Abstract
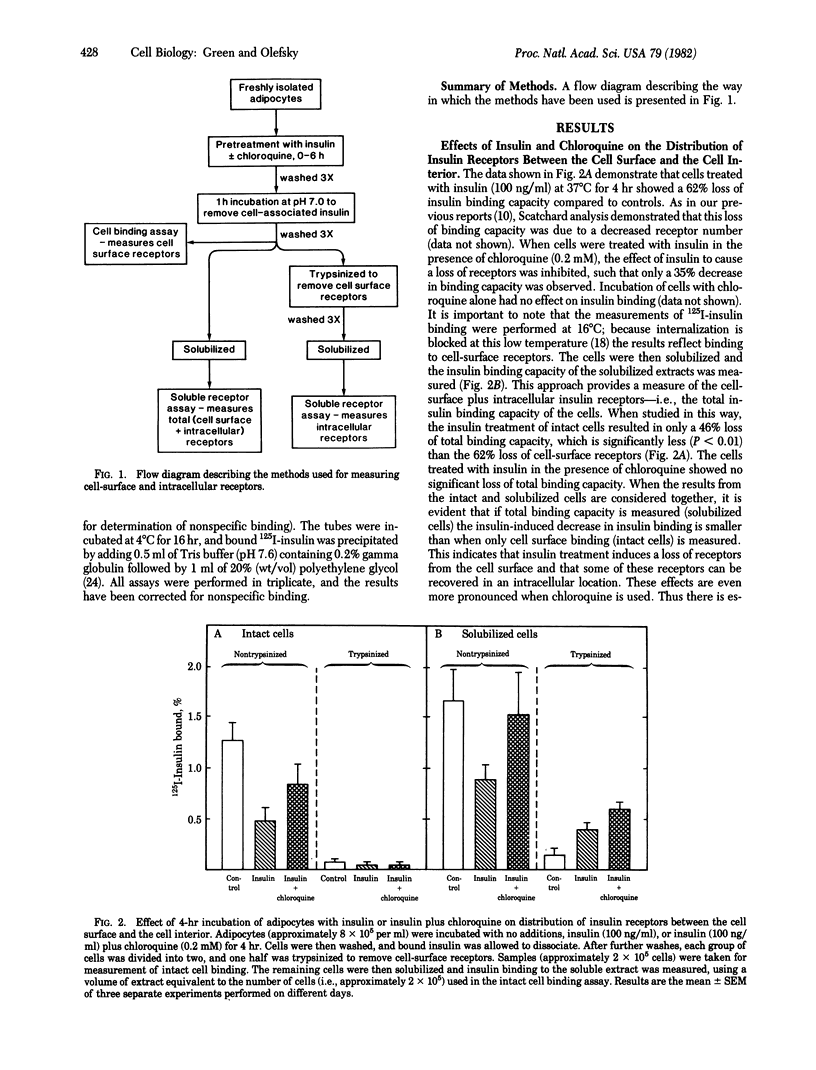
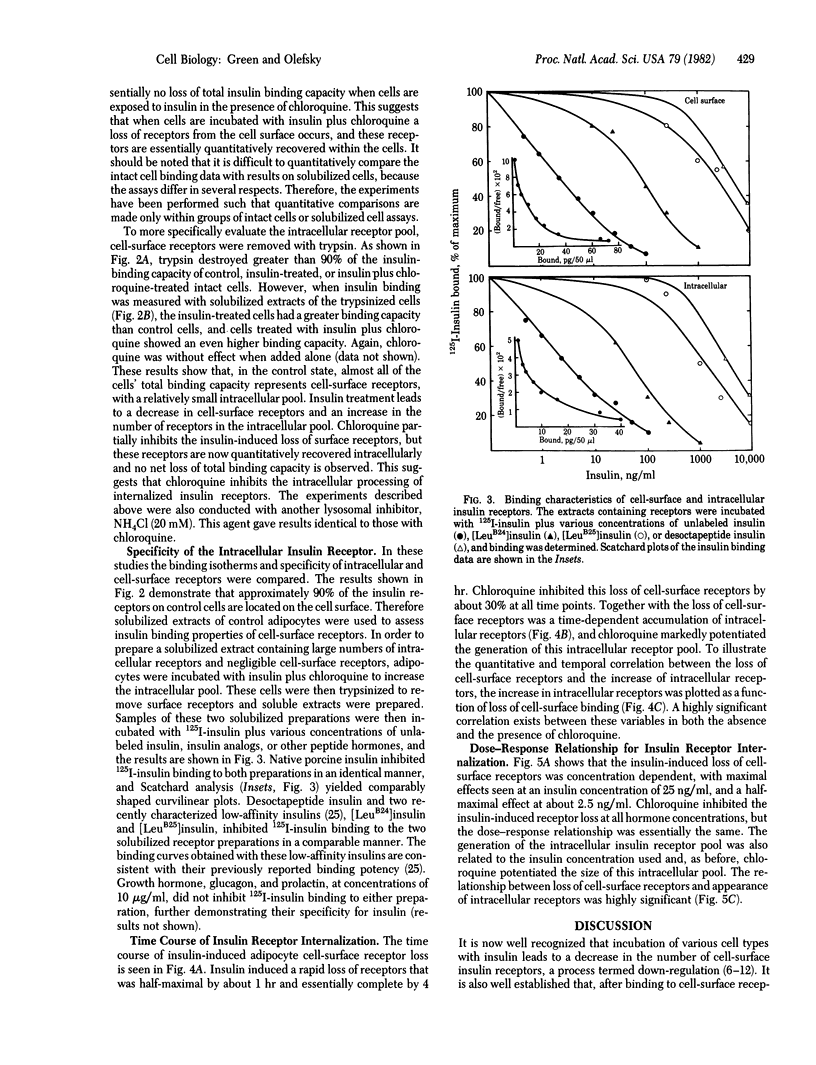
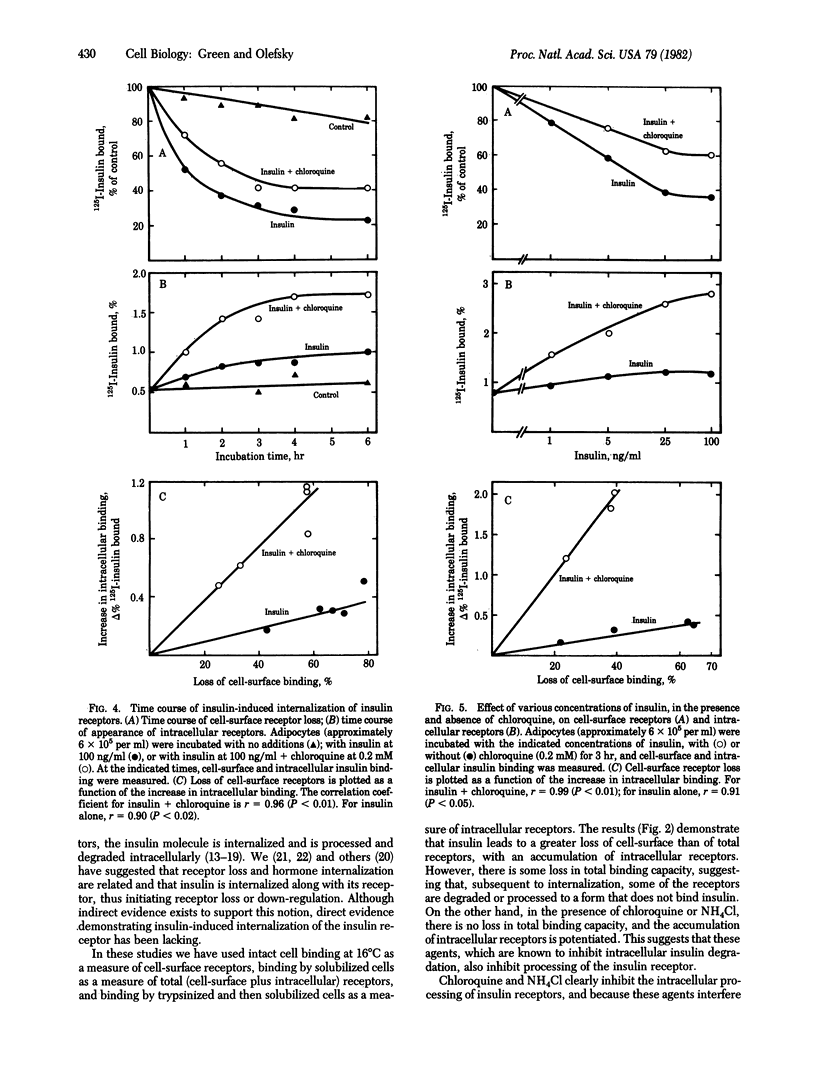
We have investigated the theory that the insulin-induced loss of insulin binding from adipocytes is due to internalization of insulin receptors. Cell-surface receptors were assessed by the binding capacity of intact cells at 16 degrees C. Total (i.e., cell-surface plus intracellular) receptors were assessed by solubilizing the cells in 1% Triton X-100 and then measuring binding by the solubilized extract. Intracellular receptors were measured by treating the cells with trypsin before solubilizing them. The trypsin treatment removed greater than 90% of the cell-surface binding, so that any significant binding by soluble extracts of these cells must represent intracellular receptors. Adipocytes were incubated with insulin (100 ng/ml) with or without chloroquine (0.2 mM) for 4 hr. Insulin alone resulted in a 62% loss of cell-surface receptors, but only a 46% loss of total receptors, and a 170% increase in intracellular receptors, suggesting that the lost cell-surface receptors were internalized, where some were degraded. Insulin in the presence of chloroquine resulted in a 34% loss of cell-surface receptors, but no loss of total receptors, and a 300% increase in intracellular receptors. Thus, in the presence of chloroquine receptors were internalized but not degraded. The loss of cell-surface receptors and appearance of intracellular receptors were time and dose dependent and were linearly related. These results demonstrate that insulin causes translocation of insulin receptors from the cell surface to the cell interior, where they can be degraded (or inactivated) by a chloroquine-sensitive process.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archer J. A., Gorden P., Roth J. Defect in insulin binding to receptors in obese man. Amelioration with calorie restriction. J Clin Invest. 