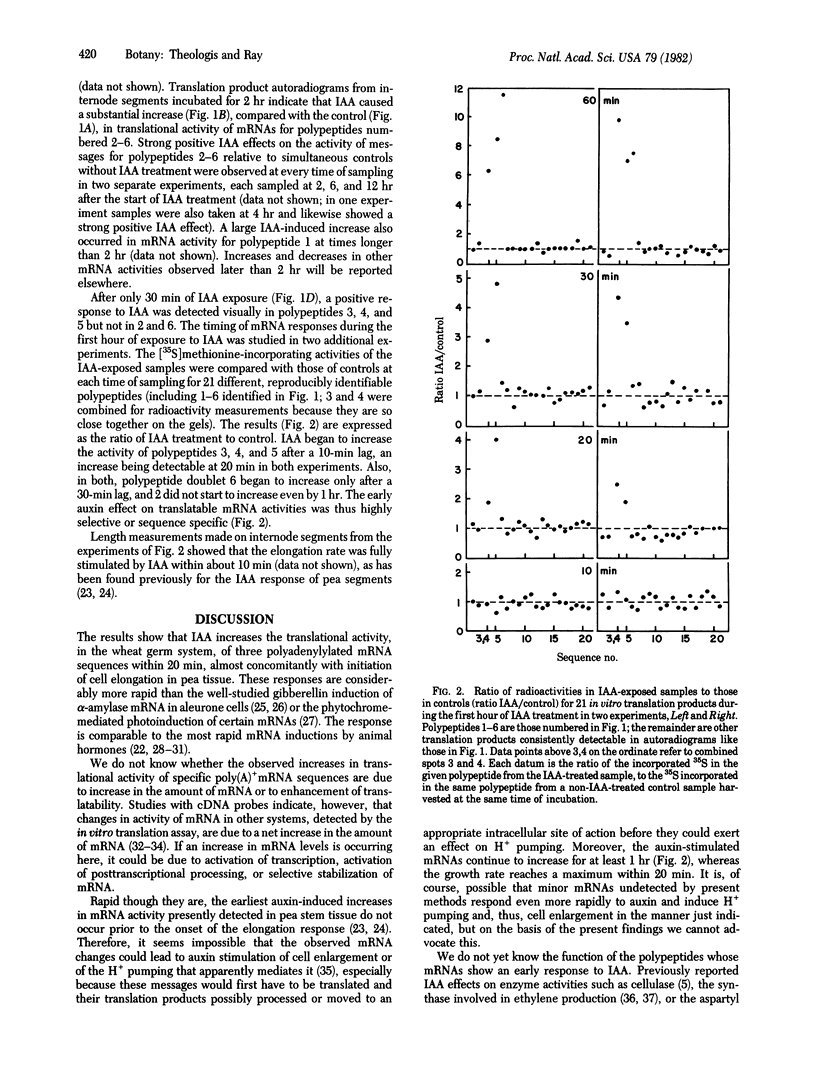
Abstract
Polyadenylylated mRNA from etiolated pea stem segments treated with or without 20 μM indoleacetic acid (IAA) for various periods of time was assayed by translating it in a wheat germ extract containing [35S]methionine and separating the translation products by two-dimensional gel electrophoresis. Within 2 hr IAA causes at least five mRNA sequences to increase in translational activity, relative to initial levels and to simultaneous controls; three of these rise significantly within 20 min after exposure of tissue to IAA but are apparently not elevated at 10 min, whereas the others begin to increase at successive times later than 30 min, and still others begin to change only later than 2 hr. These observations indicate an early, highly selective IAA regulation of mRNA amounts or activities, becoming progressively more extensive with time. The earliest detected enhancement seems close to the primary action of IAA but appears not to be rapid enough to be responsible for auxin induction of cell enlargement.
