Abstract
We investigated the roles of Salmonella pathogenicity island 2 (SPI-2) and two SPI-2 effectors in Salmonella colitis and diarrhea in genetically resistant BALB/c.D2Slc11a1 congenic mice with the wild-type Nramp1 locus. Wild-type Salmonella enterica serovar Typhimurium 14028s caused a pan-colitis, and the infected mice developed frank diarrhea with a doubling of the fecal water content. An ssaV mutant caused only a 26% increase in fecal water content, without producing the pathological changes of colitis, and it did not cause weight loss over a 1-week period of observation. However, two SPI-2 effector mutants, the spvB and sifA mutants, and a double spvB sifA mutant caused diarrhea and colitis, even though the sifA mutant was sensitive to killing by bone marrow-derived macrophages from BALB/c.D2 mice and was severely impaired in extraintestinal growth but not in growth in the cecum. These results demonstrate that systemic S. enterica infection and diarrhea/colitis are distinct pathogenic processes and that only the former requires spvB and sifA.
INTRODUCTION
Salmonella enterica infections are a worldwide health problem that is common in both developing countries and industrialized nations. As a consequence of the increased globalization and industrialization of food production, large populations are vulnerable to food-borne Salmonella outbreaks arising from both point sources of contamination and contaminated foods that are sometimes distributed over great distances, even across continents (35, 38, 53). Salmonella infections in the United States are estimated to cost over $1.4 billion/year, and costs are likely to increase, with larger outbreaks leading to expensive recalls and expensive litigation, as evidenced in the recent peanut butter contamination episode (45).
Salmonella strains produce three distinct clinical syndromes in people: gastroenteritis, sepsis with or without metastatic infections, and typhoid (enteric) fever (15). Salmonella enterica subsp. enterica strains are genetically diverse, with over 2,000 serovars based on variations in the structures of the O and H antigens. Extensive work in the Salmonella pathogenesis field has shown that serovars differ in their repertoire of virulence genes and in their pathogenic potential. Only S. enterica serovar Typhi and a few S. enterica serovar Paratyphi strains can cause typhoid fever. Similarly, only a few serovars cause sepsis and/or metastatic infections, and the ones that do generally carry large plasmids that carry the salmonella plasmid virulence (spv) operon (19). In contrast, gastroenteritis is caused by a large number of serovars that characteristically lack spv genes (57), as well as two serovars (S. enterica serovars Enteritidis and Typhimurium) that may or may not carry virulence plasmids.
Despite the high incidence of gastroenteritis caused by nontyphoid Salmonella infections, very little is known of the pathogenesis and mechanisms of diarrhea in this disease. The central problem for the study of Salmonella gastroenteritis has been the lack of a small animal model that reproduces the intestinal disease. Murine infections with Salmonella are a very powerful tool for elucidating the pathogenesis of systemic nontyphoid Salmonella infections. However, oral infection of mice does not produce diarrhea or gut pathology consistent with the pathology of Salmonella gastroenteritis in people, even if the mice carry a G169D mutation of Nramp1 (Slc11a1) or a mutation of Toll-like receptor 4 (TLR4). Even though Salmonella strains are able to infect polarized intestinal epithelial cells in vitro, S. enterica in mice appears to enter preferentially through M cells into Peyer's patches or through uptake and dissemination by CD18-positive cells in the small intestine (29, 54).
Much of the virulence of Salmonella is due to horizontally acquired genes, including several so-called Salmonella pathogenicity islands (SPI) that are inserted into the chromosome (36), as well as accessory genetic elements such as lysogenic phages and large plasmids. The roles of SPI-1, SPI-2, and the spv locus in the pathogenesis of gastroenteritis have been investigated in a calf enteritis model. S. Typhimurium and S. Dublin produce an acute enteritis in calves (the natural host of S. Dublin) that models severe intestinal salmonellosis in humans (58). Unfortunately, there are many limitations of this model: it is expensive, so only small numbers of animals can be studied; large inocula are often used to ensure disease in all of the animals; and the extensive genetic resources and immunologic reagents available for studying mice are not available for bovines. For technical and financial reasons, investigators have made and then infected multiple intestinal blind loops in calves, which allows several mutants to be tested in a single animal. This method also facilitates measurements of fluid accumulation, acute inflammation, and bacterial invasion in the loops. This allows one to study several mutant bacteria in a single animal, but there are potential artifacts in this system due to loss of intestinal motility in the blind loops, and the experiments are necessarily brief. Overall, studies in these calf models have established the primary importance of the SPI-1 type 3 secretion system (TTSS) and the secreted effectors SipA, SopA, SopB, SopD, SopE2, and SopE (in isolates that carry sopE) (58) in bovine intestinal disease (24). Limited studies on SPI-2 seem to indicate that it plays a lesser role than that of SPI-1 in these acute models of intestinal inflammation and fluid loss (51). The role of the spv locus in calf intestinal disease appears to be serovar and strain dependent (34, 51).
A mouse model of Salmonella colitis was developed 50 years ago by Bohnhoff et al., who used oral streptomycin pretreatment to alter the normal intestinal flora, which facilitated Salmonella infection of the colon (6). Recently, Hardt and colleagues revived this model in the context of contemporary knowledge of Salmonella pathogenesis (2). Streptomycin-pretreated BALB/c and C57BL/6 mice (both are Nramp1D169 mutants) develop acute inflammation of the cecum 24 to 48 h after oral infection with S. Typhimurium or S. Enteritidis (50). The ceca of these mice are shrunken and show acute inflammatory changes, with cellular infiltrates in the mucosa and submucosa, submucosal edema, loss of mucus-secreting cells, and epithelial ulceration. Changes in the proximal colon are milder, indicating that this infection is primarily a model of cecal inflammation, or typhlitis, rather than colitis. In this model, diarrhea is not pronounced (2), possibly because Nramp1 mutant mice die from systemic Salmonella infection in 3 to 5 days. However, the model does facilitate the study of the pathogenesis of cecal inflammation through the use of Salmonella virulence mutants and mice with mutations in various host response pathways. These studies have shown that the SPI-1 TTSS is required to produce cecal inflammation but is not needed for systemic dissemination to mesenteric lymph nodes or the spleen (6, 26). The roles of individual effectors have been more difficult to establish, but SipA, SopE, and SopE2 contribute to cecal disease. SopB does not have an effect in this model (23). Bacterial chemotaxis and motility enhance early induction of cecal disease, but this effect disappears at 48 h (47). Recently, TLR5 was shown to have a role in preserving epithelial integrity and decreasing cecal pathology in the streptomycin pretreatment model (55). Interestingly, in 129Sv mice, S. Typhimurium caused earlier and more severe inflammation than that in an isogenic Nramp1D169 mutant of 129Sv, even though the former could better control the growth of the pathogen (52). Diarrhea was not examined in that study.
SPI-2 is also required for full virulence in Nramp1 mutant mice with typhlitis, with effects being more evident 3 to 5 days following infection (11, 13, 26). After 3 days of infection, SPI-1-dependent inflammation is not affected by the absence of MyD88 in the host, but SPI-2-dependent disease is markedly reduced in MyD88-deficient mice (26). As anticipated, SPI-1 mutant bacteria are not found in epithelial cells, and SPI-1 and SPI-2 mutant bacteria in the lamina propria appear to be inside different inflammatory cell populations. The results of these studies indicate that both SPI-1 and SPI-2 are required for production of inflammatory cecal disease at 3 to 4 days and that SPI-1 directs bacteria into epithelial cells and induces MyD88-independent inflammation, largely confirming earlier findings with cultured epithelial cells (59). In contrast, Salmonella with a SPI-1 mutation may pass through the colonic epithelium by the CD18 cell-dependent mechanism described by Vazquez-Torres and coworkers (54) and can then interact with macrophages, dendritic cells, and mesenchymal cells to induce proinflammatory cytokines through well-described TLR/MyD88-mediated pathways. However, this process is not passive and requires the function of SPI-2 to promote intracellular infection (26). SPI-2 is also required for S. Typhimurium to transit through cecal epithelial cells to enter the lamina propria, where it is taken up by local phagocytic cells (39).
We modified the model of Salmonella colitis by using BALB/c.D2Slc11a1 congenic mice (Nramp1 wild type [WT]) in order to facilitate analysis of both the pathophysiology of gastroenteritis and the roles of specific components of the host response (56). We found that the mice developed severe diarrhea and progressive, fatal pan-colitis after infection with S. Typhimurium and that an invA mutant lacking SPI-1 function infected the mice but did not cause diarrhea. In the present study, we analyzed the virulence of an ssaV (SPI-2) mutant and the roles of two SPI-2 effectors in the pathogenesis of diarrhea.
