Abstract
Porphyromonas gingivalis is a Gram-negative obligately anaerobic bacterium associated with several forms of periodontal disease, most closely with chronic periodontitis. Previous studies demonstrated that OxyR plays an important role in the aerotolerance of P. gingivalis by upregulating the expression of oxidative-stress genes. Increases in oxygen tension and in H2O2 both induce activation of OxyR. It is also known that P. gingivalis requires hemin as an iron source for its growth. In this study, we found that a hemin-limited growth environment significantly enhanced OxyR activity in P. gingivalis. As a result, expression of sod, dps, and ahpC was also upregulated. Using a chromatin immunoprecipitation quantitative PCR (qPCR) analysis, DNA binding of activated OxyR to the promoter of the sod gene was enhanced in P. gingivalis grown under hemin-limited conditions compared to excess-hemin conditions. Cellular tolerance of H2O2 was also enhanced when hemin was limited in the growth medium of P. gingivalis. Our work supports a model in which hemin serves as a signal for the regulation of OxyR activity and indicates that P. gingivalis coordinately regulates expression of oxidative-stress-related genes by this hemin concentration-dependent pathway.
INTRODUCTION
Many organisms, including microbial cells, require iron for various metabolic processes, such as electron transport, glycolysis, and DNA synthesis (22, 27). Like other organisms, P. gingivalis, a well-known periodontal pathogen associated with several forms of periodontitis, requires iron for its growth (5, 19, 32). In vitro, P. gingivalis can grow well only in media supplemented with hemin, the oxidized form of ferrous iron bound to tetrapyrrole. This bacterium possesses a complex system for iron acquisition, and at least 50 proteins of P. gingivalis are likely involved directly or indirectly in iron utilization (16, 19). One of the characterized virulence features of P. gingivalis is its ability to accumulate hemin on its surface. It appears that its hemin binding ability is higher when it grows under excess-hemin conditions than under hemin-limited conditions (24).
Virulence gene expression in P. gingivalis is regulated by several environmental cues, including temperature, the presence of other bacteria, and iron/hemin concentrations (30). We reported earlier that expression of fimA, a gene encoding a major subunit protein of long fimbriae, is repressed in P. gingivalis grown in hemin-limited media (30). Previous studies, using proteomic and transcriptomic analyses to identify differentially expressed proteins and genes, showed that expression of 70 proteins and 160 genes was significantly altered when P. gingivalis was grown under hemin-limited conditions compared to excess-hemin conditions (6). Another recent study indicated that at least 3% of the genes in P. gingivalis genome were modulated in response to a change in hemin concentration (11). These differentially expressed genes or proteins were linked to bacterial invasion, iron transport, and an oxidative-stress response. A recent study showed that inorganic polyphosphates (potential antibacterial agents) inhibited energy-driven uptake of hemin by P. gingivalis and repressed the expression of oxidative stress-induced proteins, including superoxide dismutase (SOD) (15), which provides indirect evidence linking hemin to SOD expression. To better understand the response of P. gingivalis to hemin-limited conditions and the association between iron/hemin concentrations and oxidative stress, we investigated the expression and activity of OxyR in P. gingivalis under hemin-limited as well as excess-hemin conditions.
OxyR represents the first redox-regulatory protein that was well characterized in Escherichia coli (8). OxyR is a tetrameric DNA-binding protein that is activated under oxidative stress by forming a disulfide bond between two Cys residues that do not seem to form covalently cross-linked multimers, suggesting that the Cys199-Cys208 disulfide bond is intramolecular (21, 33). The disulfide bonds are then rereduced by thioredoxin or glutathione to form inactivated OxyR, suggesting that Cys disulfide bonds are crucial for OxyR activation. The oxyR gene is widely distributed in most Gram-negative and some Gram-positive bacteria (14), and OxyR homologs have been identified in more than a dozen other bacterial species, including P. gingivalis. P. gingivalis OxyR is a 308-amino-acid protein containing 5 cysteines (Cys24, Cys131, Cys171, Cys198, and Cys207). The two Cys residues at the C-terminal end have positions similar to those of the two Cys residues of E. coli OxyR but shifted by one amino acid. Previous studies from different laboratories indicated that OxyR is required for activation of the oxidative stress-related genes of P. gingivalis, such as sod and ahpC, encoding alkyl hydroperoxide reductase (7, 13, 18). In this study, we report that activation of OxyR occurs when the hemin concentration in the growth environment of P. gingivalis is limited and that this OxyR activation leads to alteration of expression of some genes in the OxyR regulon. Our results also demonstrated that the activated OxyR induced by hemin-limited conditions has a higher promoter binding activity. Equally importantly, we were able to show that the ability of P. gingivalis to survive under oxidative stress was greatly enhanced when the bacteria were grown under hemin-limited conditions.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Bacterial strains used in this study are listed in Table 1. P. gingivalis strains were grown from frozen stocks in Trypticase soy broth (TSB) or on TSB blood agar plates supplemented with yeast extract (1 mg/ml), hemin (5 μg/ml), and menadione (1 μg/ml) and incubated at 37°C in an anaerobic chamber (85% N2, 10% H2, 5% CO2). Antibiotics were used when appropriate, at the following concentrations: gentamicin (100 μg/ml) and erythromycin (5 μg/ml). For growth of P. gingivalis under hemin-limited conditions, the bacteria were first grown in standard TSB supplemented with hemin to reach an optical density at 600 nm (OD600) of 0.8 (mid-exponential phase). P. gingivalis cells were then subcultured in TSB without hemin supplement for another three passages; cells collected from the third passage were considered to have been grown under hemin-limited conditions.
Table 1.
Bacterial strains used in this study
| P. gingivalis strain | Relevant characteristicsa | Source or reference |
|---|---|---|
| 33277 | Type strain from ATCC | Lab collection |
| Pgn0373E | P. gingivalis mutant with the trx gene inactivated by insertion; ermF-ermAM cassette; Emr | This study |
| Pgn0368E | P. gingivalis mutant with the oxyR gene inactivated by insertion; ermF-ermAM cassette; Emr | 28 |
| Pgn1373E | P. gingivalis mutant with the cdhR gene inactivated by insertion; a ermF-ermAM cassette; Emr | 29 |
| Pgn0558E | P. gingivalis mutant with the hmuY gene inactivated by insertion; ermF-ermAM cassette; Emr | 29 |
Emr, resistance to erythromycin.
RNA isolation and qPCR.
