Abstract
Th17 cells are increasingly being recognized as an important T helper subset for immune-mediated protection, especially against pathogens at mucosal ports of entry. In several cases, it would thus be highly relevant to induce Th17 memory by vaccination. Th17 cells are reported to exhibit high plasticity and may not stably maintain their differentiation program once induced, questioning the possibility of inducing durable Th17 memory. Accordingly, there is no consensus as to whether Th17 memory can be established unless influenced by continuous Th17 polarizing conditions. We have previously reported (T. Lindenstrøm, et al., J. Immunol. 182:8047–8055, 2009) that the cationic liposome adjuvant CAF01 can prime both Th1 and Th17 responses and promote robust, long-lived Th1 memory. Here, we demonstrate that subunit vaccination in mice with CAF01 leads to establishment of bona fide Th17 memory cells. Accordingly, Th17 memory cells exhibited lineage stability by retaining both phenotypic and functional properties for nearly 2 years. Antigen-specific, long-term Th17 memory cells were found to be mobilized from lung-draining lymph nodes to the lung following an aerosol challenge by Mycobacterium tuberculosis nearly 2 years after their induction and proliferated at levels comparable to those of Th1 memory cells. During the infection, the vaccine-induced Th17 memory cells expanded in the lungs and adapted Th1 characteristics, implying that they represent a metastable population which exhibits plasticity when exposed to prolonged Th1 polarizing, inflammatory conditions such as those found in the M. tuberculosis-infected lung. In the absence of overt inflammation, however, stable bona fide Th17 memory can indeed be induced by parenteral immunization.
INTRODUCTION
In recent years, several novel T helper subsets have been described, adding to the complexity of the Th1/Th2 dichotomy originally described 25 years ago by Mosmann et al. (35). Besides the emergence of regulatory T cells, these subsets include Th9, Th17, Th22, and Tfh. The Th17 subset has gained unprecedented attention since their first description a few years ago, which has brought about considerable knowledge on their induction, transcriptional control, effector mechanisms, and involvement during infection and disease. Hence, it has become clear that unbalanced Th17 responses play an important role in the pathogenesis of several autoimmune and allergic disorders, but there is also increasing evidence that this T helper subset partakes in antimicrobial responses against pathogens of viral, bacterial, parasitic, and fungal etiology. Initially, Th17 cells were thought to be involved primarily in immunity to extracellular pathogens (21, 33, 56) and are especially important for resolving fungal infections (23, 53). However, the list of intracellular pathogens where Th17-mediated immunity is implicated is steadily growing (24, 31, 46, 47, 58).
Whereas the polarizing conditions for Th17 immunity have become firmly established (15, 55), there is a much higher degree of uncertainty as to whether Th17 cells represent a stable, terminally differentiated Th lineage. Consequently, it is not clear whether or to what extent Th17 memory can be achieved without the influence of continued Th17 polarizing stimuli. It has, however, become increasingly clear that Th17 cells exhibit considerable plasticity due to, e.g., epigenetic regulation (36, 51), and that this is particularly the case for in vitro-generated Th17 cells (28, 38), implying that the currently used in vitro polarization conditions do not sufficiently drive Th17 cells to a state of full effector and/or memory differentiation. For example, murine studies have generally led to contradictory results in terms of whether Th17 cells are established and maintained as memory cells, presumably due to the wide-spread use of such in vitro-generated Th17 cells for adoptive transfer experiments (27, 29, 30, 38). Th17 cells generated in vivo have, on the contrary, been reported to stably express interleukin-17 (IL-17) and be refractory to Th1 and Th2 polarizing signals ex vivo (30). In vivo induction of Th17 cells has primarily been achieved through, e.g., mucosal priming or by chronic infections (11, 13, 42). Such conditions may not be favorable for optimal induction of long-term CMI (cell-mediated immunity) memory. Mucosally imprinted Th17 cells were shown to express low levels of CD27 and were characterized as short-lived effectors with low memory potential (42). Human studies have reported on Th17 memory recall responses, with Th17 cells exhibiting phenotypic characteristics of long-lived central memory T cells (34), which can form an integral part of human antimycobacterial responses (43, 46). There is therefore a need to study this important Th lineage in terms of in vivo induction, stability, and memory capacity after more conventional immunization regimens and during more quiescent and homeostatic conditions using clinically relevant adjuvants.
The CAF01 (dimethyldioctadecylammonium [DDA] and trehalose dibehenate [TDB] [DDA/TDB]) adjuvant, which is currently in two phase I clinical trials, has a long preclinical track record (3) and is known to induce multifunctional long-term Th1 memory at levels normally only attained by live vectors (32). CAF01 has been reported to drive IL-17 responses (45, 52) through the interaction of the immunostimulator TDB with its cognate receptor Mincle/Clec4E. Ligation of this receptor initiates signaling through the Syk-FcRγ-Card9-Bcl-10-Malt1 pathway, paving the way for Th17 polarization through the production of proinflammatory cytokines, such as IL-1β, IL-6, tumor necrosis factor alpha (TNF-α), and tumor growth factor beta (TGF-β) (45, 52). In the current study, we exploited the capacity of the CAF01 adjuvant to induce combined Th1 and Th17 responses and characterized the long-term memory capacity and stability of the Th17 subset. The Th17 T cells were found to differ from Th1 T cells in terms of phenotype as well as functionality and established themselves as long-lived cells that remained distinct from Th1 cells in the absence of prolonged inflammation. This study clearly demonstrates that antigen (Ag)-specific Th17, similar to Th1, can establish as stable, bona fide memory cells that can be mobilized by a Mycobacterium tuberculosis challenge close to 2 years after their induction by vaccination. However, these Th17 memory cells were found to be metastable in the lung at later stages of M. tuberculosis infection and thus gave rise to progeny with Th1-like characteristics.
MATERIALS AND METHODS
Animals.
Female C57BL/6 mice, aged 6 to 8 weeks, were purchased from Harlan Scandinavia (Allerød, Denmark). Animals were kept at the experimental animal facilities at Statens Serum Institut and handled by authorized personnel. All manipulations were conducted in accordance with the regulations of the Danish Ministry of Justice and animal protection committees under permits 2004/561-868 and 2009/561-1655 and in compliance with European Community Directive 86/609. Once infected, animals were housed in cages contained within laminar-flow safety enclosures (Scantainer; Scanbur) in a separate biosafety level 3 facility.
Antigens, adjuvant, and immunizations.
Three different model antigens were used in the current study, two tuberculosis fusion proteins, H1 and H28, and one Chlamydia fusion protein, designated CTH1. H1, a fusion protein of Ag85B and ESAT-6, was produced as a recombinant antigen as previously described (40), whereas the model antigen H28 was produced by fusing the Rv2660 antigen to the Ag85B-TB10.4 backbone using the methodology outlined for H1. CTH1 was made as a fusion of the two highly conserved immunodominant Chlamydia antigens Ct443 and Ct521 as described earlier (39). For all immunizations, CAF01 (DDA/TDB), prepared by the film method, was used as the adjuvant at a dose of 250 μg DDA/50 μg TDB (12). Other adjuvants initially tested included Montanide ISA720 (used at 70% of final vaccine volume), Quil A (20 μg/dose), and alhydrogel (500 μg/dose). Mice were immunized by the subcutaneous (s.c.) route at the base of the tail three times with a 2-week interval between immunizations. Based on prior dose-response optimization experiments, the immunization dose was 2 μg for tuberculosis (TB) fusion proteins, whereas 10 μg of the Chlamydia fusion protein was used. In all cases, the injection volume was 200 μl.
Experimental infection.
To assess the involvement of vaccine-induced, long-term Th1 and Th17 memory T cells specific for Ag85B-ESAT-6 after challenge, H1 memory mice were exposed to Mycobacterium tuberculosis and recall responses were evaluated in parallel to age-matched unvaccinated mice. To this end, mice were challenged 89 weeks postimmunization by the aerosol route using the Glas-Col inhalation exposure system (Terre-Haute, IN) calibrated to deliver 100 CFU of Mycobacterium tuberculosis Erdman. To assess the early recruitment of vaccine-induced, long-term memory cells from the lung-draining tracheobronchial lymph nodes to the lung, mice (3 to 4/group) were euthanized at day 14 postchallenge. Prior experiments had established that the initial infection-driven responses could be discerned in the nonimmunized, age-matched mice around this time point. A separate experiment was set up to examine whether Ag-specific Th1 and Th17 memory T cells were actively proliferating following challenge. To this end, memory mice were aerosol challenged with M. tuberculosis as described above ∼2 years after the last H1 immunization alongside nonimmunized, aged-matched mice. Six weeks into the infection, mice were sacrificed and immune responses in pooled lung-draining tracheobronchial lymph nodes as well as individual lungs (3/group) analyzed. Mice were administered bromodeoxyuridine (BrdU; 0.8 mg/ml) in drinking water 4 days prior to euthanization, and the degree of BrdU incorporation was used to determine the degree of proliferation.
Cell preparations, ELISA, and MSD assays.