MATERIALS AND METHODS
Mice.
Female BALB/c.D2 mice between 8 and 10 weeks of age and congenic for the wild-type Nramp1 allele from DBA/2 mice (42) were used in all experiments. They were raised under specific-pathogen-free (SPF) conditions in our animal facility. Mice were pretreated by gavage with kanamycin (Kan) (20 mg dissolved in 0.2 ml of water) before being infected by gavage at various times after antibiotic treatment.
Bacteria and infection.
S. enterica serovar Typhimurium 14028s was our WT strain. It was made Kan resistant by insertion of a kanamycin resistance cassette into a genetically silent region just distal to the spv locus on the virulence plasmid. This mutation has no effect on virulence, as previously described (20). All mutations were transduced into a single 14028s lineage by use of P22 phage. Salmonella SPI-1, SPI-2, and spv mutants have been described previously (9).
All strains were grown overnight in Trypticase soy broth (TSB) and then washed twice in sterile saline before being resuspended to approximately 5 × 108 CFU/ml. They were further diluted in 0.1 M NaHCO3, and ∼3,000 CFU (except for the invA ssaV double mutant, which required a higher inoculum as described below) was administered by gavage with a blunt-tipped feeding needle. Mice were kept nil per os (NPO) for 4 h prior to being infected. All experiments were approved by the institutional animal research committee. To induce a systemic infection with the WT strain, we grew the bacteria in the same way but diluted the culture in sterile saline to contain 6,500 CFU/ml and injected 0.1 ml into the peritoneal cavity in the same strain of BALB/c.D2 congenic mice that we used for the colitis experiments. Mice were weighed before infection and 7 days later, on the day of sacrifice. Spleens were removed and processed as described in the section on necropsy.
Fecal water.
To obtain feces, we held mice vertically for up to 3 min until they spontaneously defecated. If they did not, we allowed them to move around in a container until they defecated, waiting for up to 10 min. Mice with severe diarrhea did not defecate under those conditions, so at necropsy we identified the distal 3 cm of the colon and expressed and processed the luminal contents as done for the defecated feces. We measured the water content of those intestinal contents by use of a modification of the method described by Guttman et al. (21). Feces were placed in preweighed microcentrifuge tubes, and the tubes were reweighed before they were placed in a SpeedVac (Savant, Hicksville, NY). The next day, when the contents were dry, we reweighed the tubes to calculate the fecal water content (percentage by weight).
Necropsy.
After sacrifice, we removed the mesenteric lymph nodes, spleen, and colon under sterile conditions. The proximal half of the cecum was removed for culture; cecal contents were washed out of the lumen, and the tissue was placed in a solution of 20 μg/ml of gentamicin in RPMI medium for 30 min at room temperature and then rinsed in saline before being homogenized. Each tissue sample was placed in 1 ml of sterile saline and homogenized using a TissueLyser II instrument (Qiagen, Germantown, MD). Serial dilutions of the homogenate were made in saline and plated in duplicate on Trypticase soy agar (TSA) containing 20 μg/ml of Kan, except that ceca and feces were cultured on Hektoen enteric agar containing 20 μg/ml Kan. Cultures were incubated overnight at 37°C, and then colonies were counted. Black colonies on Hektoen enteric agar containing Kan were assumed to be Salmonella. The lower limit of sensitivity of the culture was 5 CFU/ml.
After the proximal half of the cecum was removed for culture and the ileum was cut away from the colon, we perfused the colon lumen with 4% paraformaldehyde. The colon was coiled and fixed in paraformaldehyde overnight. Fixed tissue was embedded in paraffin, cut into 5-μm-thick sections, and stained with hematoxylin and eosin (H&E).
Pathology score.
To quantify the severity of the pathology, we used a slight modification of the scoring system devised by Coburn et al. (11), adding one point for blood in the lumen. We graded the pathology in 3 segments of the colon: the cecum, the midpoint, and the end (assumed to be the rectum). Each segment in each mouse was scored separately. The maximum number of points in this scoring system is 26. The slides were coded and read by one investigator for consistency.
Isolation and infection of murine macrophages.
Murine bone marrow-derived macrophages were isolated and differentiated using supplementation with mouse L cell-conditioned medium as described previously (40). After 5 to 7 days of growth and differentiation, macrophages were seeded into 24-well plates at a density of 5 × 105 cells/well and cultured overnight in Dulbecco's modified Eagle's medium (DMEM) supplemented with glutamine, pyruvate, 10% fetal calf serum (FCS), and 10 ng/ml murine macrophage colony-stimulating factor (M-CSF) (complete DMEM). Salmonella strains used for infection (14028s, the sifA mutant, and the ssaV mutant) were grown to mid-exponential phase with vigorous shaking in low-phosphate and -magnesium (LPM) medium to induce the SPI-2 TTSS (12). Bacteria were harvested, washed once in Dulbecco's phosphate-buffered saline (DPBS) containing Ca2+ and Mg2+, and then opsonized for 20 min at 37°C in 50% (vol/vol) fresh heparinized mouse plasma. Bacteria were added to macrophages at a 1:1 ratio, and the culture plate was immediately spun at 500 × g for 5 min. Following incubation at 37°C in 5% CO2 for 1 h, the culture medium was replaced with medium containing gentamicin at 100 μg/ml. After another hour, the cells were washed with DMEM and then incubated in complete DMEM containing gentamicin at 10 μg/ml. For the time point designated “0 h,” the medium was immediately aspirated after the addition of the lower gentamicin concentration; the cells were lysed with 0.5% deoxycholate in DPBS, and the number of viable intracellular bacteria was determined by serial dilution and plating on LB agar. A duplicate plate was incubated for 16 h following replacement with the medium containing the lower gentamicin concentration and then harvested in a similar manner. Where indicated, tumor necrosis factor alpha (TNF-α; PeproTech) was added to the cells at 10 ng/ml 24 h before infection.
Statistics.
Statistical analyses were performed using GraphPad Prism for Windows, version 5.00 (GraphPad Software, San Diego, CA). When we compared multiple mutants to a control, we used one-way analysis of variance (ANOVA) with Dunnett's posttest correction.
RESULTS
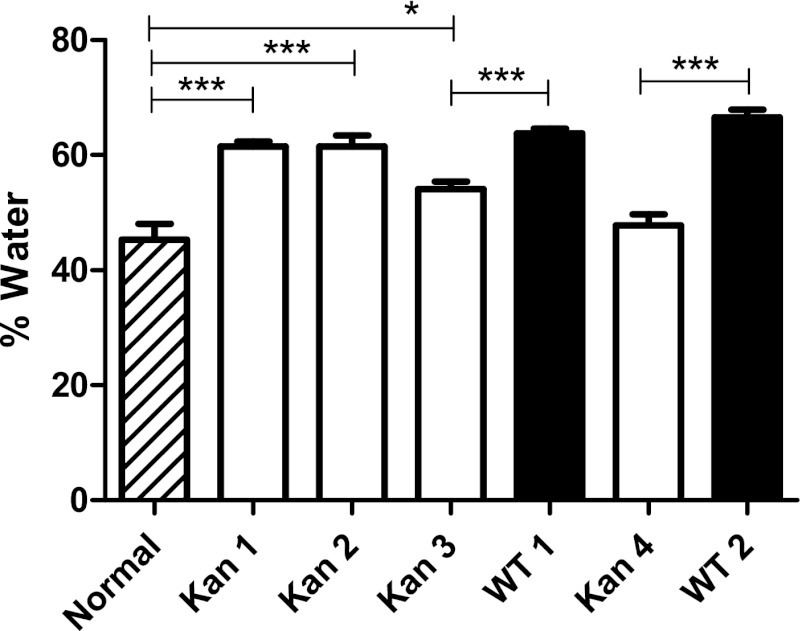
We first administered oral Kan to mice and then measured fecal water contents for the next 5 days (Fig. 1). Although all mice had normal-looking, formed stools, the percentage of fecal water was 28% higher than baseline for the first 2 days after Kan treatment, but by day 4 the water content was back to baseline, and in uninfected mice it remained at baseline thereafter. (We found that oral streptomycin had an even greater effect on fecal water content, which is why we used kanamycin.) There was no visible pathology in H&E-stained sections of the colon to account for this temporary increase in fecal water content. Although the baseline water content was already elevated when the mice were infected 48 h after a dose of Kan, infection further increased the fecal water content as soon as 24 h after infection, and the difference was maintained or increased for the first 48 h after infection (Fig. 1). This result shows that despite the change in intestinal physiology provoked by oral Kan, we could still detect an effect of Salmonella infection on intestinal water excretion. It also shows that the Kan effect on water absorption in the colon was transient.