P. gingivalis strains were grown anaerobically in 5 ml of TSB. Bacteria were harvested by centrifugation at 10,000 × g and homogenized in TRIzol reagent (Invitrogen, Carlsbad, CA). The RNA in the supernatant was then purified using an RNeasy mini-spin column (Qiagen, Valencia, CA). RNA samples were digested on the column with RNase-free DNase. The total RNA was tested using an Agilent 2100 Bioanalyzer to ensure the quality of the samples. Real-time reverse transcription-PCR (RT-PCR) analysis was performed by using a QuantiTect SYBR green RT-PCR kit (Qiagen) on an iCycler MyiQ real-time PCR detection system (Bio-Rad Laboratories, Inc., Hercules, CA) according to the manufacturer's instructions. Primers are listed in Table 2. Amplification reactions consisted of a reverse transcription cycle at 50°C for 30 min, an initial activation at 95°C for 15 min, and 40 cycles of 94°C for 15 s, 58°C for 30 s, and 72°C for 30 s. The melting curve profile was analyzed to verify a single peak for each sample, which indicated primer specificity. The expression levels of the investigated genes for the test sample were determined relative to the untreated calibrator sample by using the comparative cycle threshold (ΔCT) method. The ΔCT values were calculated by subtracting the average CT value of the test sample from the average CT value of the calibrator sample and were then used to calculate the ratio between the two by assuming 100% amplification efficiency. Finally, expression levels of testing genes were normalized by expression of a control gene (glk).
Table 2.
Oligonucleotide primers used in this study
| Gene | Primer | Primer sequence (5′–3′)a | Application |
|---|---|---|---|
| pgn0373 | Pgn0373-81F | TTGGCAGGGATTTTCGGTGTCAGA | RT-PCR |
| Pgn0373-81R | GCCCATCGTTTGCGTCGGTATTC | ||
| 33277-0275(1)R-erm | GATGTTGCAAATACCGATGAGCGCCAAGAAATATGCCGGTAA | Creating the pgn0373 mutant of 33277 | |
| 33277-0275(2)F-erm | CCTCTAGAGTCGACCTGCAGCTTTCTCTCCCTTGCTTTCG | ||
| sod | Sod-235F | AATTCCACCACGGTAAGCAC | RT-PCR |
| Sod-235R | GAGCCGAATTGTTTGTCGAT | ||
| glk | Glk-131F | ATGAATCCGATCCGCCACCAC | RT-PCR |
| Glk-131R | GCCTCCCATCCCAAAGCACT | ||
| oxyR | oxyR-132F | TCGCAAGCCAAGCAAATAC | RT-PCR |
| oxyR-132R | GACACGAGGCAGGAGATAGG | ||
| pgn0033 | PGN0033-91F | GCACCCACGAGCTTCTTTAC | RT-PCR |
| PGN0033-91R | ATACGGAATTGCCCATGAAG | ||
| pgn1232 | PGN1232-121F | CTCAACCCCATCCTCTACGA | RT-PCR |
| PGN1232-121R | GCAGGTCTTCCATCAGTTCC | ||
| pgn1373 | pgn1373-144F | GTTTGCTCACTACGCCGATT | RT-PCR |
| pgn1373-144R | GTTCTTCTCCGCTCTCTTCG | ||
| pgn2075 promoter | Pgn2075Prom-F | GCTTTCCTGCTGCTTCATTC | Real-time PCR |
| Pgn2075Prom-R | GTTCGATTGGGCATCCTCTT | ||
| sod promoter | sodProm-F | GTTTTCGCCCGAAGGTTTTATC | Real-time PCR |
| sodProm-R | GACGTCTGATTTTTATTGTAATTAAG |
The sequences corresponding to those of the erythromycin resistance (Emr) gene are underlined.
Construction of pgn0373 (trx) mutants.
An insertional trx (pgn0373) mutant was generated by using an overlap extension PCR method (20). A 2.1-kb ermF-ermAM cassette was introduced into the trx gene by three steps of PCR to yield a trx-erm-trx DNA fragment as described previously (29). Specific primers used are listed in Table 2. The final PCR products were then introduced into P. gingivalis 33277 by electroporation. The trx-deficient mutant was generated via a double-crossover event that replaced trx with the trx-erm-trx DNA fragment into the 33277 chromosome. The mutants were selected on TSB plates containing erythromycin (5 μg/ml). The insertional mutation was confirmed by PCR analysis, and the mutants were designated P. gingivalis 0373E.
Chromatin immunoprecipitation (ChIP) qPCR assay.
ChIP assays were conducted as previously described (17, 28). Briefly, formaldehyde (final concentration, 1%) was added to 20 ml of a P. gingivalis 33277 culture. The cross-linking reaction was stopped by the addition of glycine (125 mM, final concentration). Cells were resuspended in 2 ml lysis buffer (20 mM Tris-HCl [pH 8.0], 10 mM EDTA, 0.5 mg/ml TLCK [Nα-p-tosyl-l-lysine chloromethyl ketone], 10 mg/ml lysozyme) for 10 min at room temperature, followed by the addition of an equal volume of 2× immunoprecipitation (IP) buffer (0.1 M Tris-HCl [pH 7.0], 0.3 M NaCl, 2% Triton X-100, 0.2% sodium deoxycholate). Cells were sonicated to fragment chromosomal DNA; these samples were used as the input fraction for the ChIP assay.
Anti-OxyR antibodies (25 μl of the pooled sera) were added to the input fraction, and the mixture was rotated overnight at 4°C; complexes were then incubated for 1 h with a 20-μl bed volume of pre-equilibrated protein A Sepharose CL-4B beads (Sigma, St. Louis, MO). After washing, antibody-protein-DNA complexes were treated with 30 μl of elution buffer (1% sodium dodecyl sulfate [SDS], Tris-EDTA [TE; pH 8.0], 10 mM dithiothreitol [DTT]) for 30 min at 37°C. The eluates were used as the output fraction for real-time PCR analyses. Preimmune rabbit serum was used as a control. Input and output fractions were treated with RNase A (10 μg/ml) and proteinase K (2 μg/ml, Sigma). DNA samples were precipitated by ethanol and resuspended in 30 μl distilled H2O.
Real-time PCR was performed by using a QuantiTect SYBR green PCR kit (Qiagen) on an iCycler MyiQ real-time PCR detection system (Bio-Rad Laboratories, Inc.), according to the manufacturer's instructions. Primers were designed to amplify the promoter regions of sod and pgn2075. Primers are listed in Table 2. Amplification reactions consisted of an initial activation at 95°C for 15 min and 40 cycles of 94°C for 15 s, 58°C for 30 s, and 72°C for 30 s. Enrichment values (fold) were calculated according to the output/input ratio relative to that of a housekeeping gene, glk, encoding glucokinase.
Bacterial survival assay.