Blood was obtained by submandibular bleeding, and the peripheral blood mononuclear cells (PBMCs) were purified using Lympholyte (Cederlane, Burlington, NC). Splenic and lymph node cell preparations were obtained by homogenization through a metal mesh and washed twice in RPMI 1640 (Gibco Invitrogen, Taastrup, Denmark). All cell cultures were performed in Nuclon microtiter plates (Nunc, Roskilde, Denmark) containing 2 × 105 cells/well in a volume of 200 μl RPMI 1640 supplemented with 5 × 10−5 M 2-mercaptoethanol, 1 mM glutamine, 1% pyruvate, 1% penicillin-streptomycin, 1% HEPES, and 10% fetal calf serum (FCS) (all from Gibco Invitrogen, Taastrup, Denmark). Stimulants were used in concentrations of either 0.5 or 5 μg/ml, and cell culture supernatants were harvested after 72 h of incubation at 37°C. The levels of secreted antigen-specific cytokines were evaluated using enzyme-linked immunosorbent assay (ELISA) and MSD assays. For the latter, the Th1/Th2 cytokine 9-plex electrochemiluminescence assay was performed according to the manufacturer's instructions (Meso Scale Discovery, Gaithersburg, MD). The plates were read on the Sector Imager 2400 system, and the cytokine concentrations in unknown samples were determined by 4-parameter logistic nonlinear regression analysis of the standard curve. The amounts of secreted IL-21, IL-22, and IL-23 (p19/p40) were determined by ELISA in duplicate readings from 4 individual mice using Ready-Set-Go kits according to the manufacturer's instructions (eBioscience, San Diego, CA). Gamma interferon (IFN-γ) levels were likewise determined by ELISA as previously described (32). The amount of IL-17A in cell culture supernatants was similarly determined by a sandwich enzyme-linked immunosorbent assay using a purified rat anti-mouse IL-17A at a concentration of 1 μg/ml (Biolegend, San Diego, CA) as coating antibody, biotin-labeled rat anti-mouse IL-17A (Biolegend) as detection antibody (250 ng/ml), and horseradish peroxidase (HRP)-conjugated streptavidin (BD Pharmingen, San Diego, CA) for detection (diluted 1:5,000). Recombinant mouse IL-17A (Biolegend) was used in serial dilutions to obtain standard curves for determination of IL-17A levels. Reactions were visualized by TMB ready-to-use (Kem-En-Tec, Denmark) chromogen substrate.
Intracellular flow-cytometric analysis.
Intracellular staining for flow cytometry was performed essentially as described earlier (32), with the only modification being the use of the eBiosciences FoxP3 staining buffer set for cell fixation and permeabilization procedures rather than the Cytofix/Cytoperm kit (BD Pharmingen). In prior optimization experiments, we had found that the FoxP3 staining set gave better delineation of the IL-17A responses. The following antibodies were used for surface staining (in 1:200 dilutions unless otherwise stated): anti-CD3-fluorescein isothiocyanate (FITC) (clone 145-2C11; BD Pharmingen), anti-CD4-allophycocyanin (APC)-Cy7 (clone GK1.5; BD Pharmingen), anti-CXCR3-phycoerythrin (PE) (clone 220803; 1:10; RD Systems), anti-CCR6 (CD196)-Alexa 647 (clone 140706; 1:100; BD Pharmingen), and anti-CCR4 (CD194)-PE (clone 2G12; 1:50; Biolegend). For intracellular staining, the following antibodies were applied: anti-IL-17A-peridinin chlorophyll protein (PerCP)-Cy5.5 (clone eBio17B7; eBiosciences), anti-IFN-γ-PE-Cy7 (clone XMG1.2; eBiosciences), anti-TNF-α-PE or anti-TNF-α-PE-Cy7 (MP6-XT22; BD Pharmingen), anti-IL-2-APC (clone JES6-5h4; BD Pharmingen), anti-IL-21-APC (clone 149204; 1:10; RD systems), and anti-IL-22-PE (clone 1H8PWSR; 1:100; eBiosciences). BrdU staining was performed as in Elvang et al. (17). Cells were acquired and analyzed using a BD FACSCanto flow cytometer (BD Biosciences) and analyzed using FlowJo software for Macintosh (v.8.7.6; Tree Star Inc.).
Statistical analysis.
For comparative analysis of immunogenicity, data were either tested by Student's t test or by one-way analysis of variance (ANOVA). When significant differences were found, differences between means were determined by Tukey's multiple comparison tests. In cases of multiple variable parameters, a two-way ANOVA was performed. All statistical analyses were carried out in GraphPad Prism (version 5.03; GraphPad Software Inc., La Jolla, CA).
RESULTS
Immunization with CAF01 leads to responses dominated by expression of Th1 and Th17 cytokines.
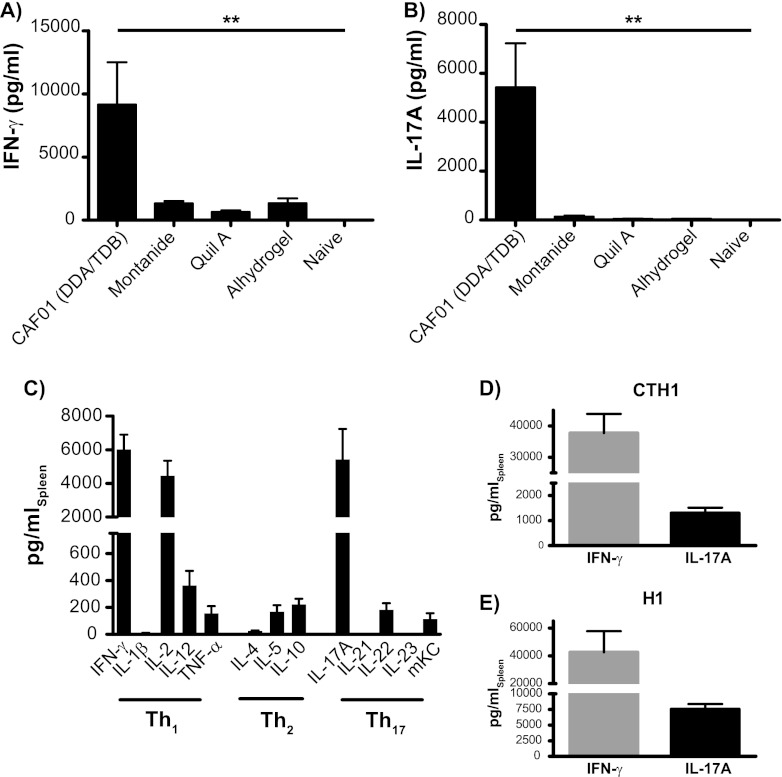
We initially characterized and compared the Th1-Th17-inducing capacity of CAF01 to that of selected adjuvants by measuring the levels of secreted IFN-γ and IL-17A after immunization with the TB fusion model antigen H28 (Fig. 1A and B). In agreement with earlier reports, we found CAF01 to promote high levels of the Th1 cytokine IFN-γ and notable Th17 responses characterized by prominent levels of IL-17A that were significantly higher than those induced by any of the other adjuvants tested (P < 0.01 by one-way ANOVA with Tukey's multiple-comparison posttest) (Fig. 1A and B). In order to fully characterize the cytokine profile in mice to the H28 fusion protein adjuvanted by CAF01, we subsequently measured 13 cytokines by electrochemiluminescence and conventional ELISA (Fig. 1C). Using the recombinant vaccine antigen 13 weeks after three s.c. immunizations confirmed that CAF01 induces prominent levels of the Th1 cytokines IFN-γ and IL-2, as well as lower but detectable levels of IL-12 and TNF-α and similarly low levels of the Th2 cytokines IL-5 and IL-10. Additionally, Th17 cytokines, especially IL-17A but also IL-22 and CXCL1 (mKC), were found to be an integral part of the recall response (Fig. 1C). The combined induction of IFN-γ and IL-17A is a general trait of CAF01-adjuvanted responses, as seen by the fact that these signature cytokines were obtained following immunization with several other antigens, including both TB-related (fusion protein H1 [Ag85B-ESAT-6]) (Fig. 1E) and non-TB-related antigens (Chlamydia fusion protein CTH1 [Ct521-Ct443]) (Fig. 1D).
Fig 1.
Immunization with CAF01 leads to responses dominated by expression of Th1 and Th17 cytokines. Groups of four C57BL/6 mice were immunized s.c. three times with 2 μg H28 plus the indicated adjuvant. Levels of Ag-specific IFN-γ (A) and IL-17A (B) were measured by ELISA in splenocyte cultures 13 weeks after immunization. Data are means ± standard errors (SE). **, P < 0.01 by one-way ANOVA using Tukey's posttest. The experiment was performed twice with comparable results. (C) Levels of antigen-specific cytokine release were measured 13 weeks after immunization by either MSD multiplex analysis or conventional ELISA (IFN-γ, IL-17A, IL-21, IL-22, and IL-23 [p19/40]); data are means ± SE. (D) Groups of four C57BL/6 mice were immunized s.c. three times with 10 μg CTH1 plus CAF01. Levels of antigen-specific IFN-γ and IL-17A were measured 2 weeks after immunization by conventional ELISA. Data are means ± SE. (E) Groups of four C57BL/6 mice were immunized s.c. three times with 2 μg H1 plus CAF01. Three weeks postimmunization, levels of antigen-specific IFN-γ and IL-17A were measured as described above. Data are means ± SE and are representative of three repetitive experiments.