1975 Jan;55(1):166–174. doi: 10.1172/JCI107907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin D., Jr, Prince M., Marshall S., Davies P., Olefsky J. M. Regulation of insulin receptors: evidence for involvement of an endocytotic internalization pathway. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5975–5978. doi: 10.1073/pnas.77.10.5975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackard W. G., Guzelian P. S., Small M. E. Down regulation of insulin receptors in primary cultures of adult rat hepatocytes in monolayer. Endocrinology. 1978 Aug;103(2):548–553. doi: 10.1210/endo-103-2-548. [DOI] [PubMed] [Google Scholar]
- Carpentier J. L., Gorden P., Freychet P., Le Cam A., Orci L. Lysosomal association of internalized 125I-insulin in isolated rat hepatocytes. Direct demonstration by quantitative electron microscopic autoradiography. J Clin Invest. 1979 Jun;63(6):1249–1261. doi: 10.1172/JCI109420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies P. J., Davies D. R., Levitzki A., Maxfield F. R., Milhaud P., Willingham M. C., Pastan I. H. Transglutaminase is essential in receptor-mediated endocytosis of alpha 2-macroglobulin and polypeptide hormones. Nature. 1980 Jan 10;283(5743):162–167. doi: 10.1038/283162a0. [DOI] [PubMed] [Google Scholar]
- Freychet P., Laudat M. H., Laudat P., Rosselin G., Kahn C. R., Gorden P., Roth J. Impairment of insulin binding to the fat cell plasma membrane in the obese hyperglycemic mouse. FEBS Lett. 1972 Sep 15;25(2):339–342. doi: 10.1016/0014-5793(72)80519-2. [DOI] [PubMed] [Google Scholar]
- Gavin J. R., 3rd, Roth J., Neville D. M., Jr, de Meyts P., Buell D. N. Insulin-dependent regulation of insulin receptor concentrations: a direct demonstration in cell culture. Proc Natl Acad Sci U S A. 1974 Jan;71(1):84–88. doi: 10.1073/pnas.71.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammons G. T., Jarett L. Lysosomal degradation of receptor-bound 125I-labeled insulin by rat adipocytes: its characterization and dissociation from the short-term biologic effects of insulin. Diabetes. 1980 Jun;29(6):475–486. doi: 10.2337/diab.29.6.475. [DOI] [PubMed] [Google Scholar]