Bacterial resistance to hydrogen peroxide (H2O2) was determined using a bacterial survival assay as previously described (7). Briefly, 200 μl of an overnight culture of P. gingivalis 33277 was inoculated into 10 ml of TSB medium. Hydrogen peroxide (H2O2; 0, 200, or 400 μM) (Sigma) was added to the cultures at 8 h after the initial inoculation. Growth curves over a 48-h period were determined by measuring optical densities (OD600) in 200-μl samples at 8- to 16-h intervals using a Benchmark Plus microplate reader (Bio-Rad). All experiments were performed in triplicate. The doubling time of bacterial growth was calculated using the formula (T2 − T1)ln2/[ln(OD2) − ln(OD1)], where T1 and T2 are starting and ending times and OD1 and OD2 are optical densities at these two times. The length of the lag phase was determined by monitoring the time required for bacteria to reach an OD600 of 0.2.
Enzyme-linked immunosorbent assay (ELISA).
ELISA was performed according to our previous description, but with some modifications (31). Briefly, 96-well microtiter plates (Thermo Scientific, Rochester, NY) were coated with proteins (25 μg) extracted by sonication of samples of P. gingivalis 33277 grown under excess-hemin or limited-hemin conditions for 16 h at 4°C. The unbound proteins were removed by washing with PBS containing 0.1% Tween 20, pH 7.4. The plates were blocked with 3% bovine serum albumin in PBS-Tween 20 for 2 h at 37°C. A series dilutions of anti-Trx polyclonal antibodies (Thermo Scientific) or anti-OxyR antibodies (28) were then applied to the plates, which were then incubated for 3 h at room temperature. The plates were washed and incubated with horseradish peroxidase-conjugated antibodies against rabbit IgG (1:3,000; Amersham Biosciences, Piscataway, NJ) for 1 h at room temperature. After the wells were washed five times with PBS, peroxide substrate (Sigma) was added to each well. The reaction was stopped by the addition of 100 μl of 1 N H2SO4. The results were read at 450 nm on a Benchmark Plus microplate reader (Bio-Rad). All samples were assayed in triplicate for each sample.
Statistical analyses.
Student's t test was used to determine statistical significance of the differences in gene expression profiles and growth rates of P. gingivalis strains. A P value of <0.05 was considered significant.
RESULTS
Activation of OxyR in P. gingivalis in response to hemin limitation.
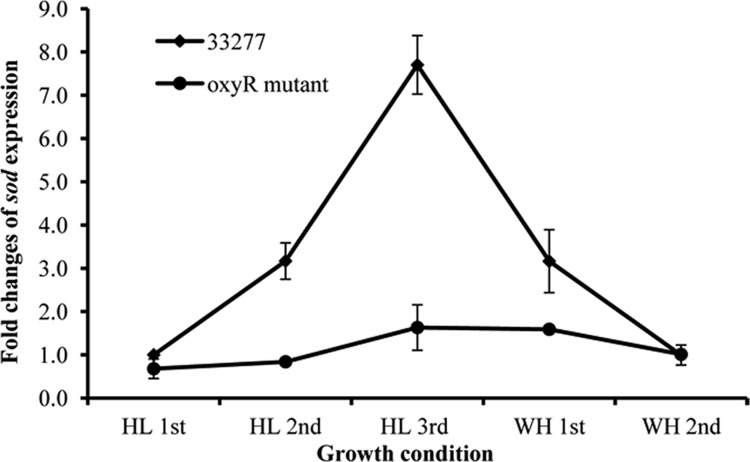
To test the role of hemin in OxyR activation, P. gingivalis wild-type strain 33277 and the oxyR mutant were first grown in TSB with hemin (WH, 5 μg/ml) and then grown without supplemental hemin for three passages (HL). Finally, both strains were inoculated into TSB containing hemin for another two passages. Because of its ability to accumulate hemin on its surface, the growth rate of P. gingivalis with hemin was not significantly different from that seen in TSB without supplemental hemin during the first two passages. However, the third passage in TSB without hemin showed a slightly lower growth rate. There was also no significant difference in growth rates between the wild type and the oxyR mutant when they were incubated under anaerobic conditions (data not shown). P. gingivalis cells were harvested from each passage at mid-log phase (OD600 = 0.8). The expression level of sod, a gene that is well known for being positively controlled by OxyR (7, 28), was determined by quantitative RT-PCR (qRT-PCR) and used as an indicator of OxyR activation. As shown in Fig. 1, expression of sod increased as much as 8-fold after P. gingivalis 33277 (wild type) was cultured for three passages under hemin-limited conditions compared to that in the cells grown with hemin. The expression of sod in 33277 after it had been grown for two additional passages in standard TSB declined to the baseline level seen in P. gingivalis grown with hemin, indicating a reversible hemin-dependent regulation of OxyR activation. In contrast, this differential expression of sod was not observed in the oxyR mutants under excess-hemin or hemin-limited conditions, suggesting an involvement of OxyR in hemin-dependent regulation of sod expression.
Fig 1.
Differential expression of sod in P. gingivalis strains. P. gingivalis strains were first grown in TSB medium without hemin for three generations (HL, hemin limitation) and then grown in standard TSB medium for another two generations (WH, with hemin). Expression of the sod gene in both the wild-type strain 33277 and the oxyR mutant was determined using real-time RT-PCR analysis. The change in expression levels was calculated by the ΔΔCT method, where ΔCT = CT(cells grown under standard conditions) − CT(cells grown under hemin limitation), and normalized to that of glk. Standard deviations are indicated (n = 3).
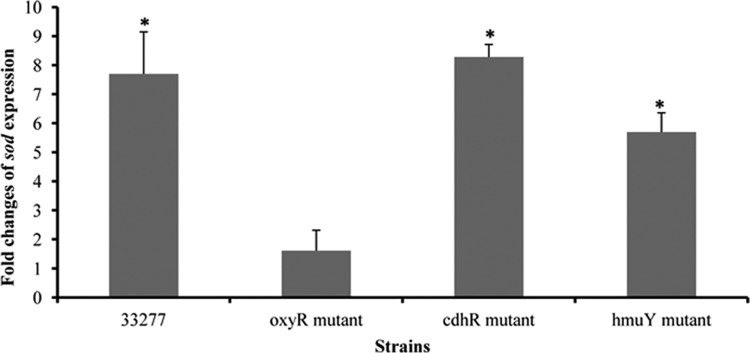
Our previous studies identified a community development and hemin regulator (CdhR) that acted as a transcriptional activator of the hmu locus, which encodes an iron acquisition system (4, 29). In those studies, the expression of both cdhR and the genes of the hmu loci was significantly decreased when P. gingivalis cells were grown at a higher cell density. To test if CdhR and HmuY, encoded by the first gene (hmuY) in the hmu locus, play a role in OxyR activation, expression of sod was measured in cdhR and hmuY mutants grown in the presence or absence of hemin. As shown in Fig. 2, similar to the sod expression pattern in wild-type strain 33277, an elevated expression of sod was observed in cdhR- and hmuY-deficient strains under hemin-limited conditions. These data suggest that cell density-dependent expression of cdhR and hmuY is not essential for OxyR activation.