CAF01 induces distinct Th1 and Th17 subsets with clear functional differences in terms of homing potential and cytokine multifunctionality.
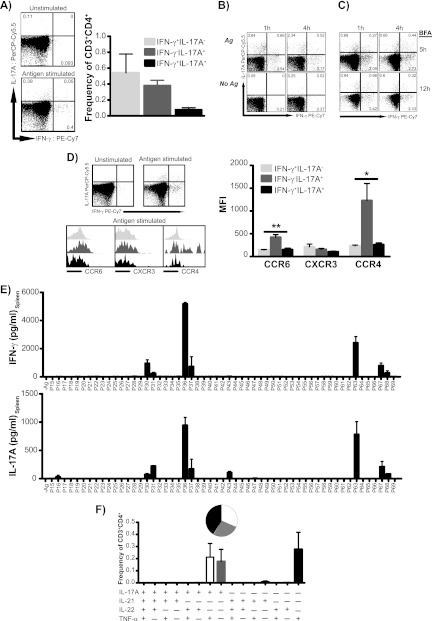
Subsequently, we addressed whether CAF01-induced CD4 T cells coexpressed IFN-γ and IL-17A or were manifested as distinct Th1 and Th17 subsets. By intracellular flow cytometry, we found that although a minor population of coexpressing Th1/Th17 cells did appear after CAF01 immunization, vaccine-driven Th1 and Th17 responses displayed a clear dichotomy (Fig. 2A). This pattern was observed irrespective of the intracellular staining (ICS) protocol used (Fig. 2B and C), and the Th1-Th17 dichotomy was likewise seen using the Chlamydia fusion protein CTH1 (data not shown). Further, CAF01 immunization was not found to induce IL-17A-secreting γδ-T cells beyond the levels seen in nonimmunized mice (data not shown). We next examined whether chemokine receptors were expressed in a lineage-specific manner in the two effector subsets and in the minor population of the IFN-γ/IL-17A-coexpressing Th1/Th17 subset. Whereas no clear differences were found for CXCR3, we consistently found Th17 cells to express significantly higher levels of the mucosal homing marker CCR6 (CD196) (P < 0.01 by one-way ANOVA with Tukey's multiple-comparison posttest) as well as the skin-homing marker CCR4 (CD194) (P < 0.05 by one-way ANOVA with Tukey's multiple-comparison posttest) than both the Th1 and Th1/Th17 subsets (Fig. 2D). As the distinct Th1-Th17 dichotomy could be explained by differential epitope recognition within the two T cell lineages, with certain epitopes preferentially recognized by Th1 cells and others by Th17, we performed an epitope mapping of the major antigen Ag85B (Rv1886c). However, T cells producing either IFN-γ or IL-17A recognized the same regions of Ag85B (Fig. 2E).
Fig 2.
CAF01 induces distinct Th1 and Th17 subsets with functional differences. Groups of four C57BL/6 mice received three immunizations with 2 μg H28 plus CAF01. (A) Fifteen weeks after immunization, the frequency of Th1 (IFN-γ+ IL-17A−; light gray bars), Th17 (IFN-γ− IL-17A+; dark gray bars), and Th1/Th17 (IFN-γ+ IL-17A+; black bars) within the CD3+ CD4+ population was determined by intracellular flow cytometry (ICS). The left panels show representative plots of IL-17A versus IFN-γ expression in CD4 lymphocytes (singlets > lymphocytes > CD3+ > CD4+) from unstimulated and H28 stimulated splenocytes. The experiment was independently performed three times with similar results. (B) Distinct Th1 and Th17 cell populations predominate postvaccination, regardless of ex vivo stimulation and the intracellular staining protocol used. Mice received 2 to 3 s.c. immunizations of 2 μg H1 plus CAF01. PBMCs pooled from 8 C57BL/6 mice after 22 weeks were stimulated with H1 (top) or were left unstimulated for either 1 h (left) or 4 h (right) before 6 h of incubation in the presence of brefeldin A (BFA). (C) Splenocytes pooled from 4 B6 × BALB/c F1 mice 3 weeks postimmunization were stimulated with H1 for either 1 h (left) or 4 h (right) before 5 or 12 h of incubation in the presence of BFA. Representative plots of gated CD4 T cells are shown. (D) Expression levels of CCR6, CXCR3, and CCR4 were determined in H28-specific Th1 (IFN-γ+ IL-17A−; light gray), Th17 (IFN-γ− IL-17A+; dark gray), and Th1/Th17 (IFN-γ+ IL-17A+; black) cells within the CD3+ CD4+ lymphocyte gate by their geometric mean fluorescent intensity. The left panels show representative plots of IL-17A versus IFN-γ expression in CD4 lymphocytes (singlets > lymphocytes > CD3+ > CD4+) from unstimulated and H28-stimulated splenocytes and histogram overlays of chemokine receptor expression of H28-specific Th1, Th17, and Th1/Th17 subsets. Geometric mean MFI values of each marker were compared by one-way ANOVA with Tukey's posttest (right). *, P < 0.05; **, P < 0.01. The graph is representative of three independent experiments. (E) Splenocytes from 3 individual mice were restimulated with overlapping peptides spanning the entire Ag85B protein. Levels of secreted IFN-γ and IL-17A were subsequently measured by ELISA. Data are means ± SE. Peptides recognized by Th1 and Th17 included P30/P31 (SSFYSDWYSPACGKA/DWYSPACGKAGCQTY), P36/P37 PQWLSANRAVKPTGS/ANRAVKPTGSAAIGL), P63 QDAYNAAGGHNAVFN), and P67 (THSWEYWGAQLNAMK). (F) Cytokine coexpression profiles in vaccinated mice 15 weeks postimmunization. By Boolean gating, cytokine-producing cells (IL-17A, TNF-α, IL-21, and IL-22) within the CD3+ CD4+ population were divided into 15 distinct subpopulations based on their production of these cytokines in any combination, and their frequency was determined. The relative contribution of each of these subpopulations to the responding T cell population is shown by the inserted pie chart. The experiment was performed three times with comparable results.
Finally, a functional characterization of the Th17 cells in terms of their cytokine coexpression profiles was performed by ICS. Some Th17 cells showed coexpression of IL-2 (not shown), but because the great majority of Th17 cells did not coexpress IL-17A with IFN-γ (Fig. 2A to C), we focused on the classical Th17 cytokines IL-21 and IL-22 as well as TNF-α. Thus, using an intracellular staining panel against IL-17A, IL-21, IL-22, and TNF-α, antigen-specific CD4 T cells were found to express IL-17A and TNF-α (Fig. 2F). The modest IL-22 expression observed by ELISA (Fig. 1) was not detected by ICS. Looking at their coexpression, Th17 cells were found to express IL-17A either alone or in combination with TNF-α (Fig. 2F).
Th17 cells induced by CAF01 are maintained as both an intermediary and long-term (>1.5 years) stable memory subset.
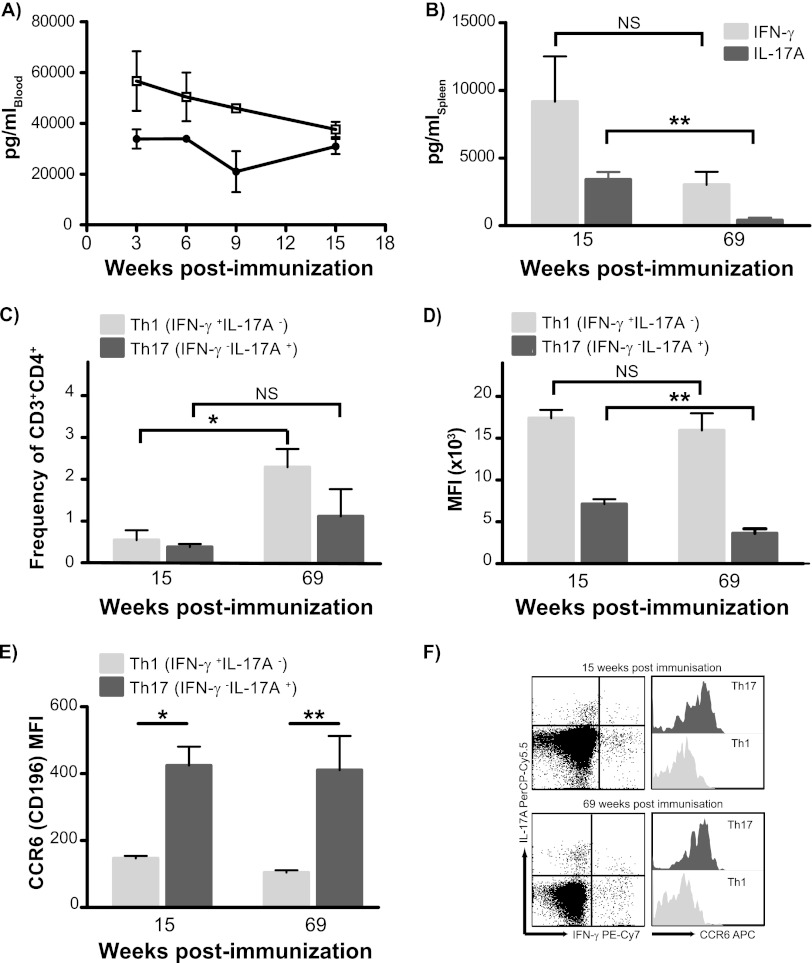
The CAF01 adjuvant has been shown earlier to promote strong and sustained multifunctional Th1 responses (16, 32). To investigate whether Th17 cells play an integral part in this memory response, we examined the IFN-γ and IL-17A recall responses after short and long intervals after immunization with CAF01. Within the first 3 months after immunization, antigen-specific levels of IL-17A and IFN-γ in the blood were relatively constant (Fig. 3A). At week 69 (long term), there were diminished levels of IFN-γ (not significant) and, in particular, lower levels of secreted IL-17A by splenocytes (P = 0.007 by Student's t test) (Fig. 3B). In contrast, the frequency of antigen-specific Th1 cells was significantly increased at 69 weeks postimmunization (the Th lineage parameter was not significant by two-way ANOVA, P < 0.01 for the time parameter, and parameter interaction was not significant) (Fig. 3C). At this late time point, the frequency of the Th17 memory subset remained stable while exhibiting a nonsignificant increase (Fig. 3C). As no increase in the frequency of IFN-γ+/IL-17A+ double-positive Th1/Th17 cells was found (not shown), lineage stability of Th1 and Th17 was maintained throughout the 69 weeks after immunization. The drop in IL-17A secretion observed from 15 to 69 weeks after immunization was reflected at the single-cell level such that IL-17A production on a per-cell basis, as measured by the average geometric mean fluorescent intensity (MFI) values of IL-17-positive CD3+ CD4+ T cells, was significantly lower at week 69 than at week 15 postimmunization. No such reduction in IFN-γ MFI values was observed for the Th1 subset (Fig. 3D). However, the Th17 cells stably retained their distinct mucosal homing properties, as shown by their significantly higher expression of mucosal homing marker CCR6 compared to the Th1 subset (according to two-way ANOVA, P < 0.001 for differences in CCR6 expression for Th lineage, and the change over time was not significant) (Fig. 3E and F).