Fig 1.
Kanamycin induces a self-limited increase in fecal water. Compared to those of untreated mice (hatched bar), fecal water contents of mice treated orally with kanamycin (open bars) were significantly higher for 48 h (P < 0.001; ANOVA with Dunnett's posttest modification). The number of days after kanamycin treatment is indicated under each open bar. We infected half of the mice with S. Typhimurium (WT) (closed bars) 48 h after the dose of kanamycin. The results for those mice are paired with the results for the mice receiving kanamycin alone for the first 2 days after infection. The difference in fecal water content of infected compared to kanamycin-treated control mice was also statistically significant for the 2 days after infection (***, P < 0.001; unpaired t test with Welch's correction).
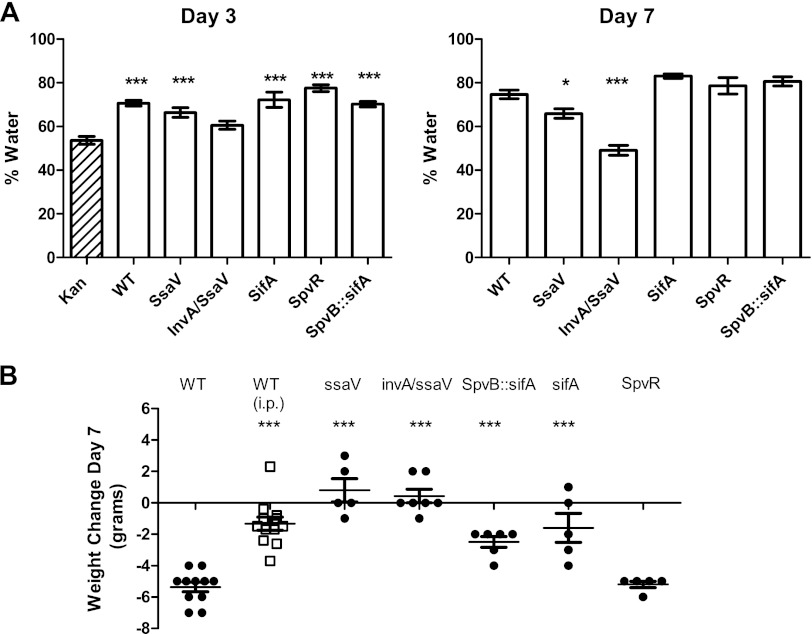
In subsequent experiments, we measured fecal water content beginning 3 days after infection, after the Kan effect had dissipated. Figure 2A shows the mean percentages of water in feces 3 and 7 days after infection by the WT and various mutants of 14028s (for simplicity, we do not show results for intervening days, but the percentage of water increased gradually over time). The control for infected mice on day 3 after infection was uninfected mice 5 days after Kan treatment, which were essentially equivalent to healthy untreated mice. As shown in Fig. 2A, 3 days after infection with WT S. Typhimurium, the fecal water content increased from 55% to 71%. At this time point, there was no significant difference between the WT and the ssaV mutant or either of the SPI-2 effector mutants. By day 7, most of the WT-infected mice had feces caked on their perirectal fur, and the fecal water content had increased to 79%, which was almost certainly an underestimate, as the mice with the most severe colitis had no fecal material to analyze in their distal colons. In contrast, the water content from ssaV mutant infections did not increase over this period (67% on both days). A great deal is known about the roles of various SPI-1 effectors in gastroenteritis (23, 59), but relatively little is known about which SPI-2 effectors are important in the colitis model (25). SifA and the spv-encoded effector SpvB are SPI-2 effectors that have major effects on virulence in mouse models of systemic Salmonella infections. However, mutants lacking these effectors caused diarrhea, and there was no significant difference between the fecal water contents of WT-infected mice and mice infected with the spvR (eliminates all spv gene expression), sifA, or spvB sifA mutant. Only the invA ssaV double mutant was unable to cause diarrhea, even though the mice were infected with 108 CFU (we were unable to establish an infection with our standard dose of 3 × 103 or with the higher dose of 3 × 104 CFU).
Fig 2.
Fecal water content and weight loss after Salmonella infection. (A) Fecal water content was measured as described in Materials and Methods. There were 6 to 12 infected mice/group and 10 uninfected Kan-pretreated controls. Each bar shows the mean ± 1 standard error of the mean (SEM). Day 3 postinfection results were compared to results for controls 5 days after Kan treatment (***, P < 0.001). On day 7, we compared each mutant to the WT (*, P < 0.05; ***, P < 0.001). (B) Weight change, in grams, 7 days after infection. Each closed symbol represents an individual mouse infected orally, and the bars indicate the mean ± 1 SEM. There were only 4 mice in the orally infected WT group because 2 moribund mice had to be euthanized on day 6. Weight losses of mice infected i.p. with the WT are shown with open squares. All mutant bacterial infections except for those with the spvR mutant caused significantly less weight loss than WT infections (***, P < 0.001; ANOVA with Dunnett's posttest modification).
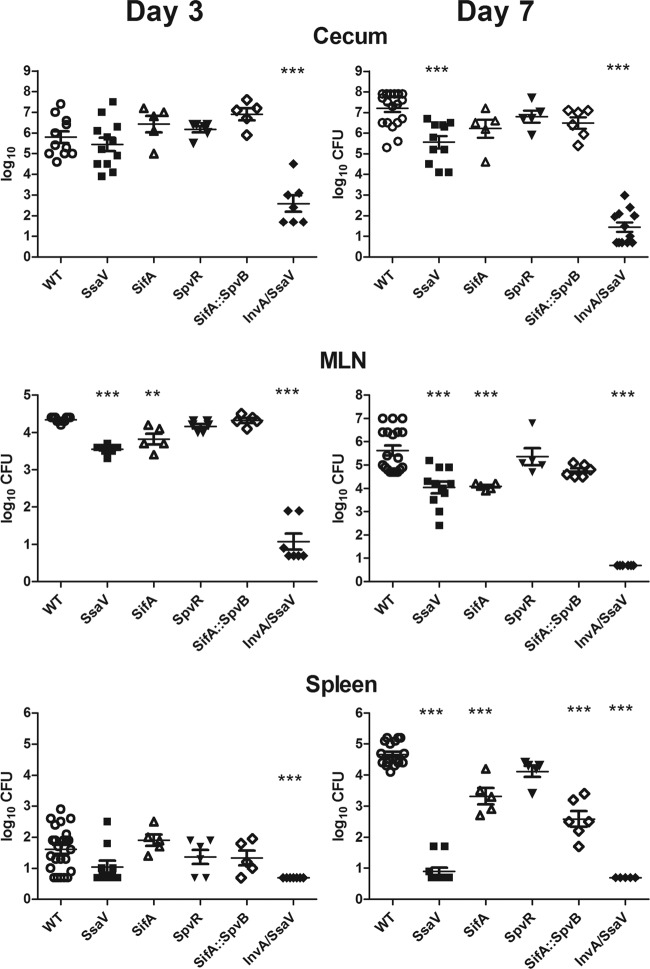
Fig 3.
Infectivity of WT and mutant Salmonella 3 and 7 days after infection. The numbers of CFU in the tissues were determined as described in Materials and Methods. Each symbol represents 1 mouse, and the horizontal lines show the geometric mean ± 1 SEM. Each mutant was compared to the WT (**, P < 0.01; ***, P < 0.001; ANOVA with Dunnett's posttest modification). MLN, mesenteric lymph nodes.
We expected diarrhea to lead to weight loss, and WT-infected mice lost nearly 1/3 of their body weight by day 7 of infection, whereas infections with the ssaV mutant and the invA ssaV double mutant did not cause weight loss (Fig. 2B). The WT infection also caused a more severe systemic infection than that caused by the ssaV mutant, and it is likely that some of the difference in weight loss could be attributed to the difference in severity of systemic infection. To estimate how much of the weight loss was due to systemic infection or resulted from the diarrhea, we infected the mice intraperitoneally (i.p.) with 650 CFU of the WT strain and sacrificed them 7 days later. They had nearly exactly the same number of CFU/spleen as did the orally infected mice, but they lost an average of only 2 g of weight. This suggests that most of the weight loss was indeed due to the diarrhea, but it is very likely that the systemic infection contributed to the inability of the WT-infected mice to keep themselves hydrated by drinking. This was further supported by the case for sifA spvB mutant-infected mice, which had diarrhea equivalent to that of WT-infected mice but only ∼1% of the number of CFU in the spleen and lost less weight than WT-infected mice (Fig. 3). In contrast, the severe weight loss caused by spvR mutant infection was probably caused by a combination of colitis and a substantial systemic infection similar to WT infection.