Fig 2.
Comparison of sod expression in P. gingivalis 33277 and its mutant strains. The oxyR mutant, the cdhR mutant, the hmuY mutant, and the parent strain 33277 were grown with or without hemin for three passages. Expression of sod was measured using real-time RT-PCR. Each bar represents the increase of sod expression in P. gingivalis grown without hemin compared to expression in strains grown with hemin, which was normalized to the change in glk expression. Error bars represent standard deviations. Asterisks indicate a statistically significant difference in sod expression levels in P. gingivalis grown with and without hemin (t test; P < 0.05).
Expression of oxidative stress-related genes in P. gingivalis under hemin-limited conditions.
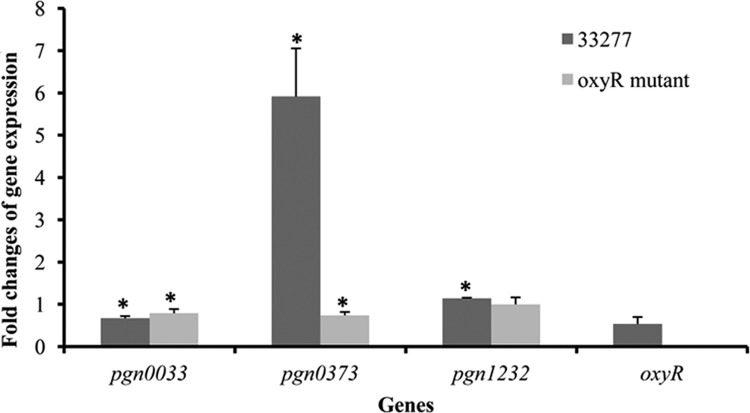
To test the possibility that transcription of oxyR is elevated in P. gingivalis in response to hemin limitation, we compared mRNA levels of oxyR in P. gingivalis grown in standard TSB supplemented with hemin to that in hemin-limited TSB. Interestingly, expression of oxyR was not altered despite a change in hemin concentration (Fig. 3). The results demonstrate that OxyR activity but not oxyR expression was activated in P. gingivalis in response to hemin-limited conditions. Thioredoxins are known for their fundamental roles in different cellular processes, including OxyR activation (32a). Expression of trx genes is also known to be under the control of OxyR. Based on their DNA sequences, two genes of P. gingivalis, pgn0033 and pgn0373, are annotated as encoding thioredoxins (Trx), and pgn1232 is annotated as encoding a thioredoxin reductase (TrxB) (20). As shown in Fig. 3, one of the trx genes, pgn0373, was expressed at 6-fold-higher levels in P. gingivalis 33277 grown under hemin-limited conditions than in the same strain under excess-hemin conditions. This alteration of gene expression induced by hemin-limited conditions was not found in the oxyR mutant, indicating that P. gingivalis OxyR serves as a transcriptional activator of a particular trx gene (pgn0033). Although the difference is statistically significant, expression of pgn0033 and pgn1232 was decreased only by 25% in both wild-type strain 33277 and the oxyR mutant and increased by 40% in 33277, in contrast to the 6-fold increase in pgn0373 expression (Fig. 3).
Fig 3.
Gene expression in P. gingivalis in response to hemin limitation. Total RNA was extracted from P. gingivalis 33277 and its oxyR mutant grown with or without hemin. Expression of glk, pgn0033 (thioredoxin), pgn0373 (putative thioredoxin), pgn1232 (thioredoxin reductase), and oxyR was measured by real-time RT-PCR. Each bar represents the change in P. gingivalis strains grown without hemin compared to that in strains grown with hemin, which was normalized to the change in glk expression. Error bars represent standard deviations. Asterisks indicate a significant difference gene expression in P. gingivalis strains grown under hemin-limited verses excess-hemin conditions (t test; P < 0.05).
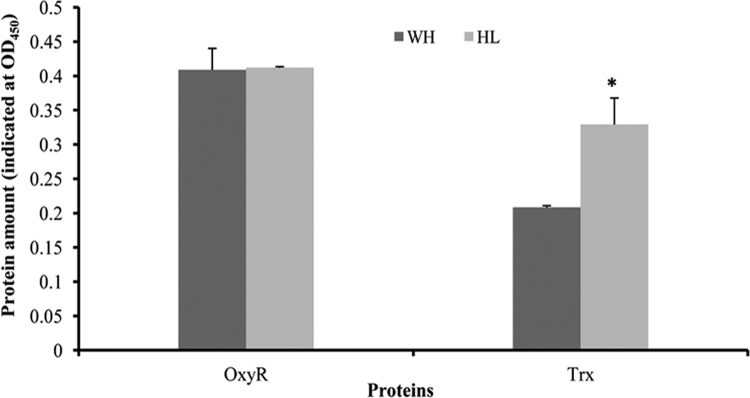
To determine if OxyR and Trx production are modified in P. gingivalis grown under hemin-limited conditions, we compared protein levels of OxyR and Trx in bacteria grown in different hemin concentrations using ELISA. As shown in Fig. 4, synthesis of OxyR, consistent with its mRNA level, was not significantly different between P. gingivalis cultures grown under excess-hemin and hemin-limited conditions. The results confirm that OxyR activity but not oxyR expression was activated in P. gingivalis in response to hemin-limited conditions. In contrast, thioredoxin (TRX) levels in P. gingivalis grown under hemin-limited conditions were 58% higher than those in bacteria grown under excess-hemin conditions (Fig. 4).
Fig 4.
Differential expression of OxyR and Trx (thioredoxin) proteins. P. gingivalis 33277 cells were grown under excess-hemin (WH) or hemin-limited (HL) conditions. OxyR and Trx levels were determined by ELISA and reported as the mean absorbance from three independent experiments. Error bars represent standard deviations. Asterisks indicate a statistically significant difference in protein expressions in P. gingivalis grown under different hemin concentrations (t test; P < 0.001).
DNA binding affinity of OxyR regulated by hemin concentrations.