Fig 3.
CAF01-induced Th17 cells are established as a distinct and stable memory subset. (A) C57BL/6 mice received 3 s.c. immunizations of 2 μg H28 plus CAF01 spaced 2 weeks apart. Blood was obtained at weeks 3, 6, 9, and 15 after immunization by submandibular bleeding. PBMCs pooled from 8 mice were stimulated with H28 at a concentration of 0.5 μg/ml, and cell culture supernatants were harvested after 72 h of incubation. The levels of secreted antigen-specific IFN-γ and IL-17A were determined by capture ELISA in triplicate readings; data are means ± standard deviations. Open squares, IFN-γ; black circles, IL-17A. Groups of four mice received three immunizations with 2 μg H28 plus CAF01, and Th1 versus Th17 memory responses were measured at short (15 weeks) and long (69 weeks) intervals after vaccination. (B) Release of IL-17A and IFN-γ from spleen cells isolated after H28 immunization was determined by ELISA; data are means ± SE. P values were calculated using Student's t test (*, P < 0.05; **, P < 0.01). (C) The frequency of Th1 (IFN-γ+ IL-17A−; light gray bars) and Th17 (IFN-γ− IL-17A+; dark gray bars) at short (15 weeks) and long (69 weeks) intervals after vaccination was determined by ICS. The percentage of each subpopulation within the CD3+ CD4+ gated lymphocyte population was determined. Significant differences were calculated by two-way ANOVA with Bonferroni's posttest (*, P < 0.05). (D) Expression levels of IL-17A in H28-specific Th17 (IFN-γ− IL-17A+; dark gray bars) and of IFN-γ in Th1 (IFN-γ+ IL-17A−; light gray bars) cells within the CD3+ CD4+ lymphocyte gate were determined in four individual mice by their geometric mean fluorescent intensity at both short (15 weeks) and long (69 weeks) intervals. Differences in mean MFI values over time within each population were compared by Student's t test. **, P < 0.01. (E) Expression levels of CCR6 were determined in H28-specific Th1 (IFN-γ+ IL-17A−; light gray bars) and Th17 (IFN-γ− IL-17A+; dark gray bars) cells within the CD3+ CD4+ lymphocyte gate by their geometric mean fluorescent intensity. Significant differences in MFI values were calculated by two-way ANOVA with Bonferroni's posttest. *, P < 0.05; **, P < 0.01. (F) Representative plots of distinctive antigen-specific Th1 (IFN-γ+ IL-17A−) and Th17 (IFN-γ− IL-17A+) cells and histogram overlays of their CCR6 expression levels at 15 and 69 weeks postimmunization. This experiment was repeated once with comparable results, though with H1 (Ag85B-ESAT-6) as the antigen.
Long-term Th17 memory cells proliferate following challenge with Mycobacterium tuberculosis and are mobilized from lung-draining lymph nodes to the lung.
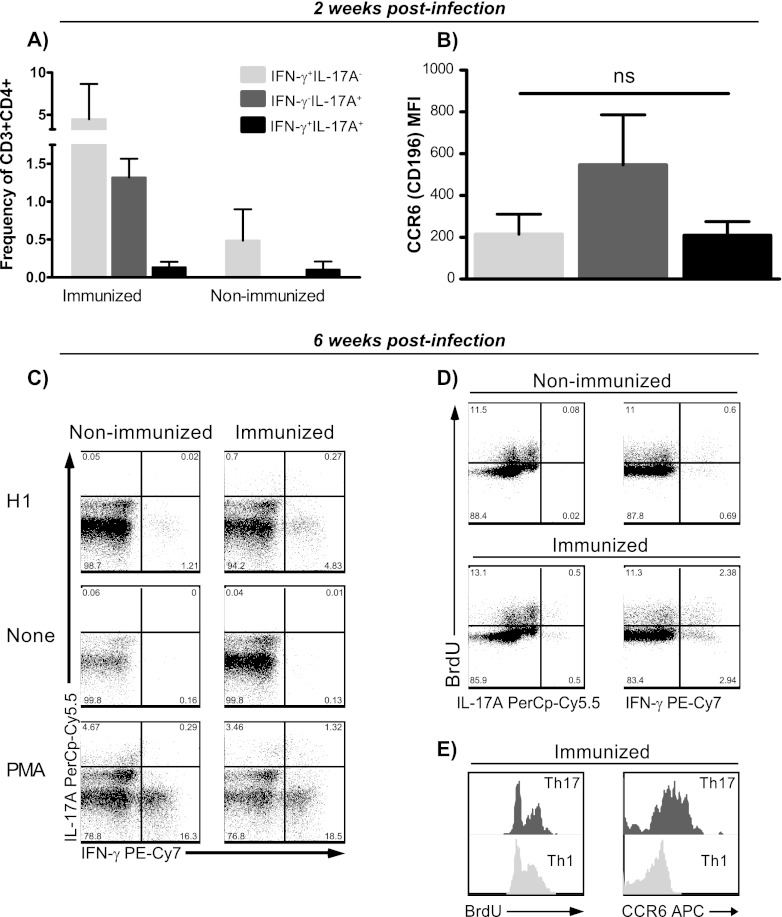
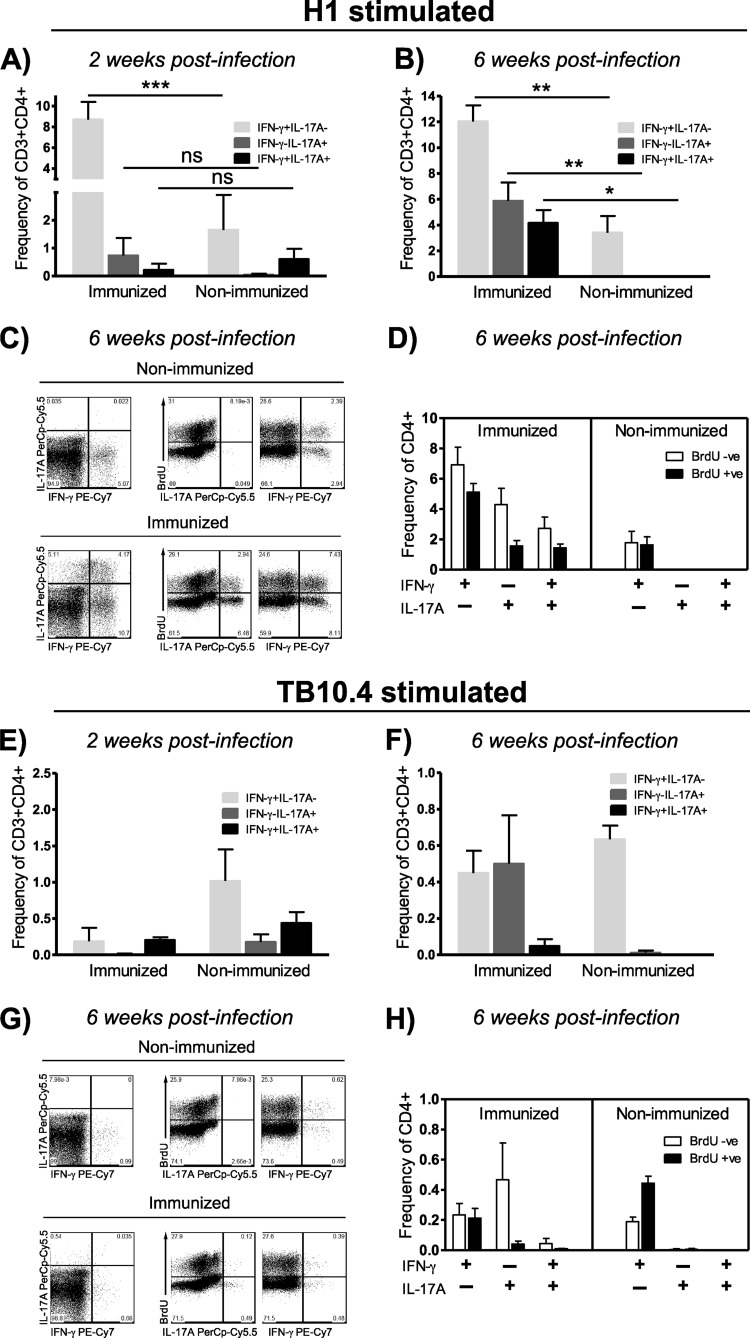
Having established that CAF01 can promote long-term Th17 memory cells exhibiting lineage stability in terms of both cytokine production and mucosal homing properties, we next addressed whether vaccine-induced Th17 memory cells were still functional and could be mobilized following challenge with Mycobacterium tuberculosis. To this end, mice were immunized three times with the TB fusion model protein H1 at 2-week intervals, and 89 weeks later they were challenged by the aerosol route. Two weeks into the infection, when the initial infection-driven responses could be detected in the lung-draining lymph nodes of nonimmunized mice, mice were euthanized and antigen-specific Th1 and Th17 responses determined by intracellular flow cytometry in both immunized and aged-matched nonimmunized animals. We found that long-term Th17 memory cells were mobilized after challenge to the lung-draining tracheobronchial lymph nodes, as we observed a significantly higher frequency of antigen-specific Th17 cells in the draining lymph nodes from memory mice than from nonimmunized animals (P = 0.003 by Student's t test). Further, the Th17 cells from the memory animals clearly maintained their dichotomy relative to Th1 (Fig. 4A). This was in contrast to the infection-driven Th17 response in nonimmunized mice, which were of a distinctive Th1/Th17-coexpressing profile (Fig. 4A). Although antigen-specific Th1 cells were likewise mobilized from memory mice, frequencies were not significantly higher than in nonimmunized animals at this early time point (Fig. 4A). In terms of CCR6 levels, the Th17 cells from the tracheobronchial lymph nodes in the immunized mice had somewhat higher mucosal homing potential than the Th1 and the Th1/Th17 counterparts, although differences were not significant (P > 0.05 by one-way ANOVA) (Fig. 4B).
Fig 4.
Long-term Th17 memory cells are rapidly mobilized from lung-draining lymph nodes following challenge with Mycobacterium tuberculosis. (A and B) C57BL/6 mice were immunized three times with 2 μg H1 plus CAF01 and 89 weeks later were aerosol challenged with M. tuberculosis Erdman. Mice were euthanized 2 weeks into the infection, and H1-specific Th1 and Th17 responses were determined by ICS in both immunized (3 mice) and aged-matched, nonimmunized animals (4 mice). (A) The frequency of Th1 (IFN-γ+ IL-17A−; light gray bars), Th17 (IFN-γ− IL-17A+; dark gray bars), and Th1/Th17 (IFN-γ+ IL-17A+; black bars) cells mobilized from the tracheobronchial lymph nodes was determined by ICS in both long-term memory and aged-matched, nonimmunized mice. (B) Expression levels of CCR6 were determined by geometric CCR6 mean fluorescent intensity in infection-mobilized, H1-specific Th1 (IFN-γ+ IL-17A−; light gray bars), Th17 (IFN-γ− IL-17A+; dark gray bars), and Th1/Th17 (IFN-γ+ IL-17A+; black bars) cells within the CD3+ CD4+ lymphocyte gate from the tracheobronchial lymph nodes of memory immune mice. Differences were found to be nonsignificant (ns; one-way ANOVA). (C to E) C57BL/6 mice were immunized three times with 2 μg H1 plus CAF01 and ∼2 years later were aerosol challenged with M. tuberculosis Erdman. Mice were euthanized 6 weeks into the