Since there is likely a correlation between the invasiveness of the mutants and the severity of the diarrhea they induce, we compared the infectivities of the WT strain and the ssaV and SPI-2 effector mutants after oral infection and assessed the amounts of colon pathology they caused. The infection begins in the cecum, so we determined the numbers of Salmonella organisms that invaded the proximal half of the cecum (defined as the bacteria not killed by gentamicin). As shown in Fig. 3, 3 days after infection there was an average of ∼106 CFU of the WT or the ssaV mutant in the cecum, and there was no significant difference between the two strains. This is consistent with the similar increases in fecal water content caused by those strains on day 3 (Fig. 2A). The number of ssaV mutant bacteria in mesenteric lymph nodes was slightly but statistically significantly lower than that of WT bacteria at that time. The number of sifA mutant bacteria was also significantly lower in the mesenteric lymph nodes, and the invA ssaV mutant did not infect the nodes. Only a small number of WT bacteria (geometric mean, <200 CFU) reached the spleens of these genetically resistant mice by day 3, and none of the mutants were significantly different from the WT except for the invA ssaV double mutant, which did not infect the spleen. On day 7 postinfection, the counts of WT S. Typhimurium were more than a log higher than those of the ssaV mutants in the cecum, 2 log higher in the mesenteric lymph nodes, and 4 log higher in the spleen, consistent with impaired virulence of the ssaV mutants both locally and systemically (4, 49), even in mice with functional Nramp1. The ssaV invA double mutant was essentially unable to infect the cecum until we increased the inoculum 10,000-fold, and even then the counts in the cecum were 4 log lower than those of either the WT or ssaV mutant strain, and the ssaV invA mutant infection was essentially eradicated from the cecum by day 7, as there was no growth from half of the mice and none of the others had counts reaching <103 CFU (Fig. 3). The inability of the invA ssaV double mutant to infect the colon shows that there is at least an additive effect of SPI-1 and SPI-2 in this model. The sifA and sifA spvB mutants were also recovered in smaller numbers from the mesenteric lymph nodes and spleens, but the numbers were not as low as those of the ssaV mutant. Since these strains caused severe colitis (Fig. 4 and 5) and were present in large numbers in the cecum, this shows that they were more impaired in the ability to grow in the spleen than in the intestine.
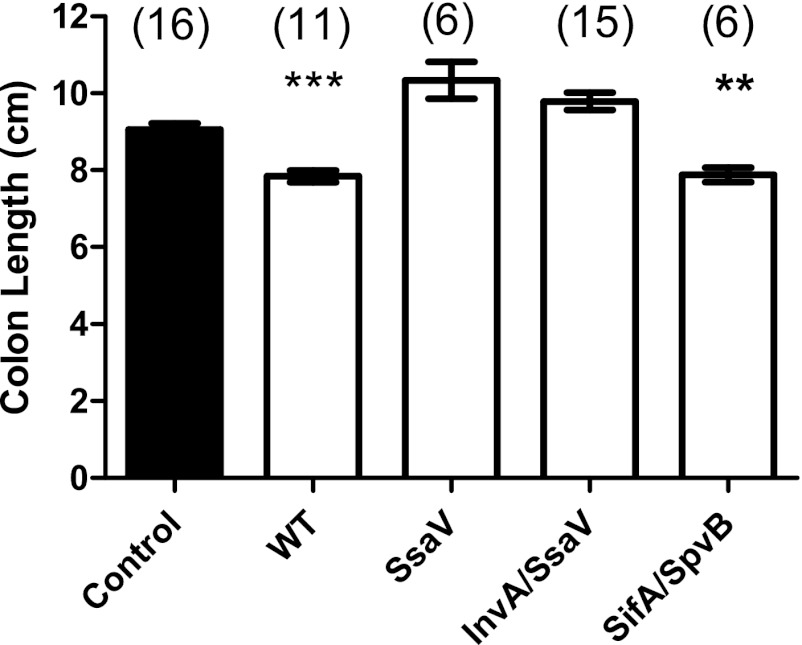
Fig 4.
Colon length 7 days after infection. Length was measured from the end of the cecum to the rectum. Numbers of mice in the groups are shown in parentheses. The filled bar shows the mean colon length ± 1 SEM for uninfected mice of the same age and sex. Open bars represent values for infected mice.
Fig 5.
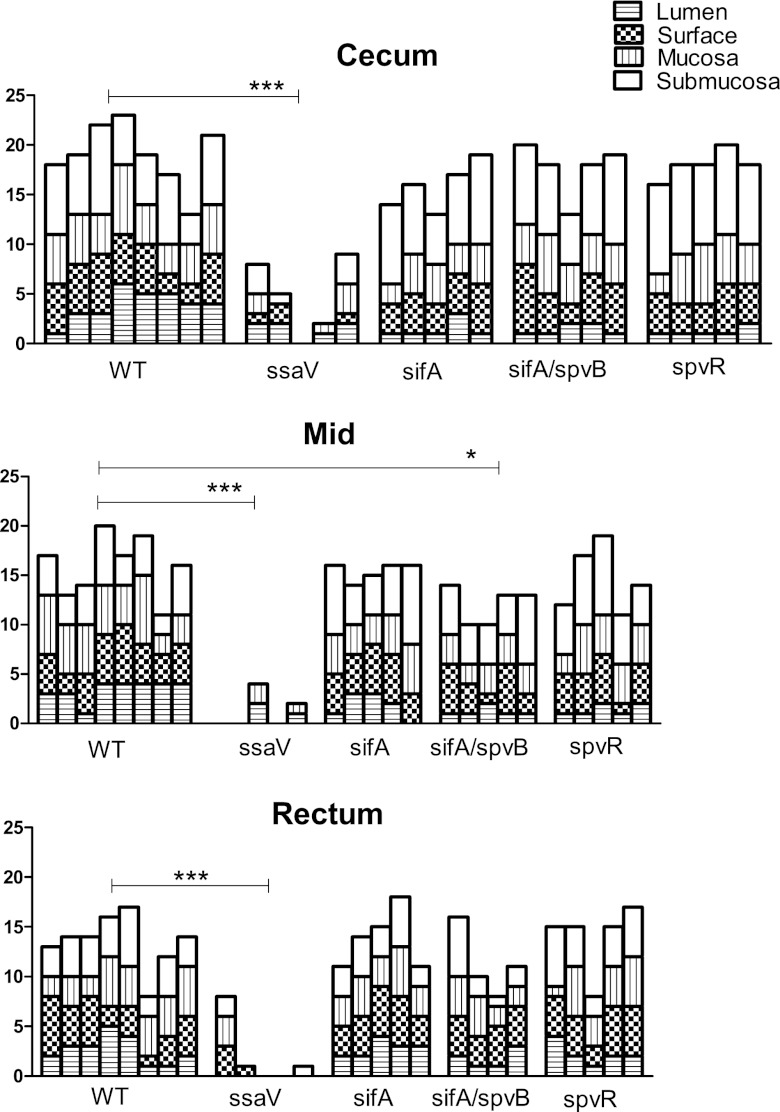
Pathology scores for WT and mutant bacteria at day 7 postinfection. The pathological scores were determined as described in Materials and Methods. Each column shows data for a single mouse. The rectum was assumed to be the most distal segment of the colon on the slide. The midportion was the closest area to one full turn beyond the cecum and was cut so at least some of the crypts were displayed longitudinally. The mean of the composite scores for the mutant salmonellae in each of the three segments was determined and compared to the mean of the WT infection scores (ANOVA with Dunnett's posttest modification). The ssaV pathology scores were all very much lower than the WT scores. The double mutant (spvB sifA) had a slightly lower score than the WT in the midsection of the colon (***, P < 0.001; *, P < 0.05).
One measure of the severity of colitis is the length of the colon, which is inversely proportional to the severity of colitis. We measured colon lengths on day 7 after infection. As shown in Fig. 4, WT infection resulted in marked shortening of the colon, while infection with the ssaV mutant did not. The spvB sifA mutant also caused significant shortening of the colon, providing further evidence that the two important SPI-2 effectors encoded by these genes are not necessary for S. Typhimurium to cause colitis.