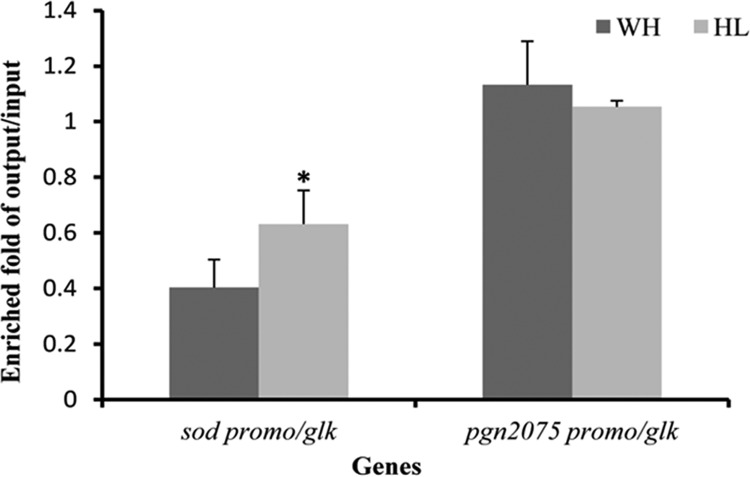
We previously showed that expression of sod by OxyR involves direct interaction of OxyR and the promoter region of the sod gene (28). The fact that OxyR is activated in P. gingivalis grown under hemin-limited conditions suggests that a modified form of OxyR may exist under this conditions, one that has a higher affinity for the promoter of the sod gene. To test this hypothesis, we carried out a chromatin immunoprecipitation (ChIP) qPCR assay to assess the interaction of OxyR and the sod promoter in vivo. P. gingivalis 33277 cells were grown under hemin-limited or excess conditions. OxyR-DNA complexes were immune-precipitated with anti-OxyR antibodies and measured using a qPCR analysis with specific primers corresponding to the promoter region of sod. As shown in Fig. 5, enrichment of the promoter region of sod in P. gingivalis grown under the hemin-limited conditions was significantly greater than that in P. gingivalis grown under excess-hemin conditions, suggesting that interaction of OxyR and the sod promoter was enhanced in P. gingivalis grown under hemin-limited conditions. Interaction between OxyR and the promoter of pgn2075, a gene encoding excinuclease, was tested as a control. There was no significant difference in immune precipitation of the pgn2075 promoter by OxyR antibodies. Moreover, the differential enrichment of the sod promoter was not observed in parallel samples using preimmune serum from the same rabbit (data not shown).
Fig 5.
Effect of hemin on DNA binding activity of OxyR. The ability of OxyR to bind to the promoter regions of sod and pgn2075, encoding excinuclease, was determined using a chromatin immunoprecipitation (ChIP)-qPCR assay. P. gingivalis 33277 cells were grown under excess-hemin (WH) or hemin-limited (HL) conditions. Immunoprecipitations were performed in the presence of anti-OxyR antibodies. The precipitated DNAs were amplified using the promoter sequence-specific primers for sod and pgn2075 (as a control). Enrichment values were calculated according to the output/input ratio relative to that of glk (encoding glucokinase). Error bars represent standard deviations. The asterisk indicates a statistically significant difference between values for excess-hemin (WH) versus hemin-limited (HL) conditions (t test; P < 0.05).
Role of hemin in survival ability of P. gingivalis under oxidative stresses.
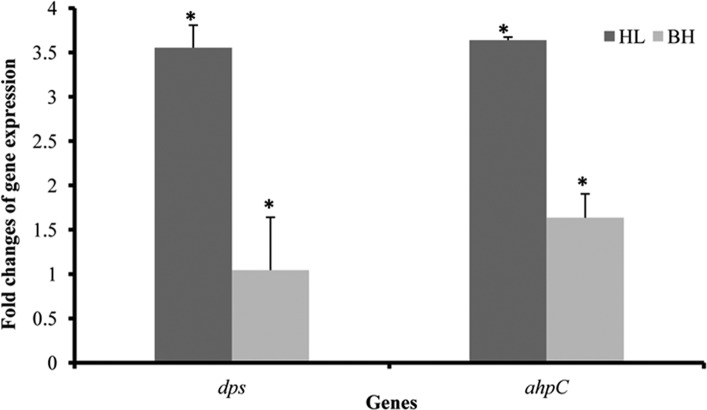
P. gingivalis OxyR plays a central role in the bacterial aerotolerance by activating transcription of oxidation-related genes under anaerobic conditions, including sod, dps (encoding a DNA-binding protein), and ahpC (encoding alkyl hydroperoxide reductase) (7, 28). A previous study suggested that SOD was responsible for tolerance to atmospheric oxygen but did not appear to protect P. gingivalis against hydrogen peroxide (H2O2) (12). On the other hand, P. gingivalis Dps was found to be essential for protection of the organism from H2O2 (26). Therefore, the expression of dps (pgn2037) and ahpC (pgn0060) in P. gingivalis 33277 grown with or without hemin was determined using qRT-PCR. As expected, expression of dps and ahpC was enhanced approximately 2.5- and 3.5-fold, respectively, in response to hemin limitation (Fig. 6). Similar to the change in sod expression (Fig. 1), expression of dps and ahpC was also reduced to baseline levels when P. gingivalis cells grown under hemin-limited conditions for three passages were cultured in a standard TSB medium.
Fig 6.
Differential expression of dps and ahpC in P. gingivalis 33277 in response to hemin concentrations. Total RNAs were extracted from P. gingivalis grown under hemin-limited (HL) conditions or those grown under hemin-limited conditions first and then cultured in standard TSB with hemin (BH). Expression of dps and ahpC in P. gingivalis grown without hemin was measured using qRT-PCR and compared to that in P. gingivalis with hemin after normalized with expression level of glk. Error bars represent standard deviations. An asterisk indicates a statistically significant difference between expression levels of genes in P. gingivalis grown without hemin and those in P. gingivalis grown in TSB with hemin (t test; P < 0.05).