infection, and H1-specific Th1 and Th17 responses and their proliferative capacity were determined by ICS in pooled tracheobronchial lymph nodes from both immunized and aged-matched, nonimmunized animals. (C) IFN-γ versus IL-17A expression in pooled lung-draining lymph node CD4+ T cells after stimulation with H1, medium, and phorbol myristate acetate-ionomycin. Numbers denote the frequency of CD4+ T cells. (D) Proportion of BrdU-incorporating H1-specific Th17 (left plots) versus Th1 (right plots) CD4 T cells from lung-draining lymph nodes 6 weeks into M. tuberculosis infection in nonimmunized (upper part) and immunized mice (lower part). Numbers denote the frequency of CD4+ T cells. (E) Histogram overlay showing the degree of BrdU incorporation (left) and CCR6 expression levels (right) in H1-specific Th1 (IFN-γ+ IL-17A−) and Th17 (IFN-γ− IL-17A+) CD4 T cells from tracheobronchial lymph nodes isolated from memory immune mice 6 weeks into infection.
We subsequently wanted to investigate whether M. tuberculosis infection led to proliferation within the Th17 memory compartment in the tracheobronchial lymph nodes and, further, if Th17 cells were subsequently recruited to the lungs during infection. To address these issues, memory mice, approximately 2 years after the last H1 immunization, were aerosol challenged, and the frequency of antigen-specific Th1 and Th17 and their degree of proliferation were determined in both lung-draining lymph nodes as well as in lungs 6 weeks into infection. At this time point, immunized mice still mounted combined Th1 and Th17 responses in the tracheobronchial lymph nodes, with these responses still being predominantly distinct from each other (Fig. 4C). However, one-third of the IL-17A-producing CD4 T cells were IFN-γ coproducers at this time. Nonimmunized mice were devoid of H1-specific Th17 cells in the lung-draining lymph nodes 6 weeks into infection, and responses thus were exclusively of the Th1 subset (Fig. 4C). We found that vaccine-induced Th17 memory cells proliferated to the same extent as Th1 following infection. Thus, in the tracheobronchial lymph nodes, approximately 50% of both subsets of H1-specific CD4 T cells were found to incorporate BrdU (Fig. 4D). Further, each of these subsets exhibited a similar degree of incorporation (Fig. 4E, left). In addition to their expansion in the draining lymph nodes, Th17 cells were still found to express higher levels of the mucosal homing marker CCR6 6 weeks into the infection (Fig. 4E, right).
After showing that Th17 memory cells are mobilized to the lung-draining lymph nodes, maintain their polarization, stably express CCR6, and proliferate to the same extent as Th1 memory cells following challenge, we next looked at their involvement at the site of infection. Early H1 responses in the lung (2 weeks) (Fig. 5A) were dominated by Th1 and Th17 cells, with only a minor contribution of Th1/Th17 coproducers in immunized mice. In nonimmunized animals, significantly lower levels of Th1 responses could be detected (P < 0.001 by two-way ANOVA using Bonferroni's posttest) and Th17 responses were similar to what was observed in the draining lymph nodes at week 2, dominated by IFN-γ/IL-17A-coexpressing cells (Fig. 5A). Six weeks into the infection, H1-specific Th17 cells were nearly absent from nonimmunized mice, whereas these cells had greatly expanded in the immunized group (differences between nonimmunized and immunized in Th1 cells were significant at P < 0.01, those in Th17 cells at P < 0.01, and those in Th1/Th17 cells at P < 0.05, each determined by two-way ANOVA using Bonferroni's posttest) (Fig. 5B). Both Th1 and Th17 CD4 T cells thus were found to have incorporated BrdU during the preceding 4 days (Fig. 5C and D). However, and strikingly, at this time during infection, both IFN-γ−/IL-17A+ and IFN-γ+/IL-17A+ subsets were found to contribute almost equally to the Th17 response (Fig. 5B to D). In terms of their proliferative capacity, we found no differences between these two Th17 subsets, as bona fide Th17 and Th1/Th17 cells had incorporated BrdU to almost the same extent (Fig. 5D). These findings raised the question of whether the prominent population of Th1/Th17 in the inflamed tissue at the later stages of M. tuberculosis infection could be regarded as progeny of metastable Th17 memory cells displaying plasticity in the face of prolonged inflammation or represented effector cells primed and maintained by the infection. Although the M. tuberculosis infection seemed to prime H1-specific Th1/Th17 cells in nonimmunized mice, this subset was found to be neither maintained nor expanded by the infection (compare Fig. 4A to 5A and 4C to 5B and C). To further elaborate on this, we next looked at responses to the M. tuberculosis antigen TB10.4, which was not contained in the subunit vaccine and would therefore be primed and maintained/expanded solely by the infection in both the immunized and nonimmunized group. Two weeks after challenge, infection primed both classical Th1 and the Th1/Th17 subsets (Fig. 5E) (differences between immunized and nonimmunized mice were not significant as determined by two-way ANOVA), but this IFN-γ/IL-17A-coexpressing subset was not found to be maintained or expanded later during infection (Fig. 5F to H) (differences between immunized and nonimmunized mice were not significant by two-way ANOVA). In the immunized group, approximately 0.5% TB10.4-specific, bona fide Th17 cells were observed in the lungs at week 6 (Fig. 5F), but this infection-driven response had low proliferative capacity and exhibited no or very limited BrdU incorporation (Fig. 5G and H). Hence, Th17 cells primed by the M. tuberculosis infection appear to be short-lived effectors and consequently seem unlikely to account to any significant degree for the mixed H1-specific Th1/Th17 cells that emerge during later stages of infection in the lungs of memory mice.