We also compared the severities of colitis caused by the various mutants by scoring the histopathological changes induced by the infections (Fig. 5). Because we were concerned with damage to the whole colon rather than only to the cecum, we applied a scoring system (11) to three parts of the colon: cecum, midcolon, and rectum. WT S. Typhimurium caused the most damage in the cecum, but there was substantial pathology throughout the colon, as indicated by scores of >15 for most mice in that group. This scoring system gives much weight to the presence of polymorphonuclear leukocytes (PMN) in tissues and the lumen, but by day 7 after infection the predominant inflammatory cells in the submucosa were macrophages, so the scores are lower than might be expected if we had evaluated the mice earlier in the infection. There was mucosal ulceration as well as sloughed epithelial cells throughout the colons of WT-infected mice. Note that while submucosal edema and mucosal infiltrates nearly always occurred together in the cecum, there was little or no submucosal edema in the more distal parts of the colon, despite extensive mucosal inflammatory cell infiltration. Infection with the WT resulted in damage throughout the colon, and there was also evidence of repair, including inflammatory pseudopolyps (Fig. 6A). In comparison, the ssaV mutant caused only a small amount of damage in the cecum in 3/5 mice, with essentially no damage in the more distal parts of the colon. The pathological scores for the three mutants were not significantly different from WT scores.
Fig 6.
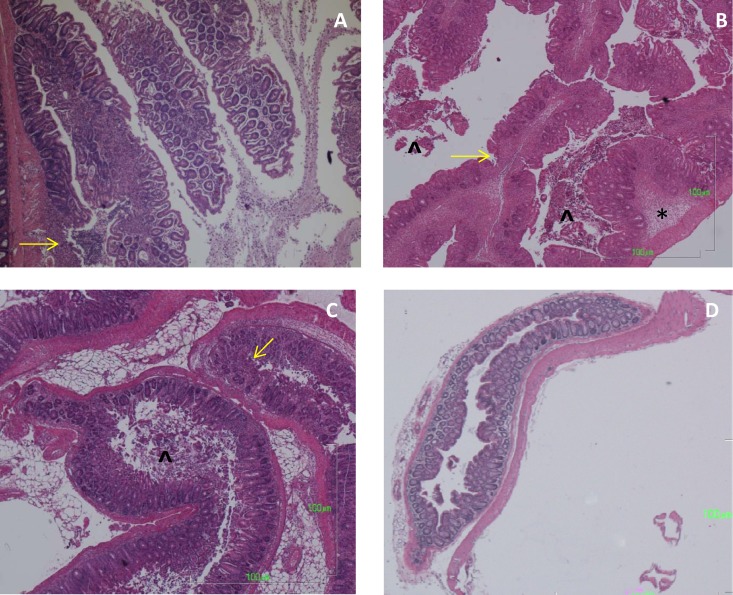
H&E-stained colons from mice at 7 days postinfection. (A) Inflammatory polyps in the proximal colon of a mouse infected with WT S. Typhimurium 14028s. There is an inflammatory cell infiltrate in each of the polyps and an area of mucosal ulceration on the lower polyp. (B) Rectum from a mouse infected with WT S. Typhimurium 14028s, with submucosal edema (asterisk), sloughed epithelial cells in the lumen (arrowhead), and inflammatory infiltrates in the mucosa. (C) Rectum from a mouse with a sifA mutant infection, showing intense inflammation, a lesser degree of submucosal edema, mucosal ulcerations, and epithelial cells in the lumen. (D) Rectum from a mouse infected with the ssaV mutant; the tissue is normal. Yellow arrows point to areas of mucosal erosions. Magnification for all photos, ×40.
It is difficult to compare our results directly to those of Hapfelmeier et al. (26) because we used a different scoring system to measure the pathological changes and scored the mice 7 days rather than 4 days after infection. However, we are in agreement that a SPI-2 mutant caused significantly less pathology than the WT in the mouse colitis model.
We show examples of the pathology in the rectum to emphasize that WT infection produced a pan-colitis (Fig. 6B and C). The changes produced by the sifA mutant were similar to those induced by the WT (Fig. 6C). The ssaV mutant caused essentially no pathology in the rectum, other than small areas of focal mixed mononuclear and PMN infiltrates (Fig. 6D).
Macrophage sensitivity of the sifA mutant.
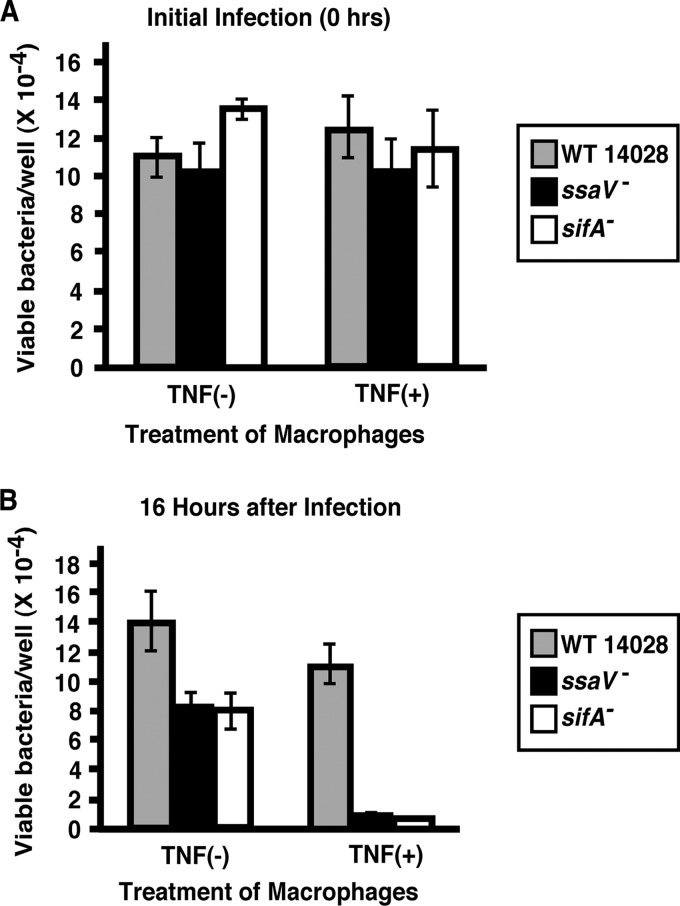
The results obtained with our colitis model indicated that sifA was not required to produce diarrhea during Salmonella enteritis. In mouse models of systemic infection, sifA mutants are significantly attenuated, and they are also less able to spread to and/or grow in the spleen in this colitis model. SifA has long been recognized as a major virulence effector of the SPI-2 TTSS (37, 49). In cell culture, sifA mutants are associated with an unstable Salmonella-containing vacuole (SCV) and tend to be released into the cytoplasm, at least in epithelial cells (4). In these cells, they show an enhanced rate of intracellular growth. However, in macrophages, sifA mutants survive poorly compared to wild-type strains (5, 10). These observations help to substantiate the established paradigm that macrophage survival is essential for Salmonella virulence in systemic infection (14). Since we did not find that sifA was required to produce colitis, we considered the possibility that macrophages from wild-type BALB/c.D2Slc11a1-congenic mice were equally bactericidal for WT and sifA mutant Salmonella. In order to test this possibility, we performed a macrophage survival assay to compare the sifA mutant to WT 14028s and the ssaV mutant as controls. Resting and TNF-activated bone marrow-derived macrophages from BALB/c.D2Slc11a1-congenic mice were infected with the Salmonella strains, and bacterial survival 16 h after inoculation was determined and compared to that at the initial time point. As shown in Fig. 7, both the sifA and ssaV mutants showed decreased survival in nonactivated macrophages at 16 h compared to that of wild-type bacteria. Activation of macrophages with TNF resulted in a further decrease in survival of both the sifA and ssaV mutants relative to the wild type. These results indicate that even in macrophages from wild-type Nramp1-carrying mice, a sifA mutant is more susceptible to macrophage killing than WT Salmonella, and a sifA mutant is as susceptible to macrophage killing as an ssaV mutant.
Fig 7.
SifA and SsaV mutants are more susceptible than WT S. Typhimurium to killing by bone marrow-derived macrophages. Macrophages were grown from the bone marrow of BALB/c.D2Slc11a1 congenic mice as described in Materials and Methods. There was no significant difference in the numbers of WT and ssaV and sifA mutant bacteria ingested by the macrophages. After 16 h of incubation, the survival of the two mutant strains was significantly less than the survival of WT Salmonella in both untreated cells and cells that were activated by pretreatment with TNF-α. The activated macrophages were bactericidal for all three strains.