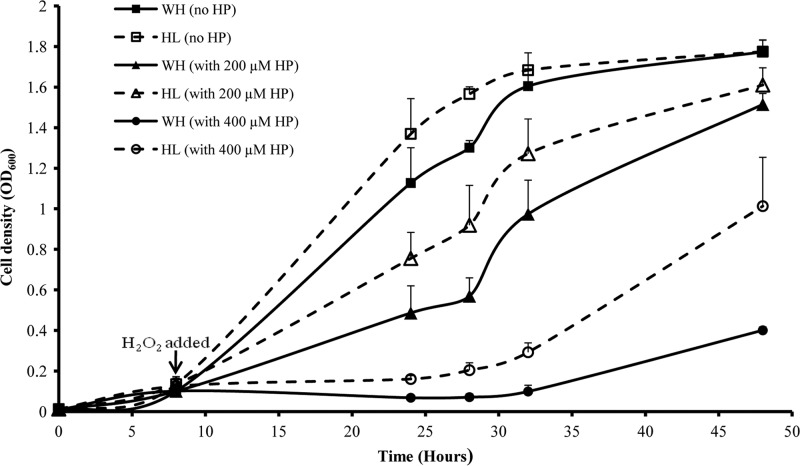
Based on these observations, we speculate that P. gingivalis grown under hemin-limited conditions may have a higher tolerance to H2O2 due to OxyR activation. To determine the hemin concentration-dependent tolerance to H2O2, we compared growth curves of P. gingivalis 33277 in the absence or presence of hemin and exogenous H2O2. P. gingivalis was grown in TSB with or without supplemental hemin for 48 h. There was no significant difference (t test) in the lengths of the lag phase (9.25 h versus 9 h) or doubling times (4.6 h versus 4.7 h) between P. gingivalis cultures grown with and without hemin (Fig. 7). This indicates that accumulation of hemin on the surface of P. gingivalis is sufficient to support growth over a 48-h period; this is true even though consumption of the resident hemin on the surface affected gene expression of some proteins. Addition of 200 and 400 μM H2O2 at 8 h after initial inoculations had a significant impact on the growth rates of P. gingivalis. Thus, in the presence of H2O2, P. gingivalis required a significantly longer time to reach its log phase, which is in agreement with a previous report (7). More interestingly, there was a significantly increased ability to resist H2O2 in P. gingivalis grown under hemin-limited conditions compared to bacteria grown under excess-hemin conditions. As shown in Fig. 7, P. gingivalis grown under hemin-limited conditions reached its log phase in 10 h in the presence of 200 μM and 26 h in the presence of 400 μM H2O2, while P. gingivalis grown under excess-hemin conditions required 12 h in the presence of 200 μM H2O2 and 36 h in the presence of 400 μM H2O2 to reach log phase. Similar patterns were observed in the bacterial doubling times. These data demonstrate that resistance to H2O2 was enhanced in P. gingivalis in response to hemin-limited conditions, which was likely due to activation of OxyR and the proteins of the OxyR regulon.
Fig 7.
Comparison of growth curves and tolerance to hydrogen peroxide of P. gingivalis under different growth conditions. P. gingivalis 33277 cells were grown under excess-hemin (WH) or hemin-limited (HL) conditions for 48 h. Hydrogen peroxide (HP) was added to the medium 8 h after the initial inoculation. Data are the mean optical densities (OD600) of bacterial cultures from four experiments. Error bars represent standard deviations.
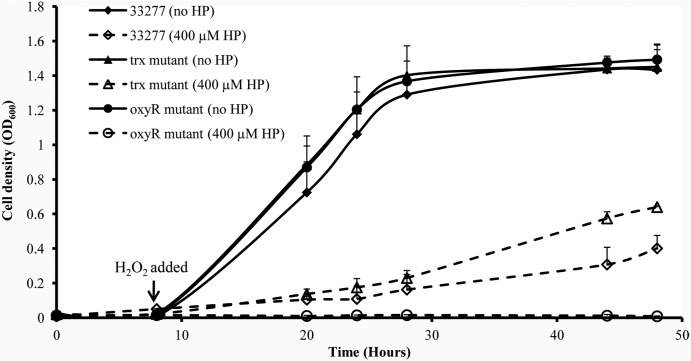
To establish a role for Trx (PGN0373) in P. gingivalis OxyR activation, sensitivities to H2O2 of the wild-type strain 33277, the oxyR mutant, and the trx (pgn0373) mutant were determined and compared. There was no significant difference in growth rates when all three strains were grown in standard TSB (Fig. 8). However, significant differences in sensitivities to H2O2 (400 μM) were found among these three strains. The oxyR mutant was not able to recover from the H2O2 attack at all, while 33277 recovered gradually. The highest recovery rate was found in the trx mutant, likely due to increased OxyR activity in this mutant.
Fig 8.
Sensitivities of P. gingivalis strains to hydrogen peroxide. P. gingivalis 33277, the oxyR mutant, and the trx (pgn0373) mutant were grown in standard TSB for 48 h. Hydrogen peroxide (HP, 400 μm) was added to each bacterial culture 8 h after the initial inoculation. Data are the mean optical densities (OD600) of bacterial cultures from four experiments. Error bars represent standard deviations.
DISCUSSION
Ferrous iron, hemin, and OxyR activation.
Iron is essential to most organisms, including bacteria. Iron metabolism in bacteria is associated with their ability to overcome oxidative stress, since iron serves as a cofactor of enzymes such as SOD. P. gingivalis cells also contain cytosolic SOD that requires both Fe and Mn for activity (12). Iron is also known as a main determinant of the cellular response to oxidative stress. Excessive free iron can generate the highly reactive and extremely damaging hydroxyl radical (2). In E. coli, an increased sensitivity to redox stress agents was observed when cells were grown under iron-rich conditions (1). In addition, deregulation of iron metabolism inactivated the fur gene in E. coli and led to an increased sensitivity of the organism to redox stress, an effect that could be reversed by either iron chelation or a tonB mutation that blocked iron uptake (25).
OxyR activity in many Gram-negative bacteria is known to be regulated under oxidative stresses, such as H2O2. Previous studies from independent laboratories have shown that the activity of OxyR in P. gingivalis was enhanced when the organism was exposed to H2O2 and atmospheric oxygen (7, 28). In this study, we revealed that expression of several oxidative-stress-related genes, including sod, trx, dps, and ahpC, fluctuated in P. gingivalis in response to a hemin-limited environment. Apparently, this hemin-dependent alteration of the expression of oxidative-stress-related genes is controlled by OxyR, since the phenomenon was not observed in the oxyR mutant (Fig. 1). Only baseline expression of the sod gene was found in the oxyR mutant grown with either excess or limited hemin. A recent study showed that iron significantly reduced expression of PerR, which, like OxyR, is a transcriptional regulator controlling transcription of oxidative-stress-related genes in Campylobacter jejuni (10). Most interestingly, the effect of iron on perR transcription was unique, and other metal ions, including copper, cobalt, manganese, and zinc, were ineffective. In contrast, our data demonstrate that expression of the oxyR gene in P. gingivalis was not altered as a function of fluctuating hemin concentrations in the growth media and rather that OxyR activity was enhanced under hemin-limited conditions. It is possible that ferrous iron and hemin play different roles in regulating anti-oxidative stress in P. gingivalis. According to previous reports, ferrous iron acts as a cofactor for SOD and Dps and is required for the activity of these anti-oxidative stress proteins (12, 26). We demonstrate here that a low hemin concentration acts as a signal for the positive regulation of OxyR activity, which in turn enhances the expression of anti-oxidative stress genes.