Fig 5.
Challenge with Mycobacterium tuberculosis mediates proliferation of long-term Th17 memory cells and recruitment to the lung, where they adopt Th1 cell characteristics as infection progresses into chronic stages. (A) C57BL/6 mice were immunized three times with 2 μg H1 plus CAF01, and 89 weeks later aerosol was challenged with M. tuberculosis Erdman. Mice were euthanized 2 weeks into the infection, and the frequency of H1-specific Th1 (IFN-γ+ IL-17A−; light gray bars), Th17 (IFN-γ− IL-17A+; dark gray bars), and Th1/Th17 (IFN-γ+ IL-17A+; black bars) cells in both immunized (3 mice) and aged-matched, nonimmunized animals (4 mice) were determined by ICS. Significant differences were calculated by two-way ANOVA with Bonferroni's posttest (***, P < 0.001). (B to H) C57BL/6 mice were immunized three times with 2 μg H1 plus CAF01 and ∼2 years later were aerosol challenged with M. tuberculosis Erdman. Mice were euthanized 6 weeks into the infection, and antigen-specific Th1 and Th17 responses and their proliferative capacity were determined by ICS in individual lungs from both immunized and aged-matched, nonimmunized animals (3 mice/group). (B) Frequency of H1-specific CD3+ CD4+ Th1 (IFN-γ+ IL-17A−; light gray bars), Th17 (IFN-γ− IL-17A+; dark gray bars), and Th1/Th17 (IFN-γ+ IL-17A+; black bars) cells from the lung 6 weeks into infection in both long-term memory and aged-matched, nonimmunized mice. Significant differences were calculated by two-way ANOVA with Bonferroni's posttest (***, P < 0.001; **, P < 0.01; *, P < 0.05). (C) Representative plots showing IL-17A versus IFN-γ expression (left) and the proportion of BrdU incorporating H1-specific Th17 (middle) and Th1 (right) cells 6 weeks into infection. (D) Mean frequencies of H1-specific Th1 (IFN-γ+ IL-17A−), Th17 (IFN-γ− IL-17A+), and Th1/Th17 (IFN-γ+ IL-17A+) subsets being either BrdU negative or BrdU positive 6 weeks into challenge. (E) Frequency of TB10.4-specific Th1 (IFN-γ+ IL-17A−; light gray bars), Th17 (IFN-γ− IL-17A+; dark gray bars), and Th1/Th17 (IFN-γ+ IL-17A+; black bars) cells 2 weeks after infection (ns, not significant by two-way ANOVA). (F) Frequency of TB10.4-specific Th1 (IFN-γ+ IL-17A−; light gray bars), Th17 (IFN-γ− IL-17A+, dark gray bars), and Th1/Th17 (IFN-γ+ IL-17A+; black bars) cells 6 weeks into infection. (G) Representative plots showing IL-17A versus IFN-γ expression (left) and the proportion of BrdU incorporating TB10.4-specific Th17 (middle) and Th1 (right) cells 6 weeks into infection. (H) Mean frequencies of TB10.4-specific Th1 (IFN-γ+ IL-17A−), Th17 (IFN-γ− IL-17A+), and Th1/Th17 (IFN-γ+ IL-17A+) subsets being either BrdU negative or BrdU positive 6 weeks into challenge.
DISCUSSION
The ultimate goal of any vaccination procedure is to establish a pool of memory cells of adequate size and quality to promote long-lived protection against subsequent challenge. Recent human and murine studies have indeed shown that subunit vaccination with novel adjuvant platforms can promote long-lived Th1 memory with durability similar to or exceeding the levels normally attained only by live vectors (32, 49, 50). Memory-phenotype Th17 cells have been reported from human infections or disease conditions (34, 43, 46), and a recent report has confirmed that human Th17 memory cells are long lived and confined to the memory T cell compartment as they are not exhausted (PD-1−), not regulatory/suppressive (FoxP3−; IL-10−), and not senescent (CD28+ CD57+ KLRG-1−) in nature (26). It was further shown that human Th17 memory cells have high proliferative capacity (i.e., express high levels of Ki67) and are resistant to apoptosis, but at the same time they are terminally differentiated as they are CD27low (26). Low levels of CD27 expression have also been reported from mouse Th17 cells (37, 42), and Th17 memory cells from mice have accordingly been reported to be short-lived with a limited capacity to persist (42). Thus, there is no clear consensus in the field as to whether long-lived Th17 memory with equivalence to Th1 can be induced by immunization (5, 27, 29, 30, 37, 38, 42).
In the current work, we show that CAF01-induced Th17 cells can be stably maintained in mice up to 1.5 years after immunization in a fashion similar to what has been reported earlier for CAF01-induced Th1 memory (32). This observation is in keeping with reports that in vivo-generated Th17 cells represent a stable and distinct lineage of Th cell differentiation (30) and that in vitro-generated Th17 cells can maintain their differentiation program after transfer into a normal host, even in the absence of antigen or continued Th17 polarizing conditions (38). Likewise, Muranski et al. (37) reported that murine Th17 could persist for at least 2 months as self-renewing, IL-17A-secreting cells despite their low expression of CD27. In a contrasting report, CD27low Th17 cells with a central memory phenotype (CCR7+) from mice were described to be shorter lived than antigen-specific Th1 cells due to inferior Th17 survival and a lower degree of homeostatic proliferation (42). In contrast to our experiment, in that case Th1 and Th17 cells were induced by incommensurate routes of delivery, such as Th17 cells by a mucosal Listeria monocytogenes infection, whereas Th1 cells were promoted by intravenous inoculation with the bacteria (42). Th17 cells are known to display substantial chromosome instability of key transcription factors and cytokine genes (36, 51), and fate mapping in Th17 reporter mice has also unequivocally shown that certain inflammatory conditions can induce rapid transition of Th17 cells into, e.g., Th1 (8, 22, 28). In the absence of chronic inflammation, Th17 cells were found to be stable and not deviate into other T cell lineages (22). In agreement with this, we found the induced Th17 cells to be stable and to maintain their dichotomy relative to Th1, thus implying that during the inflammatory quiescent 69-week period from final immunization, the Th17 memory cells did not adopt Th1 phenotypic characteristics. At all times we found the Th17 cells to express significantly higher levels of the mucosal chemokine receptor CCR6 (CD196) than the Th1 T cell lineage, which further reinforces that the vaccine-induced Th17 memory subset is phenotypically stable (2, 48).
The capacity of CAF01 to induce prominent Th17 responses in addition to Th1 was not seen with any other of the tested adjuvants and might be a distinctive feature. Thus, immunization with CpG, another strong Th1 adjuvant, did not give rise to Th17 responses either and elicited a markedly different transcriptome profile compared to TDB administration (52). The induction of bona fide Th17 cells using CAF01 is in contrast to a recent publication within the Chlamydia field, where Th17 responses were highly dominated by an IFN-γ/IL-17A-coexpressing subset after immunizations with DDA/TDB (58). We repeatedly observed clear Th1-Th17 dichotomy using different antigens as well as different ICS protocols, implying that this discrepancy cannot be ascribed to these factors. However, in the work by Yu et al. (58), the DDA/TDB adjuvant was formulated by the aqueous heat method, where TDB is less efficiently incorporated into the lipid bilayer than the current CAF01 (DDA/TDB), which is formulated by the thin-film lipid rehydration method (12). As TDB is essential for the Th17 adjuvant activity of CAF01 (DDA/TDB) (45, 52), an uneven distribution of TDB in DDA liposomes might in part explain the incongruity.