DISCUSSION
The hallmark of Salmonella enteritis in people is diarrhea, yet this key clinical manifestation has generally not been amenable for study in the mouse model of oral infection. We previously described a new model for the study of Salmonella-induced colitis and diarrhea that reproduces many of the salient features of the disease found in humans and susceptible animals (55). Diarrhea can be defined either as an increase in the number of bowel movements/day over the norm for an individual or as an increase in the water content of the stools. Since it is not feasible to count the number of times/day that mice defecate, we measured the water content of their feces as a metric for the severity of diarrhea. We made two changes in the standard mouse model of Salmonella colitis that enabled us to study diarrhea: we pretreated mice with kanamycin rather than streptomycin, and we infected BALB/c.D2Slc11a1 congenic mice that are genetically resistant to S. enterica infections because they express a functional Nramp1 protein (56). We used Kan rather than streptomycin because the former had a smaller effect on fecal water content (not shown). By using mice that have a functional Nramp1 protein, we were able to observe the infection for a week, long enough for the mice to develop frank diarrhea. Although the mice died from the infection, it was not primarily because of the systemic spread of the infection but rather from the severe pan-colitis that they developed. It is possible that the mice we used also developed more inflammation than would have been seen in Nramp1 mutant strains (52), but the latter do not survive long enough after infection with WT Salmonella to make that comparison. We also used a much smaller inoculum to infect the mice, but we do not know if that affected the extent or timing of the pathology, because by 24 h after infection WT Salmonella had already proliferated to >108 CFU/g of feces, and that number did not change significantly during the course of the infection (not shown).
One drawback of this model for the study of Salmonella-induced diarrhea was that an oral dose of Kan itself caused a measurable increase in fecal water content that persisted for 2 to 3 days. Despite that, we were able to show that infection with WT Salmonella caused a further increase in fecal water. Using this model, we previously reported (56) that mice infected with WT S. Typhimurium 14028s have severe typhlitis by 48 h after infection but that the increase in the fecal water content is not greater than background until 3 days after infection, perhaps because the baseline water content is already elevated above normal by the Kan pretreatment. From day 3 to day 7 after WT infection, the fecal water content increased further, until by the fifth or sixth day of infection mice had frankly watery diarrhea. The development of colitis and frank diarrhea and the systemic spread of the infection resulted in a 25 to 30% loss of weight by day 7 after WT infection, at which time we terminated the experiment. By then, WT Salmonella had caused a pan-colitis with submucosal edema, inflammatory cell infiltration, and ulcerations throughout the colon. Another indication of the severity of the colitis was the 30% shortening of the colons.
Stecher et al. recently reported that S. Typhimurium infection in streptomycin-treated 129Sv/Ev (Slc11a1G169) mice leads to chronic cecal and colonic inflammation and to biliary disease with cholangitis that appears after 14 days of infection (48). They also found that DBA/2 mice, which also have wild-type Nramp1, have more severe colitis than 129 mice and that the former have more systemic spread of infection and do not survive beyond 7 to 10 days. It is not clear why 129 mice have milder colitis than DBA/2, BALB/c.D2Slc11a1 congenic (18), or C57BL/6Slc11a1 (J. Fierer, unpublished observation) transgenic mice, but it was recently discovered that 129 mice have a spontaneous mutation in caspase 11, which reduces interleukin-1α (IL-1α) and IL-1β secretion by bone marrow-derived macrophages that have ingested Gram-negative bacteria (31). This mutation would be expected to reduce inflammation after Salmonella infection (17), but the role of caspase 11 in colitis has not yet been studied.
We found that an ssaV (SPI-2) mutant also caused a typhlitis by day 3 after infection, as well as an increase in fecal water, that was not significantly different from that caused by WT infection. In contrast to WT infection, the diarrhea caused by the ssaV mutant strain did not worsen between days 3 and 7, and the mice infected with the ssaV mutant continued to have formed feces throughout the experiment. On day 3 postinfection, inflammation caused by ssaV mutant bacteria was largely confined to the cecum, although there were scattered foci of inflammation in the more proximal colon (not shown), as we reported previously for an invA mutant (56). In keeping with the pathology, there was no significant difference between the numbers of WT and mutant Salmonella bacteria recovered from cecal tissue on day 3 after infection. However, between days 3 and 7 after infection, there was a 10-fold increase in the number of WT Salmonella organisms in the cecum but no significant increase in the numbers of ssaV mutant bacteria, and the inflammation score was much lower in ssaV mutant infections than in WT infections (Fig. 5). Mice infected with the ssaV mutant lost little, if any, weight over the course of the infection and did not have significantly shortened colons, further evidence that this strain did not produce pan-colitis. Thus, it appears that the ssaV mutant is able to infect and inflame the cecum but not the rest of the colon in these genetically resistant mice, and as a result the diarrhea induced is much milder. An invA ssaV double mutant was almost unable to infect the colon, caused no pathology, and did not cause diarrhea. This implies that acquisition of both SPI-1 and SPI-2 is necessary for S. enterica to be a successful enteric pathogen. This conclusion is consistent with human epidemiological studies, as nearly every clinical isolate of S. enterica from patients with gastroenteritis that has been studied has both SPI-1 and SPI-2, with the exception of one outbreak due to a strain of S. enterica that lacked SPI-1 (27). How the two type 3 secretion systems and their effectors interact in vivo has not been established, but there is evidence that SipA, a SPI-1 effector, cooperates with SifA, a SPI-2 effector, to form and move SCVs toward a perinuclear position, which is adventitious to intracellular replication of Salmonella (8). However, we found that SifA is not required to produce colitis or diarrhea. This finding casts doubt on the relevance of SifA for the infection of epithelial cells during Salmonella enteritis (8).
Cecal inflammation caused by a SPI-1 but not a SPI-2 mutant of S. Typhimurium is abrogated in MyD88 knockout mice (26), so it appears that the mechanisms of inflammation and perhaps diarrhea are different in the two mutants. We were unable to test the role of MyD88 in our model because the mutation is on a C57BL/6 background. However, we did show that a mutant expressing only SPI-2 did not cause frank diarrhea, even though it was significantly more virulent in systemic tissues than a mutant expressing only SPI-1 (56), as measured by the numbers of CFU in mesenteric lymph nodes and the spleen. Our results were largely in accord with those of Hapfelmeier et al. showing that SPI-1 and SPI-2 mutants can infect the cecum (26) but that the SPI-2 mutant is impaired in the ability to survive in mesenteric lymph nodes and the spleen.
There is relatively little known about the roles of the more than 20 SPI-2 effectors in the pathogenesis of gastroenteritis (28). We tested the ability of sifA and spv mutants to cause diarrhea, since the sifA and spv genes have been shown to greatly affect the course of systemic infections (7, 43), and we found that neither effector was required to produce diarrhea or colitis in this model. SifA interacts with host kinesin and is required for endosomal tubulation and the formation of Salmonella-induced filaments (Sifs), which are filamentous structures within infected epithelial cells that contain lysosomal membrane glycoproteins (32), and it stabilizes the modified phagosome in macrophages, termed the “Salmonella-containing vacuole” (SCV), where the ingested Salmonella organisms replicate (8). SifA also interacts directly with the host GTPase RhoA, and its C-terminal part has a fold similar to that of a guanine nucleotide exchange factor (41).
We confirmed that both ssaV and sifA mutants are susceptible to killing by both nonactivated and TNF-α-activated macrophages. Nevertheless, the SifA strain caused colitis and the SsaV strain did not. This implies either that macrophage survival is not required for Salmonella to produce enteritis and diarrhea in BALB/c.D2 congenic mice or that the intestinal macrophages of these mice are not able to kill a sifA mutant, whereas bone marrow-derived macrophages are bactericidal to the mutant. Intestinal macrophages are quite varied as assessed by surface antigens, and they are downregulated for secretion of proinflammatory cytokines (1). However, it has been reported that human intestinal macrophages are both phagocytic and bactericidal for S. Typhimurium LT2L (46).
Since the ssaV mutant lacking secretion of all SPI-2 effectors survived less well than WT Salmonella in the cecum (Fig. 3) and the growth of both the sifA mutant and the spvB sifA double mutant was not significantly different from WT growth, there must be other SPI-2 effectors that specifically promote survival inside intestinal macrophages. Furthermore, continued growth of the sifA mutant in epithelial cells may provide a reservoir of bacteria promoting epithelial cell death and continued inflammatory stimuli even after activated macrophages migrate into the intestine. Cell culture studies have shown that SPI-2 TTSS mutants neither grow nor induce cell death in intestinal epithelial cells (19).