P. gingivalis utilizes hemin through hemin-binding proteins. The best described are HmuY and HmuY, encoded by hmuY and pg1552. We were the first to identify a transcriptional activator of the hmu gene, CdhR (PGN1373) (29). Thus, we hypothesized in this study that expression of the sod gene would be increased in the cdhR and the hmuY mutants due to an impaired ability to take up hemin, resulting in a decreased intracellular hemin concentration, and that the fold change of sod expression observed in P. gingivalis 33277 in response to a limited hemin concentration would not be detected in the mutants. Unexpectedly, the hmuY and the cdhR mutants showed a pattern of enhancing sod expression similar to that in 33277 (Fig. 2). There could be two explanations for this phenomenon. The first one is that other surface proteins are involved in uptake of hemin. At least two other proteins in P. gingivalis, hemin uptake system protein A (HusA) and hemin-binding protein 35 (HBP35), appear to possess hemin binding activity (9, 23). These proteins may compensate for the function of HmuY and thus fulfill the hemin requirements of P. gingivalis. The other explanation is that P. gingivalis senses a lower hemin concentration in its growth environment, but not a lower intracellular hemin concentration, and then adjusts its cellular response accordingly, in this case to enhance OxyR activity. An investigation of the mechanism by which P. gingivalis senses hemin-limited conditions is under way in our laboratory.
It is noteworthy that in a previous study, a rapid, OxyR-dependent response to H2O2 was observed in E. coli, such that several OxyR-regulated genes (e.g., dps, ahpC, and trx) were induced more than 20-fold (35). In contrast, another study used real-time PCR analysis and found that the expression of the OxyR-dependent genes, including sod, dps, ahpC, and trx, was either repressed or unchanged in P. gingivalis treated with H2O2 (10). Those authors suggested that H2O2 is not a strong inducer of OxyR activation in these cells and that OxyR constitutively activated transcription of oxidative-stress-related genes in P. gingivalis. The observations reported here, however, indicate that activation of OxyR is inducible in P. gingivalis by a low hemin concentration in the growth environment.
Thioredoxins and OxyR.
Activation of bacterial transcription factor OxyR is best described in E. coli. A series of studies suggested the presence of a thiol-based on-off switch in E. coli (3, 33, 34). They revealed that active OxyR contains an intramolecular disulfide bond between two cysteine residues, whereas the inactive form loses this disulfide bond through disulfide reductases such as thioredoxin (Trx). OxyR activation in P. gingivalis appears to also be controlled by this thiol-based on-off switch. We demonstrated here that a trx mutant has an increased tolerance to H2O2 (Fig. 8), which probably results from an accumulation of the active form of OxyR due to a lack of Trx. Interestingly, the trx genes are also members of the oxyR regulon in some bacteria, including P. gingivalis (7). Expression of the trx genes is elevated by OxyR, likely via a feedback mechanism crucial for regulation of OxyR activity. We tested the trx regulation of P. gingivalis in response to different hemin concentrations. An elevated trx expression at both the transcriptional and translational levels was found when P. gingivalis was grown under hemin-limited conditions, which is presumably due to activation of OxyR. Our observation of an enhanced expression of sod, trx, and ahpC as a consequence of OxyR activation further underscores the essential role of OxyR in P. gingivalis against oxidative stresses.
Role of OxyR regulation in pathogenicity.
The ability to regulate activation of OxyR appears to be an important feature for the survival of P. gingivalis in the oral cavity. Our in vitro studies indicated that P. gingivalis cells grown under hemin-limited conditions showed much higher tolerance to H2O2 than cells grown under excess-hemin conditions (Fig. 7). This was likely due to an activation of OxyR which upregulated expression of oxidative stress defense genes. In fact, expression of sod, dps, and ahpC genes was enhanced severalfold in P. gingivalis grown under hemin-limited conditions (Fig. 1 and 6). Previously, Smalley et al. observed that P. gingivalis binds to hemin in a μ-oxo dimeric form, as monitored by Mössbauer spectroscopy (24). Presumably, this dimeric form on the surface of P. gingivalis ties up free oxygen species and protects the organism from reactive oxidants. In this study, we provided direct evidence and support for a critical role of hemin in OxyR activation in P. gingivalis and in the organism's survival under oxidative stress, which indicates that there is a sophisticated regulation system in P. gingivalis to protect the bacteria against harsh environments.
When P. gingivalis cells enter the oral cavity, the bacteria are suspended in saliva and to oral surfaces, where the oxygen tension is higher and the hemin concentration is relatively lower than in periodontal pockets. Therefore, P. gingivalis may adjust expression of some oxidative-stress-related genes in response to this low hemin signal, which likely greatly increases its ability to survive in this environment. In contrast, at a late stage of infection, P. gingivalis lives with other bacteria in more complex mixed microbial communities in deep periodontal pockets, an environment bathed by gingival crevicular fluid, which exudes from vessels of the microcirculation or is present in blood from periodontal inflammation. Proteins in gingival crevicular fluid or blood, such as hemoglobin, are an important source of hemin for P. gingivalis. Therefore, hemin concentrations in the periodontal pocket are much higher than those in the oral cavity of a periodontally healthy individual. In the meantime, the oxygen tension is much lower in a mature microbial community and in a deep periodontal pocket. P. gingivalis, therefore, represses expression of most oxidative-stress-related genes in response to a higher hemin concentration signal and a lower oxygen tension.
In conclusion, as an anaerobic bacterium, P. gingivalis possesses an OxyR that controls expression of some well-known anti-oxidative stress genes, such as sod, trx, dps, and ahpC. We revealed that activity of OxyR is inducible in response to a hemin-limited environment, which is likely crucial for P. gingivalis' survival and pathogenicity. The findings presented here provide a foundation for further investigation into a novel mechanism of OxyR activation induced by hemin. Future studies may establish a strategy to block this pathway of OxyR activation, thus diminishing P. gingivalis' ability to survive in the oral cavity.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grant DE020915 (H.X.) from the National Institute of Dental and Craniofacial Research and U54RR026140 (H.X.) from NCRR.