On a functional level, a substantial part of the CAF01-induced Th17 cells were found to coexpress TNF-α. TNF-α and IL-17A coexpression has been reported previously and found to act in synergy by promoting IL-23p19 and IL-6 expression, the latter through synergistic activation of transcription factors belonging to the C/EBP family (19, 44). Further, TNF-α has been shown to increase the expression of CD54 (ICAM-1), which would allow CCR6-positive Th17 cells to extravasate at mucosal sites such as the lung through engagement with CCL20 (MIP-3α) (18). Along this line, Khader et al. (24) reported on rapid accumulation of IL-17-producing T cells in the lungs of vaccinated mice after challenge with Mycobacterium tuberculosis that preceded the corresponding IFN-γ-producing population. They proposed that vaccine-induced T cells producing IL-17 could rapidly respond to infection and accelerate the recruitment of Th1 cells through expression of CXCL9, CXCL10, and CXCL11 chemokines. In the current experiments, we found that the CAF01-induced Th17 memory cells were functionally competent and could be mobilized from the lung-draining lymph nodes after M. tuberculosis challenge close to 2 years after their induction. The Th17 cells recruited from the tracheobronchial lymph nodes were likewise stable, did not coexpress IFN-γ, displayed infection-mediated proliferation at levels comparable to Th1, and exhibited somewhat higher mucosal homing potential than both the Th1/Th17 and Th1 subsets. However, in this case the levels of CCR6 were not significantly different between the three T helper subsets, probably reflecting that the Th17 memory cells had experienced Ag-specific activation during the infection by lung-draining dendritic cells, a process known to downregulate CCR6 expression (18). Once in the lung, vaccine-induced Th17 cells kept their distinction from Th1 early in infection, but, under the influence of prolonged inflammation, mixed Th17 and Th1 phenotype cells emerged which then constituted approximately half of the Th17 response during the later stages of infection. M. tuberculosis infection did prime responses with mixed Th1/Th17 cells, but this population was neither maintained nor expanded in the nonimmunized mice. Thus, although IL-17A-producing cells could be observed in the lungs of nonimmunized mice 2 weeks into infection (Fig. 5A and E), very limited recruitment from the tracheobronchial lymph nodes was observed at any time point (Fig. 4), leading to the near absence of Th17 cells at week 6 in nonimmunized animals. This trait, with no or limited maintenance of infection-driven Th17 cells, was also reflected in responses to the infection marker TB10.4, where IFN-γ+ IL-17A+ CD4 T cells were primed but not sustained in any of the groups. The infection-elicited Th17 cells did not incorporate any noticeable degree of BrdU, and the infection therefore seems to prime short-lived Th17 effector cells with limited proliferative capacity. We therefore interpret the emergence of mixed Th17 and Th1 phenotype cells during the chronic infection as progeny of the vaccine-induced Th17 memory cells, which in such case would represent a metastable memory population that exhibits plasticity when exposed to prolonged Th1 polarizing inflammatory conditions. This interpretation would be in congruence with reports stating that the duration (and polarizing type) of stimulation of Th17 cells determines whether Th17 cells maintain their phenotype or begin to adopt Th1 cell characteristics, with chronic and/or repeated infection leading to the latter (13, 22, 37).
The definite role of Th17 responses for protection against mycobacterial infections has not been fully established and is beyond the scope of this paper. Although Th17 cells have been found to be dispensable for overall protection against mycobacterial infections (4, 25), IL-17-producing γδ-T cells do play a certain role in shaping innate and adaptive responses leading to granuloma formation (57), whereas classical (αβ CD4+) Th17 cells have been associated with early and IFN-γ-independent protection (54). In humans, long-lived Ag-specific Th17 cells with a central memory phenotype and distinct from Th1 have been identified in PBMCs from healthy, mycobacterium-exposed individuals and from individuals with latent tuberculosis infection, whereas significantly reduced Th17 responses were found in patients with active TB (6, 46). This correlation, with lower Th17 responses and disease progression, was not due to relocation to the lungs, as Th17 responses were found to be low to undetectable in bronchoalveolar lavage fluid and in pleural effusion from TB patients, possibly reflecting infection-mediated Th17 downregulation (6, 43, 46). However, since their discovery (20, 41) it has become clear that exaggerated and uncontrolled Th17 responses can be associated with autoimmunity and pathological conditions (7, 9, 14). Hence, there could be a risk that repeated vaccinations promoting Th17 immunity during chronic infection or in postexposure settings circumvent the regulatory effect of IFN-γ, eventually leading to exaggerated Th17 responses and associated immunopathology. Along these lines, Cruz et al. (9) recently showed that repeated Mycobacterium bovis BCG administration on top of an ongoing M. tuberculosis infection led to excess IL-17 expression associated with pathological lung inflammation, and it was suggested that such unregulated Th17 responses could in part be responsible for the Koch phenomenon (9). However, using CAF01 in a multistage vaccine tested in murine models of latent TB has, for the first time, shown promising results in terms of controlling reactivation without any signs of such adverse pathological effects (1). In this regard, the coinduction of Th1 responses could be a significant advantage of the CAF01 adjuvant, as IFN-γ is known to control Th17 expansion during mycobacterial infection (10). Dissecting the protective versus pathological roles of Th17 cells in preventive as well as postexposure vaccination against chronic infections in particular, including TB, should have continued priority. Still, Th17 cells play an important role in protective immunity against several pathogens, and in such cases it would be highly relevant and beneficial to induce preexisting Th17 memory by vaccination. We have shown that adjuvanted subunit vaccination given by the s.c. route can establish such stable, long-term Th17 memory responses with a predilection to target mucosal sites and thereby exert their direct effect as well as accelerate the recruitment of Th1 cells with an overall improved protective efficacy of novel vaccines.
ACKNOWLEDGMENTS
We thank Linda Christensen, Rune Fledelius Jensen, and Vivi Andersen for their excellent technical help. The animal technicians at the Statens Serum Institut are likewise gratefully acknowledged for their excellent technical assistance.
This work was supported by the European Commission contract FP7-HEALTH-F3-2009-241745 under the NEWTBVAC consortium.
P.A. and E.M.A. are coinventors of patented processes relating to cationic liposomes as vaccine adjuvants. P.A. is the coinventor of patented processes relating to tuberculosis fusion protein Ag85B-ESAT-6 and Chlamydia fusion protein CTH1. P.A., C.A., and J.D. are coinventors of patented processes relating to tuberculosis vaccines containing TB10.4. All rights have been assigned to the Statens Serum Institut. Ongoing clinical development of an Ag85B-TB10.4/IC31 vaccine is done in partnership with Sanofi Aventis and is supported by the Aeras global vaccine foundation.
Funding bodies had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. We have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Footnotes
Published ahead of print 30 July 2012
REFERENCES
- 1. Aagaard C, et al. 2011. A multistage tuberculosis vaccine that confers efficient protection before and after exposure. Nat. Med. 17:189–194 [DOI] [PubMed] [Google Scholar]
- 2. Acosta-Rodriguez EV, et al. 2007. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat. Immunol. 8:639–646 [DOI] [PubMed] [Google Scholar]
- 3. Agger EM, et al. 2008. Cationic liposomes formulated with synthetic mycobacterial cordfactor (CAF01): a versatile adjuvant for vaccines with different immunological requirements. PLoS One 3:e3116 doi:10.1371/journal.pone.0003116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Aujla SJ, et al. 2008. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat. Med. 14:275–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen K, et al. 2011. Th17 cells mediate clade-specific, serotype-independent mucosal immunity. Immunity 35:997–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen XC, et al. 2010. Reduced Th17 response in patients with tuberculosis correlates with IL-6R expression on CD4+ T cells. Am. J. Respir. Crit. Care Med. 181:734–742 [DOI] [PubMed] [Google Scholar]
- 7. Cox CA, et al. 2008. Both Th1 and Th17 are immunopathogenic but differ in other key biological activities. J. Immunol. 180:7414–7422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Croxford AL, Kurschus FC, Waisman A. 2009. Cutting edge: an IL-17F-Cre(EYFP) reporter mouse allows fate mapping of Th17 cells. J. Immunol. 182:1237–1241 [DOI] [PubMed] [Google Scholar]
- 9. Cruz A, et al. 2010. Pathological role of interleukin 17 in mice subjected to repeated BCG vaccination after infection with Mycobacterium tuberculosis. J. Exp. Med. 207:1609–1616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cruz A, et al. 2006. Cutting edge: IFN-γ regulates the induction and expansion of IL-17-producing CD4 T cells during mycobacterial infection. J. Immunol. 177:1416–1420 [DOI] [PubMed] [Google Scholar]
- 11. Datta SK, et al. 2010. Mucosal adjuvant activity of cholera toxin requires Th17 cells and protects against inhalation anthrax. Proc. Natl. Acad. Sci. U. S. A. 107:10638–10643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Davidsen J, et al. 2005. Characterization of cationic liposomes based on dimethyldioctadecylammonium and synthetic cord factor from M. tuberculosis (trehalose 6,6′-dibehenate)–a novel adjuvant inducing both strong CMI and antibody responses. Biochim. Biophys. Acta Biomembr. 1718:22–31 [DOI] [PubMed] [Google Scholar]