- Kahn C. R., Neville D. M., Jr, Roth J. Insulin-receptor interaction in the obese-hyperglycemic mouse. A model of insulin resistance. J Biol Chem. 1973 Jan 10;248(1):244–250. [PubMed] [Google Scholar]
- Kobayashi M., Olefsky J. M. Effect of experimental hyperinsulinemia on insulin binding and glucose transport in isolated rat adipocytes. Am J Physiol. 1978 Jul;235(1):E53–E62. doi: 10.1152/ajpendo.1978.235.1.E53. [DOI] [PubMed] [Google Scholar]
- Kosmakos F. C., Roth J. Insulin-induced loss of the insulin receptor in IM-9 lymphocytes. A biological process mediated through the insulin receptor. J Biol Chem. 1980 Oct 25;255(20):9860–9869. [PubMed] [Google Scholar]
- Livingston J. N., Purvis B. J., Lockwood D. H. Insulin induced changes in insulin binding and insulin-sensitivity of adipocytes. Metabolism. 1978 Dec;27(12 Suppl 2):2009–2014. doi: 10.1016/s0026-0495(78)80017-1. [DOI] [PubMed] [Google Scholar]
- Livingston J. N., Purvis B. J., Lockwood D. H. Insulin-dependent regulation of the insulin-sensitivity of adipocytes. Nature. 1978 Jun 1;273(5661):394–396. doi: 10.1038/273394a0. [DOI] [PubMed] [Google Scholar]
- Marshall S., Olefsky J. M. Effects of insulin incubation on insulin binding, glucose transport, and insulin degradation by isolated rat adipocytes. Evidence for hormone-induced desensitization at the receptor and postreceptor level. J Clin Invest. 1980 Oct;66(4):763–772. doi: 10.1172/JCI109914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall S., Olefsky J. M. Effects of lysosomotropic agents on insulin interactions with adipocytes. Evidence for a lysosomal pathway for insulin processing and degradation. J Biol Chem. 1979 Oct 25;254(20):10153–10160. [PubMed] [Google Scholar]
- McKanna J. A., Haigler H. T., Cohen S. Hormone receptor topology and dynamics: morphological analysis using ferritin-labeled epidermal growth factor. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5689–5693. doi: 10.1073/pnas.76.11.5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott D. M., Howard B. V., Bennett P. H. Stoichiometric binding and regulation of insulin receptors on human diploid fibroblasts using physiologic insulin levels. J Biol Chem. 1979 Sep 25;254(18):8762–8767. [PubMed] [Google Scholar]