Footnotes
Published ahead of print 23 July 2012
REFERENCES
- 1. Abdul-Tehrani H, et al. 1999. Ferritin mutants of Escherichia coli are iron deficient and growth impaired, and fur mutants are iron deficient. J. Bacteriol. 181:1415–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Andrews SC, Robinson AK, Rodriguez-Quinones F. 2003. Bacterial iron homeostasis. FEMS Microbiol. Rev. 27:215–237 [DOI] [PubMed] [Google Scholar]
- 3. Aslund F, Zheng M, Beckwith J, Storz G. 1999. Regulation of the OxyR transcription factor by hydrogen peroxide and the cellular thiol-disulfide status. Proc. Natl. Acad. Sci. U. S. A. 96:6161–6165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chawla A, et al. 2010. Community signalling between Streptococcus gordonii and Porphyromonas gingivalis is controlled by the transcriptional regulator CdhR. Mol. Microbiol. 78:1510–1522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Choi JI, Nakagawa T, Yamada S, Takazoe I, Okuda K. 1990. Clinical, microbiological and immunological studies on recurrent periodontal disease. J. Clin. Periodontol. 17:426–434 [PubMed] [Google Scholar]
- 6. Dashper SG, et al. 2009. Response of Porphyromonas gingivalis to heme limitation in continuous culture. J. Bacteriol. 191:1044–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Diaz PI, et al. 2006. Role of oxyR in the oral anaerobe Porphyromonas gingivalis. J. Bacteriol. 188:2454–2462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Farr SB, Kogoma T. 1991. Oxidative stress responses in Escherichia coli and Salmonella typhimurium. Microbiol. Rev. 55:561–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gao JL, Nguyen KA, Hunter N. 2010. Characterization of a hemophore-like protein from Porphyromonas gingivalis. J. Biol. Chem. 285:40028–40038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kim M, Hwang S, Ryu S, Jeon B. 2011. Regulation of perR expression by iron and PerR in Campylobacter jejuni. J. Bacteriol. 193:6171–6178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lewis JP. 2010. Metal uptake in host-pathogen interactions: role of iron in Porphyromonas gingivalis interactions with host organisms. Periodontol. 2000 52:94–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lynch MC, Kuramitsu HK. 1999. Role of superoxide dismutase activity in the physiology of Porphyromonas gingivalis. Infect. Immun. 67:3367–3375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Meuric V, Gracieux P, Tamanai-Shacoori Z, Perez-Chaparro J, Bonnaure-Mallet M. 2008. Expression patterns of genes induced by oxidative stress in Porphyromonas gingivalis. Oral Microbiol. Immunol. 23:308–314 [DOI] [PubMed] [Google Scholar]
- 14. Mongkolsuk S, Helmann JD. 2002. Regulation of inducible peroxide stress responses. Mol. Microbiol. 45:9–15 [DOI] [PubMed] [Google Scholar]
- 15. Moon JH, Park JH, Lee JY. 2011. Antibacterial action of polyphosphate on Porphyromonas gingivalis. Antimicrob. Agents Chemother. 55:806–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nelson KE, et al. 2003. Complete genome sequence of the oral pathogenic bacterium Porphyromonas gingivalis strain W83. J. Bacteriol. 185:5591–5601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nishikawa K, Yoshimura F, Duncan MJ. 2004. A regulation cascade controls expression of Porphyromonas gingivalis fimbriae via the FimR response regulator. Mol. Microbiol. 54:546–560 [DOI] [PubMed] [Google Scholar]
- 18. Ohara N, Kikuchi Y, Shoji M, Naito M, Nakayama K. 2006. Superoxide dismutase-encoding gene of the obligate anaerobe Porphyromonas gingivalis is regulated by the redox-sensing transcription activator OxyR. Microbiology 152:955–966 [DOI] [PubMed] [Google Scholar]
- 19. Olczak T, Simpson W, Liu X, Genco CA. 2005. Iron and heme utilization in Porphyromonas gingivalis. FEMS Microbiol. Rev. 29:119–144 [DOI] [PubMed] [Google Scholar]
- 20. Pogulis RJ, Vallejo AN, Pease LR. 1996. In vitro recombination and mutagenesis by overlap extension PCR. Methods Mol. Biol. 57:167–176 [DOI] [PubMed] [Google Scholar]
- 21. Pomposiello PJ, Demple B. 2001. Redox-operated genetic switches: the SoxR and OxyR transcription factors. Trends Biotechnol. 19:109–114 [DOI] [PubMed] [Google Scholar]
- 22. Schaible UE, Kaufmann SH. 2004. Iron and microbial infection. Nat. Rev. Microbiol. 2:946–953 [DOI] [PubMed] [Google Scholar]
- 23. Shoji M, et al. 2010. Characterization of hemin-binding protein 35 (HBP35) in Porphyromonas gingivalis: its cellular distribution, thioredoxin activity and role in heme utilization. BMC Microbiol. 10:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Smalley JW, Silver J, Marsh PJ, Birss AJ. 1998. The periodontopathogen Porphyromonas gingivalis binds iron protoporphyrin IX in the mu-oxo dimeric form: an oxidative buffer and possible pathogenic mechanism. Biochem. J. 331:681–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Touati D, Jacques M, Tardat B, Bouchard L, Despied S. 1995. Lethal oxidative damage and mutagenesis are generated by iron in Δfur mutants of Escherichia coli: protective role of superoxide dismutase. J. Bacteriol. 177:2305–2314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ueshima J, et al. 2003. Purification, gene cloning, gene expression, and mutants of Dps from the obligate anaerobe Porphyromonas gingivalis. Infect. Immun. 71:1170–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Weinberg ED. 1978. Iron and infection. Microbiol. Rev. 42:45–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wu J, Lin X, Xie H. 2008. OxyR is involved in coordinate regulation of expression of fimA and sod genes in Porphyromonas gingivalis. FEMS Microbiol. Lett. 282:188–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wu J, Lin X, Xie H. 2009. Regulation of hemin binding proteins by a novel transcriptional activator in Porphyromonas gingivalis. J. Bacteriol. 191:115–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Xie H, Cai S, Lamont RJ. 1997. Environmental regulation of fimbrial gene expression in Porphyromonas gingivalis. Infect. Immun. 65:2265–2271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Xie H, Kozlova N, Lamont RJ. 2004. Porphyromonas gingivalis genes involved in fimA regulation. Infect. Immun. 72:651–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ximenez-Fyvie LA, Haffajee AD, Socransky SS. 2000. Microbial composition of supra- and subgingival plaque in subjects with adult periodontitis. J. Clin. Periodontol. 27:722–732 [DOI] [PubMed] [Google Scholar]
- 32a. Zeller T, Klug G. 2006. Thioredoxins in bacteria: functions in oxidative stress response and regulation of thioredoxin genes. Naturwissenschaften 93:259–266 [DOI] [PubMed] [Google Scholar]
- 33. Zheng M, Aslund F, Storz G. 1998. Activation of the OxyR transcription factor by reversible disulfide bond formation. Science 279:1718–1721 [DOI] [PubMed] [Google Scholar]
- 34. Zheng M, et al. 2001. Computation-directed identification of OxyR DNA binding sites in Escherichia coli. J. Bacteriol. 183:4571–4579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zheng M, et al. 2001. DNA microarray-mediated transcriptional profiling of the Escherichia coli response to hydrogen peroxide. J. Bacteriol. 183:4562–4570 [DOI] [PMC free article] [PubMed] [Google Scholar]