Keywords: indoleacetic acid, cell elongation, cell-free translation, gene expression, hormone action
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker H. J., Shapiro D. J. Kinetics of estrogen induction of Xenopus laevis vitellogenin messenger RNA as measured by hybridization to complementary DNA. J Biol Chem. 1977 Dec 10;252(23):8428–8434. [PubMed] [Google Scholar]
- Baker H. J., Shapiro D. J. Rapid accumulation of vitellogenin messenger RNA during secondary estrogen stimulation of Xenopus laevis. J Biol Chem. 1978 Jul 10;253(13):4521–4524. [PubMed] [Google Scholar]
- Barkley G. M., Evans M. L. Timing of the auxin response in etiolated pea stem sections. Plant Physiol. 1970 Feb;45(2):143–147. doi: 10.1104/pp.45.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobberstein B., Blobel G. Functional interaction of plant ribosomes with animal microsomal membranes. Biochem Biophys Res Commun. 1977 Feb 21;74(4):1675–1682. doi: 10.1016/0006-291x(77)90637-4. [DOI] [PubMed] [Google Scholar]
- Evans M. L., Ray P. M. Timing of the auxin response in coleoptiles and its implications regarding auxin action. J Gen Physiol. 1969 Jan;53(1):1–20. doi: 10.1085/jgp.53.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs M., Ray P. M. Rapid Auxin-induced Decrease in Free Space pH and Its Relationship to Auxin-induced Growth in Maize and Pea. Plant Physiol. 1976 Aug;58(2):203–209. doi: 10.1104/pp.58.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martial J. A., Baxter J. D., Goodman H. M., Seeburg P. H. Regulation of growth hormone messenger RNA by thyroid and glucocorticoid hormones. Proc Natl Acad Sci U S A. 1977 May;74(5):1816–1820. doi: 10.1073/pnas.74.5.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight G. S., Palmiter R. D. Transcriptional regulation of the ovalbumin and conalbumin genes by steroid hormones in chick oviduct. J Biol Chem. 1979 Sep 25;254(18):9050–9058. [PubMed] [Google Scholar]
- Mozer T. J. Control of protein synthesis in barley aleurone layers by the plant hormones gibberellic acid and abscisic acid. Cell. 1980 Jun;20(2):479–485. doi: 10.1016/0092-8674(80)90634-0. [DOI] [PubMed] [Google Scholar]
- Noodén L. D., Thimann K. V. EVIDENCE FOR A REQUIREMENT FOR PROTEIN SYNTHESIS FOR AUXIN-INDUCED CELL ENLARGEMENT. Proc Natl Acad Sci U S A. 1963 Aug;50(2):194–200. doi: 10.1073/pnas.50.2.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson B. D., Trewavas A. J. Changes in the pattern of protein synthesis induced by 3-indolylacetic Acid. Plant Physiol. 1967 Aug;42(8):1081–1086. doi: 10.1104/pp.42.8.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Ringold G. M. Glucocorticoid regulation of mouse mammary tumor virus gene expression. Biochim Biophys Acta. 1979 Dec 19;560(4):487–508. doi: 10.1016/0304-419x(79)90014-3. [DOI] [PubMed] [Google Scholar]
- Roman R., Brooker J. D., Seal S. N., Marcus A. Inhibition of the transition of a 40 S ribosome-Met-tRNA-i-Met complex to an 80 S ribosome-Met-tRNA-i-Met- complex by 7-Methylguanosine-5'-phosphate. Nature. 1976 Mar 25;260(5549):359–360. doi: 10.1038/260359a0. [DOI] [PubMed] [Google Scholar]
- Scheele G., Blackburn P. Role of mammalian RNase inhibitor in cell-free protein synthesis. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4898–4902. doi: 10.1073/pnas.76.10.4898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder J., Kreuzaler F., Schäfer E., Hahlbrock K. Concomitant induction of phenylalanine ammonia-lyase and flavanone synthase mRNAs in irradiated plant cells. J Biol Chem. 1979 Jan 10;254(1):57–65. [PubMed] [Google Scholar]
- Shapiro D. J., Baker H. J., Stitt D. T. In vitro translation and estradiol-17beta induction of Xenopus laevis vitellogenin messenger RNA. J Biol Chem. 1976 May 25;251(10):3105–3111. [PubMed] [Google Scholar]
- Shields D., Blobel G. Cell-free synthesis of fish preproinsulin, and processing by heterologous mammalian microsomal membranes. Proc Natl Acad Sci U S A. 1977 May;74(5):2059–2063. doi: 10.1073/pnas.74.5.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaneck G. E., Kreuzaler F., Tsai M. J., O'Malley B. W. Absence of an obligatory lag period in the induction of ovalbumin mRNA by estrogen. Biochem Biophys Res Commun. 1979 Jun 27;88(4):1412–1418. doi: 10.1016/0006-291x(79)91137-9. [DOI] [PubMed] [Google Scholar]
- VENIS M. A. INDUCTION OF ENZYMATIC ACTIVITY BY INDOLYL-3-ACETIC ACID AND ITS DEPENDENCE ON SYNTHESIS OF RIBONUCLEIC ACID. Nature. 1964 May 30;202:900–901. doi: 10.1038/202900b0. [DOI] [PubMed] [Google Scholar]
- Vanderhoef L. N., Dute R. R. Auxin-regulated Wall Loosening and Sustained Growth in Elongation. Plant Physiol. 1981 Jan;67(1):146–149. doi: 10.1104/pp.67.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderhoef L. N., Stahl C. A., Williams C. A., Brinkmann K. A. Additional evidence for separable responses to auxin in soybean hypocotyl. Plant Physiol. 1976 May;57(5):817–819. doi: 10.1104/pp.57.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma D. P., Maclachlan G. A., Byrne H., Ewings D. Regulation and in vitro translation of messenger ribonucleic acid for cellulase from auxin-treated pea epicotyls. J Biol Chem. 1975 Feb 10;250(3):1019–1026. [PubMed] [Google Scholar]
- Walker M. D., Kaye A. M. mRNA for the rat uterine estrogen-induced protein. Translation in vitro and regulation by estrogen. J Biol Chem. 1981 Jan 10;256(1):23–26. [PubMed] [Google Scholar]
- Yu Y. B., Adams D. O., Yang S. F. Regulation of Auxin-induced Ethylene Production in Mung Bean Hypocotyls: Role of 1-Aminocyclopropane-1-Carboxylic Acid. Plant Physiol. 1979 Mar;63(3):589–590. doi: 10.1104/pp.63.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurfluh L. L., Guilfoyle T. J. Auxin-induced changes in the patterns of protein synthesis in soybean hypocotyl. Proc Natl Acad Sci U S A. 1980 Jan;77(1):357–361. doi: 10.1073/pnas.77.1.357. [DOI] [PMC free article] [PubMed] [Google Scholar]