- Olefsky J. M. Decreased insulin binding to adipocytes and circulating monocytes from obese subjects. J Clin Invest. 1976 May;57(5):1165–1172. doi: 10.1172/JCI108384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olefsky J. M., Kobayashi M., Chang H. Interactions between insulin and its receptors after the initial binding event. Functional heterogeneity and relationships to insulin degradation. Diabetes. 1979 May;28(5):460–471. doi: 10.2337/diab.28.5.460. [DOI] [PubMed] [Google Scholar]
- RODBELL M. METABOLISM OF ISOLATED FAT CELLS. I. EFFECTS OF HORMONES ON GLUCOSE METABOLISM AND LIPOLYSIS. J Biol Chem. 1964 Feb;239:375–380. [PubMed] [Google Scholar]
- Schlessinger J., Shechter Y., Cuatrecasas P., Willingham M. C., Pastan I. Quantitative determination of the lateral diffusion coefficients of the hormone-receptor complexes of insulin and epidermal growth factor on the plasma membrane of cultured fibroblasts. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5353–5357. doi: 10.1073/pnas.75.11.5353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K., Kono T. Internalization and degradation of fat cell-bound insulin. Separation and partial characterization of subcellular vesicles associated with iodoinsulin. J Biol Chem. 1979 Oct 10;254(19):9786–9794. [PubMed] [Google Scholar]
- Tager H., Thomas N., Assoian R., Rubenstein A., Saekow M., Olefsky J., Kaiser E. T. Semisynthesis and biological activity of porcine [LeuB24]insulin and [LeuB25]insulin. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3181–3185. doi: 10.1073/pnas.77.6.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terris S., Hofmann C., Steiner D. F. Mode of uptake and degradation of 125I-labelled insulin by isolated hepatocytes and H4 hepatoma cells. Can J Biochem. 1979 Jun;57(6):459–468. doi: 10.1139/o79-059. [DOI] [PubMed] [Google Scholar]