- 13. Dileepan T, et al. 2011. Robust antigen specific Th17 T cell response to group A streptococcus is dependent on IL-6 and intranasal route of infection. PLoS Pathog. 7:e1002252 doi:10.1371/journal.ppat.1002252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Domingues HS, Mues M, Lassmann H, Wekerle H, Krishnamoorthy G. 2010. Functional and pathogenic differences of Th1 and Th17 cells in experimental autoimmune encephalomyelitis. PLoS One 5:e15531 doi:10.1371/journal.pone.0015531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dong C. 2008. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat. Rev. Immunol. 8:337–348 [DOI] [PubMed] [Google Scholar]
- 16. Duffy D, et al. 2009. Immunological memory transferred with CD4 T cells specific for tuberculosis antigens Ag85B-TB10.4: persisting antigen enhances protection. PLoS One 4:e8272 doi:10.1371/journal.pone.0008272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Elvang T, et al. 2009. CD4 and CD8 T cell responses to the M. tuberculosis Ag85B-TB10.4 promoted by adjuvanted subunit, adenovector or heterologous prime boost vaccination. PLoS One 4:e5139 doi:10.1371/journal.pone.0005139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ghannam S, et al. 2011. CCL20 and β-defensin-2 induce arrest of human Th17 cells on inflamed endothelium in vitro under flow conditions. J. Immunol. 186:1411–1420 [DOI] [PubMed] [Google Scholar]
- 19. Goldberg M, et al. 2009. Synergism between tumor necrosis factor α and interleukin-17 to induce IL-23 p19 expression in fibroblast-like synoviocytes. Mol. Immunol. 46:1854–1859 [DOI] [PubMed] [Google Scholar]
- 20. Harrington LE, et al. 2005. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 6:1123–1132 [DOI] [PubMed] [Google Scholar]
- 21. Higgins SC, Jarnicki AG, Lavelle EC, Mills KHG. 2006. TLR4 mediates vaccine-induced protective cellular immunity to Bordetella pertussis: role of IL-17-producing T cells. J. Immunol. 177:7980–7989 [DOI] [PubMed] [Google Scholar]
- 22. Hirota K, et al. 2011. Fate mapping of IL-17-producing T cells in inflammatory responses. Nat. Immunol. 12:255–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Huang WT, Na L, Fidel PL, Schwarzenberger P. 2004. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. J. Infect. Dis. 190:624–631 [DOI] [PubMed] [Google Scholar]
- 24. Khader SA, et al. 2007. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat. Immunol. 8:369–377 [DOI] [PubMed] [Google Scholar]
- 25. Khader SA, et al. 2005. IL-23 compensates for the absence of IL-12p70 and is essential for the IL-17 response during tuberculosis but is dispensable for protection and antigen-specific IFN-γ responses if IL-12p70 is available. J. Immunol. 175:788–795 [DOI] [PubMed] [Google Scholar]
- 26. Kryczek I, et al. 2011. Human TH17 cells are long-lived effector memory cells. Sci. Transl. Med. 3:104ra100795. doi:10.1126/scitranslmed.3002949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kurschus FC, et al. 2010. Genetic proof for the transient nature of the Th17 phenotype. Eur. J. Immunol. 40:3336–3346 [DOI] [PubMed] [Google Scholar]
- 28. Lee YK, et al. 2009. Late developmental plasticity in the T helper 17 lineage. Immunity 30:92–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lexberg MH, et al. 2010. IFN-γ and IL-12 synergize to convert in vivo generated Th17 into Th1/Th17 cells. Eur. J. Immunol. 40:3017–3027 [DOI] [PubMed] [Google Scholar]
- 30. Lexberg MH, et al. 2008. Th memory for interleukin-17 expression is stable in vivo. Eur. J. Immunol. 38:2654–2664 [DOI] [PubMed] [Google Scholar]
- 31. Lin JS, Kummer LW, Szaba FM, Smiley ST. 2011. IL-17 contributes to cell-mediated defense against pulmonary Yersinia pestis infection. J. Immunol. 186:1675–1684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lindenstrøm T, et al. 2009. Tuberculosis subunit vaccination provides long-term protective immunity characterized by multifunctional CD4 memory T cells. J. Immunol. 182:8047–8055 [DOI] [PubMed] [Google Scholar]
- 33. Mangan PR, et al. 2006. Transforming growth factor-β induces development of the TH17 lineage. Nature 441:231–234 [DOI] [PubMed] [Google Scholar]
- 34. Mehling M, et al. 2010. Th17 central memory T cells are reduced by FTY720 in patients with multiple sclerosis. Neurology 75:403–410 [DOI] [PubMed] [Google Scholar]
- 35. Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. 1986. Two types of murine helper T cell clone. 1. Definition according to profiles of lymphokine activities and secreted proteins. J. Immunol. 136:2348–2357 [PubMed] [Google Scholar]
- 36. Mukasa R, et al. 2010. Epigenetic instability of cytokine and transcription factor gene loci underlies plasticity of the T helper 17 cell lineage. Immunity 32:616–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Muranski P, et al. 2011. Th17 cells are long lived and retain a stem cell-like molecular signature. Immunity 35:972–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nurieva R, Yang XXO, Chung Y, Dong C. 2009. Cutting edge: in vitro generated Th17 cells maintain their cytokine expression program in normal but not lymphopenic hosts. J. Immunol. 182:2565–2568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Olsen AW, Theisen M, Christensen D, Follmann F, Andersen P. 2010. Protection against Chlamydia promoted by a subunit vaccine (CTH1) compared with a primary intranasal infection in a mouse genital challenge model. PLoS One 5:e10768 doi:10.1371/journal.pone.0010768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Olsen AW, van Pinxteren LAH, Okkels LM, Rasmussen PB, Andersen P. 2001. Protection of mice with a tuberculosis subunit vaccine based on a fusion protein of antigen 85B and ESAT-6. Infect. Immun. 69:2773–2778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Park H, et al. 2005. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 6:1133–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pepper M, et al. 2010. Different routes of bacterial infection induce long-lived TH1 memory cells and short-lived TH17 cells. Nat. Immunol. 11:83–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Qiao D, et al. 2011. ESAT-6-and CFP-10-specific Th1, Th22 and Th17 cells in tuberculous pleurisy may contribute to the local immune response against Mycobacterium tuberculosis infection. Scand. J. Immunol. 73:330–337 [DOI] [PubMed] [Google Scholar]
- 44. Ruddy MJ, et al. 2004. Functional cooperation between interleukin-17 and tumor necrosis factor-α is mediated by CCAAT/enhancer-binding protein family members. J. Biol. Chem. 279:2559–2567 [DOI] [PubMed] [Google Scholar]
- 45. Schoenen H, et al. 2010. Cutting edge: Mincle is essential for recognition and adjuvanticity of the mycobacterial cord factor and its synthetic analog trehalose-dibehenate. J. Immunol. 184:2756–2760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Scriba TJ, et al. 2008. Distinct, specific IL-17- and IL-22-producing CD4+ T cell subsets contribute to the human anti-mycobacterial immune response. J. Immunol. 180:1962–1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sellge G, et al. 2010. Th17 cells are the dominant T cell subtype primed by Shigella flexneri mediating protective immunity. J. Immunol. 184:2076–2085 [DOI] [PubMed] [Google Scholar]
- 48. Steinfelder S, et al. 2011. Epigenetic modification of the human CCR6 gene is associated with stable CCR6 expression in T cells. Blood 117:2839–2846 [DOI] [PubMed] [Google Scholar]
- 49. van Dissel JT, et al. 2010. Ag85B-ESAT-6 adjuvanted with IC31 (R) promotes strong and long-lived Mycobacterium tuberculosis specific T cell responses in naive human volunteers. Vaccine 28:3571–3581 [DOI] [PubMed] [Google Scholar]
- 50. van Dissel JT, et al. 2011. Ag85B-ESAT-6 adjuvanted with IC31 (R) promotes strong and long-lived Mycobacterium tuberculosis specific T cell responses in volunteers with previous BCG vaccination or tuberculosis infection. Vaccine 29:2100–2109 [DOI] [PubMed] [Google Scholar]
- 51. Wei G, et al. 2009. Global mapping of H3K4me3 and H3K27me3 reveals specificity and plasticity in lineage fate determination of differentiating CD4+ T cells. Immunity 30:155–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Werninghaus K, et al. 2009. Adjuvanticity of a synthetic cord factor analogue for subunit Mycobacterium tuberculosis vaccination requires FcRγ-Syk-Card9-dependent innate immune activation. J. Exp. Med. 206:89–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wozniak KL, Hardison SE, Kolls JK, Wormley FL. 2011. Role of IL-17A on resolution of pulmonary C. neoformans infection. PLoS One 6:e17204 doi:10.1371/journal.pone.0017204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wozniak TM, Saunders BM, Ryan AA, Britton WJ. 2010. Mycobacterium bovis BCG-specific Th17 cells confer partial protection against Mycobacterium tuberculosis infection in the absence of gamma interferon. Infect. Immun. 78:4187–4194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yang XXO, et al. 2008. T helper 17 lineage differentiation is programmed by orphan nuclear receptors RORα and RORγ. Immunity 28:29–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ye P, et al. 2001. Interleukin-17 and lung host defense against Klebsiella pneumoniae infection. Am. J. Respir. Cell Mol. Biol. 25:335–340 [DOI] [PubMed] [Google Scholar]
- 57. Yoshida YO, et al. 2010. Essential role of IL-17A in the formation of a mycobacterial infection-induced granuloma in the lung. J. Immunol. 184:4414–4422 [DOI] [PubMed] [Google Scholar]
- 58. Yu H, et al. 2010. Chlamydia muridarum T cell antigens formulated with the adjuvant DDA/TDB induce immunity against infection that correlates with a high frequency of gamma interferon (IFN-γ)/tumor necrosis factor α and IFN-γ/interleukin-17 double-positive CD4+ T cells. Infect. Immun. 78:2272–2282 [DOI] [PMC free article] [PubMed] [Google Scholar]