SpvB is an ADP-ribosylating toxin expressed within the SCV and translocated by SPI-2 into the cytoplasm, where it depolymerizes F actin and causes macrophage apoptosis (9, 33). SpvB is encoded by a gene that is part of the spv operon that we inactivated directly by a deletion and indirectly by making a mutation in spvR, which abrogates expression of all genes in the operon (19). Käppeli et al. recently reported that neither a spvB nor a spvC mutation in WT S. Typhimurium decreased the amount of inflammatory damage produced 12 h after infection (30). However, when those mutations were made in an invG (SPI-2) mutant, the result was less inflammation in the cecum 3 days after infection than that with the invG mutant alone, but not lower bacterial counts in the cecum. Käppeli et al. postulated that the spv genes are needed only in the invasive phase of colonic infection, not in conjunction with the early phase of infection that they attributed to SPI-1. We also found that an spvR mutant grew as well as the WT in the cecum and caused severe pathology throughout the colon 7 days after infection. Inflammation in the proximal colon was not significantly different from that induced by the WT 3 days after infection (not shown), and the increase in water content was not significantly different from that with the WT. We did not test this mutant in a strain that was also a SPI-1 mutant, so we cannot directly compare our results to those of Käppeli et al. However, since the spvR mutant caused as much diarrhea and colon pathology as the WT, our data suggest that spv genes are not needed for induction of colitis. Although spv genes are needed for optimal replication of S. enterica in reticuloendothelial system (RES) organs, as shown by Käppeli et al., in these experiments the spv mutants were not very impaired in growth in spleens. The failure to show statistical significance may have been due to the relatively small number of animals (4) in the spvR group, but mutation of sifA clearly had a larger effect on virulence in the WT strain.
The lack of a role for spv genes in this mouse model of colitis is consistent with human epidemiological data. Many serovars that cause human gastroenteritis never carry virulence plasmids (44), and even individual stool isolates of S. Typhimurium and S. Enteritidis, serovars that are associated with virulence plasmids, may not carry a virulence plasmid (16). It is still possible that strains with virulence plasmids cause more severe gastroenteritis than strains lacking a virulence plasmid, but that has not been determined for humans. A recent study of acute cecal inflammation in the streptomycin-treated mouse model reported that SpvC appeared to downregulate the inflammatory response 1 day after Salmonella infection, but this effect was not found at later times (22). Our results with the spvR mutant demonstrate that none of the spv gene products affect diarrhea or colonic disease in our mouse model, though we did not study the mice 1 day after infection. The significance of a transient effect of SpvC on cecal inflammation is unclear.
We postulate that there may be two different mechanisms for diarrhea during Salmonella colitis. The initial increase in fecal water that was first observed 3 days after infection, at a time when inflammation was largely but not entirely confined to the cecum, may have been due to direct or indirect effects of Salmonella on the absorptive function of epithelial cells. Bertelsen et al. showed that Salmonella infection causes an increase in salt and water secretion by the small intestinal epithelium that is mediated by Cox-2 in a human xenograft model of gastroenteritis (3); in the colon, the corollary function is water absorption. Infection with WT Salmonella caused diffuse damage to the epithelium at later times, and then the mice developed frank dysentery. At this stage, there may have been water loss via paracellular channels and/or a shorter transit time, with less water reabsorption as a consequence. The early stage did not require SPI-2, but the later process required both SPI-1 and SPI-2. Our negative results with the sifA and spv mutants demonstrate that Salmonella diarrhea and colitis differ from systemic Salmonella infection in their requirements for two key SPI-2 virulence effectors.
ACKNOWLEDGMENTS
This work was supported by NIH grants AI032178 and AI077661 (D.G.G.) and by a VA merit review grant (J.F.).
We thank Patricia Hasegawa for extensive expertise in strain construction and graphics.
Footnotes
Published ahead of print 9 July 2012
REFERENCES
- 1. Bain CC, Mowat AM. 2011. Intestinal macrophages—specialised adaptation to a unique environment. Eur. J. Immunol. 41:2494–2498 [DOI] [PubMed] [Google Scholar]
- 2. Barthel M, et al. 2003. Pretreatment of mice with streptomycin provides a Salmonella enterica serovar Typhimurium colitis model that allows analysis of both pathogen and host. Infect. Immun. 71:2839–2858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bertelsen LS, Paesold G, Eckmann L, Barrett KE. 2003. Salmonella infection induces a hypersecretory phenotype in human intestinal xenografts by inducing cyclooxygenase 2. Infect. Immun. 71:2102–2109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Beuzón CR, et al. 2000. Salmonella maintains the integrity of its intracellular vacuole through the action of SifA. EMBO J. 19:3235–3249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Beuzon CR, Salcedo SP, Holden DW. 2002. Growth and killing of a Salmonella enterica serovar Typhimurium sifA mutant strain in the cytosol of different host cell lines. Microbiology 148:2705–2715 [DOI] [PubMed] [Google Scholar]
- 6. Bohnhoff M, Drake BL, Miller CP. 1954. Effect of streptomycin on susceptibility of intestinal tract to experimental Salmonella infection. Proc. Soc. Exp. Biol. Med. 86:132–137 [DOI] [PubMed] [Google Scholar]
- 7. Boucrot E, Beuzón CR, Holden DW, Gorvel J-P, Meresse S. 2003. Salmonella typhimurium SifA effector protein requires its membrane-anchoring C-terminal hexapeptide for its biological function. J. Biol. Chem. 278:14196–14202 [DOI] [PubMed] [Google Scholar]
- 8. Brawn LC, Hayward RD, Koronaksi V. 2007. Salmonella SPI1 effector SipA persists after entry and cooperates with a SPI2 effector to regulate phagosome maturation and intracellular replication. Cell Host Microbe 1:63–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Browne SH, Hasegawa P, Okamoto S, Fierer J, Guiney DG. 2008. Identification of Salmonella SPI-2 secretion system components required for SpvB-mediated cytotoxicity in macrophages and virulence in mice. FEMS Immunol. Med. Microbiol. 52:194–201 [DOI] [PubMed] [Google Scholar]
- 10. Brumell JH, Tang P, Zaharik ML, Finlay BB. 2002. Disruption of the Salmonella-containing vacuole leads to increased replication of Salmonella enterica serovar Typhimurium in the cytosol of epithelial cells. Infect. Immun. 70:3264–3270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Coburn B, Li Y, Owen D, Vallance BA, Finlay BB. 2005. Salmonella enterica serovar Typhimurium pathogenicity island 2 is necessary for complete virulence in a mouse model of infectious enterocolitis. Infect. Immun. 73:3219–3227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Coombes BK, Brown NF, Valdez Y, Brumell JH, Finlay BB. 2004. Expression and secretion of Salmonella pathogenicity island-2 virulence genes in response to acidification exhibit differential requirements of a functional type III secretion apparatus and SsaL. J. Biol. Chem. 279:49804–49815 [DOI] [PubMed] [Google Scholar]
- 13. Coombes BK, et al. 2005. Analysis of the contribution of Salmonella pathogenicity islands 1 and 2 to enteric disease progression using a novel bovine ileal loop model and a murine model of infectious enterocolitis. Infect. Immun. 73:7161–7169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fields PI, Swanson RV, Haidaris CG, Heffron F. 1986. Mutants of Salmonella typhimurium that cannot survive within the macrophage are avirulent. Proc. Natl. Acad. Sci. U. S. A. 83:5189–5193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fierer J, Guiney DG. 2001. Diverse virulence traits underlying different clinical outcomes of Salmonella infection. J. Clin. Invest. 107:775–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fierer J, Krause M, Tauxe R, Guiney DG. 1992. Salmonella typhimurium bacteremia: association with the virulence plasmid. J. Infect. Dis. 166:639–642 [DOI] [PubMed] [Google Scholar]
- 17. Geddes K, et al. 2010. Nod1 and Nod2 regulation of inflammation in the Salmonella colitis model. Infect. Immun. 78:5107–5115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Govoni G, et al. 1996. The bcg/ity/lsh locus: genetic transfer of resistance to infections in C57BL/6J mice transgenic for the Nramp1Gly169 allele. Infect. Immun. 64:2923–2929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Guiney DG, Fierer J. 2011. The role of the spv genes in Salmonella pathogenesis. Front. Microbiol. 2:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Guiney DG, Lesnick M. 2005. Targeting of the actin cytoskeleton during infection by Salmonella strains. Clin. Immunol. 114:248–255 [DOI] [PubMed] [Google Scholar]
- 21. Guttman JA, et al. 2006. Attaching and effacing pathogen-induced tight junction disruption in vivo. Cell. Microbiol. 8:634–645 [DOI] [PubMed] [Google Scholar]
- 22. Haneda T, et al. 2012. Salmonella type III effector SpvC, a phosphothreonine lyase, contributes to reduction in inflammatory response during intestinal phase of infection. Cell. Microbiol. 14:485–499 [DOI] [PubMed] [Google Scholar]
- 23. Hapfelmeier S, et al. 2004. Role of the Salmonella pathogenicity island 1 effector proteins SipA, SopB, SopE, and SopE2 in Salmonella enterica subspecies 1 serovar Typhimurium colitis in streptomycin-pretreated mice. Infect. Immun. 72:795–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hapfelmeier S, Hardt W-D. 2005. A mouse model for S. typhimurium-induced enterocolitis. Trends Microbiol. 13:497–503 [DOI] [PubMed] [Google Scholar]
- 25. Hapfelmeier S, et al. 2008. Microbe sampling by mucosal dendritic cells is a discrete, MyD88-independent step in delta invG S. typhimurium colitis. J. Exp. Med. 205:437–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hapfelmeier S, et al. 2005. The Salmonella pathogenicity island (SPI)-2 and SPI-1 type III secretion systems allow Salmonella serovar typhimurium to trigger colitis via MyD88-dependent and MyD88-independent mechanisms. J. Immunol. 174:1675–1685 [DOI] [PubMed] [Google Scholar]
- 27. Hu Q, et al. 2008. Salmonella enterica serovar Senftenberg human clinical isolates lacking SPI-1. J. Clin. Microbiol. 46:1330–1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ibarra JA, Steele-Mortimer O. 2009. Salmonella—the ultimate insider. Salmonella virulence factors that modulate intracellular survival. Cell. Microbiol. 11:1579–1586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jones BD, Ghori N, Falkow S. 1994. Salmonella typhimurium initiates murine infection by penetrating and destroying the specialized epithelial M cells of the Peyer's patches. J. Exp. Med. 180:15–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Käppeli R, Kaiser P, Stecher B, Hardt W-D. 2011. Roles of spvB and spvC in S. Typhimurium colitis via the alternative pathway. Int. J. Med. Microbiol. 301:117–124 [DOI] [PubMed] [Google Scholar]
- 31. Kayagaki N, et al. 2011. Non-canonical inflammasome activation targets caspase-11. Nature 479:117–122 [DOI] [PubMed] [Google Scholar]
- 32. Leone P, Meresse S. 2011. Kinesin regulation by Salmonella. Virulence 2:63–66 [DOI] [PubMed] [Google Scholar]
- 33. Lesnick ML, Reiner NE, Fierer J, Guiney DG. 2001. The Salmonella spvB virulence gene encodes an enzyme that ADP-ribosylates actin and destabilizes the cytoskeleton of eukaryotic cells. Mol. Microbiol. 39:1464–1470 [DOI] [PubMed] [Google Scholar]
- 34. Libby SJ, et al. 1997. The spv genes on the Salmonella dublin virulence plasmid are required for severe enteritis and systemic infection in the natural host. Infect. Immun. 65:1786–1792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mahon BE, et al. 1997. An international outbreak of Salmonella infections caused by alfalfa sprouts grown from contaminated seeds. J. Infect. Dis. 175:876–882 [DOI] [PubMed] [Google Scholar]
- 36. Marcus SL, Brumell JH, Pfeifer CG, Finlay BB. 2000. Salmonella pathogenicity islands: big virulence in small packages. Microbes Infect. 2:145–156 [DOI] [PubMed] [Google Scholar]
- 37. Miao EA, Miller SI. 2000. A conserved amino acid sequence directing intracellular type III secretion by Salmonella typhimurium. Proc. Natl. Acad. Sci. U. S. A. 97:7539–7544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mohle-Boetani JC, et al. 1999. An outbreak of Salmonella serogroup Saphra due to cantaloupes from Mexico. J. Infect. Dis. 180:1361–1364 [DOI] [PubMed] [Google Scholar]
- 39. Mueller AJ, et al. 2012. Salmonella gut invasion involves TTSS-2-dependent epithelial traversal, basolateral exit, and uptake by epithelium-sampling lamina propria phagocytes. Cell Host Microbe 11:19–32 [DOI] [PubMed] [Google Scholar]
- 40. Obonyo M, et al. 2007. Deficiencies of myeloid differentiation factor 88, Toll-like receptor 2 (TLR2), or TLR4 produce specific defects in macrophage cytokine secretion induced by Helicobacter pylori. Infect. Immun. 75:2408–2414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ohlson MB, et al. 2008. Structure and function of Salmonella SifA indicate that its interactions with SKIP, SseJ, and RhoA family GTPases induce endosomal tubulation. Cell Host Microbe 4:434–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Potter M, et al. 1983. A BALB/c congenic strain of mice that carries a genetic locus (Ity) controlling resistance to intracellular parasites. Infect. Immun. 40:1234–1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Roudier C, Fierer J, Guiney DG. 1992. Characterization of translation termination mutations in the spv operon of the Salmonella virulence plasmid pSDL2. J. Bacteriol. 174:6418–6423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sanders E, Brachman PS, Friedman EA, Goldsby J, McCall CE. 1965. Salmonellosis in the United States. Results of nationwide surveillance. Am. J. Epidemiol. 81:370–384 [DOI] [PubMed] [Google Scholar]
- 45. Sheth AN, et al. 2011. A national outbreak of Salmonella serotype Tennessee infections from contaminated peanut butter: a new food vehicle for salmonellosis in the United States. Clin. Infect. Dis. 53:356–362 [DOI] [PubMed] [Google Scholar]
- 46. Smythies LE, et al. 2005. Human intestinal macrophages display profound inflammatory anergy despite avid phagocytic and bacteriocidal activity. J. Clin. Invest. 115:66–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Stecher B, et al. 2004. Flagella and chemotaxis are required for efficient induction of Salmonella enterica serovar Typhimurium colitis in streptomycin-pretreated mice. Infect. Immun. 72:4138–4150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Stecher B, et al. 2006. Chronic Salmonella enterica serovar Typhimurium-induced colitis and cholangitis in streptomycin-pretreated Nramp1+/+ mice. Infect. Immun. 74:5047–5057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Stein MA, Leung KY, Zwick M, Garcia-del Portillo F, Finlay BB. 1996. Identification of a Salmonella virulence gene required for formation of filamentous structures containing lysosomal membrane glycoproteins within epithelial cells. Mol. Microbiol. 20:151–164 [DOI] [PubMed] [Google Scholar]
- 50. Suar M, et al. 2009. Accelerated type III secretion system 2 dependent enteropathogenesis by a Salmonella enterica serovar Enteritidis PT4/6 strain. Infect. Immun. 77:3569–3577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tsolis RM, Adams LG, Ficht TA, Bäumler AJ. 1999. Contribution of Salmonella typhimurium virulence factors to diarrheal disease in calves. Infect. Immun. 67:4879–4885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Valdez Y, et al. 2009. Nramp1 drives an accelerated inflammatory response during Salmonella-induced colitis in mice. Cell. Microbiol. 11:351–362 [DOI] [PubMed] [Google Scholar]
- 53. Van Beneden CA, et al. 1999. Multinational outbreak of Salmonella enterica serotype Newport infections due to contaminated alfalfa sprouts. JAMA 281:158–162 [DOI] [PubMed] [Google Scholar]
- 54. Vazquez-Torres A, et al. 1999. Extraintestinal dissemination of Salmonella by CD18-expressing phagocytes. Nature 401:804–808 [DOI] [PubMed] [Google Scholar]
- 55. Vijay-Kumar M, et al. 2006. Flagellin suppresses epithelial apoptosis and limits disease during enteric infection. Am. J. Pathol. 169:1686–1700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Woo H, Okamoto S, Guiney DG, Gunn JS, Fierer J. 2008. A model of Salmonella colitis with features of diarrhea in SLC11A1 wild-type mice. PLoS One 3:e1603 doi:10.1371/journal.pone.0001603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Woodward MJ, McLaren I, Wray C. 1989. Distribution of virulence plasmids within salmonellae. J. Gen. Microbiol. 135:503–511 [DOI] [PubMed] [Google Scholar]
- 58. Wray C, Sojka WJ. 1981. Salmonella dublin infection of calves: use of small doses to simulate natural infection on the farm. J. Hyg. (Lond.) 87:501–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhang S, et al. 2002. The Salmonella enterica serotype Typhimurium effector proteins SipA, SopA, SopB, SopD, and SopE2 act in concert to induce diarrhea in calves. Infect. Immun. 70:3843–3855 [DOI] [PMC free article] [PubMed] [Google Scholar]