Abstract
SecA2 is an ATPase present in some pathogenic Gram-positive bacteria, is required for translocation of a limited set of proteins across the cytosolic membrane, and plays an important role in virulence in several bacteria, including mycobacteria that cause diseases such as tuberculosis and leprosy. However, the mechanisms by which SecA2 affects virulence are incompletely understood. To investigate whether SecA2 modulates host immune responses in vivo, we studied Mycobacterium marinum infection in two different hosts: an established zebrafish model and a recently described mouse model. Here we show that M. marinum ΔsecA2 was attenuated for virulence in both host species and SecA2 was needed for normal granuloma numbers and for optimal tumor necrosis factor alpha response in both zebrafish and mice. M. marinum ΔsecA2 was more sensitive to SDS and had unique protrusions from its cell envelope when examined by cryo-electron tomography, suggesting that SecA2 is important for bacterial cell wall integrity. These results provide evidence that SecA2 induces granulomas and is required for bacterial modulation of the host response because it affects the mycobacterial cell envelope.
INTRODUCTION
Although Mycobacterium tuberculosis was identified as the causative agent of tuberculosis in humans over 100 years ago, many of the mechanisms leading to the success of this pathogen are still unclear. M. tuberculosis has infected one-third of all humans (19, 23), yet only 5 to 10% of infected people develop active disease. Nonetheless, humans are the only reservoir for M. tuberculosis, and individuals with active disease are key to the wide dissemination of the organism. Therefore, it appears that the bacterial strategy to fill its ecological niche is to limit severe pathology to those few people who will act as important vectors of dissemination. It has been known for some time that these individuals have compromised cellular immunity due to old age, malnutrition, or intercurrent illness. Thus, the ability of the vast majority of individuals to mount an immune response that contains the infection and limits disease is key to the success of M. tuberculosis as a human pathogen. Identifying host and pathogen factors that achieve this balance of susceptibility versus immunity to M. tuberculosis is of great basic biological interest and is important for efforts aimed at eradicating this disease.
M. marinum is a model species for M. tuberculosis infection that causes a chronic granulomatous disease in ectotherms that shares many features with tuberculosis (7). Less commonly, it is able to cause localized disease in humans in the form of skin lesions (22) and disseminated disease in immunocompromised humans (28, 36). M. marinum is the closest genetic relative to the M. tuberculosis complex and shares virulence factors with M. tuberculosis (40). Additionally, zebrafish and mice infected with M. marinum develop caseating granulomas that are very similar to those found in active human pulmonary disease (3, 39, 41). Granuloma formation, maturation, and maintenance are complex processes that are thought to benefit the host by containing the bacteria but may also benefit the bacteria by creating a niche for growth (9, 11, 16). Because of the genetic and pathological similarities between M. tuberculosis and M. marinum infections, as well as the fact that both can cause both primary progressive and chronic disease, it is thought that the two organisms share similar mechanisms of establishing disease and modulating the host immune response.
Several protein secretion systems are required for growth and virulence of mycobacteria (10). SecA2, found in all mycobacteria and some Gram-positive bacteria (31), is required for the secretion of several proteins, including the antioxidant enzymes SodA and KatG (2, 14). Mutation of secA2 in M. tuberculosis has revealed a role for SecA2 in the growth, survival, and virulence of the pathogen in vivo prior to the onset of adaptive immunity (2). In addition, studies in murine bone marrow-derived macrophages (BMDMs) suggest that SecA2 is required to inhibit the immune response (21). Additional studies have shown that loss of SecA2 enhances priming of antigen-specific T cells in vivo, and vaccination with a ΔsecA2 mutant consequently increases protection against M. tuberculosis infection in mice and guinea pigs (15, 30), suggesting that SecA2 plays a role in modulating host immunity.
The mechanism of immune modulation by SecA2 in vivo is not well understood, either from the perspective of host immunity or in terms of how SecA2 affects bacterial physiology. We have studied M. marinum infection in both zebrafish and mice to investigate this question. Our data suggest that SecA2 modulates adaptive immunity to promote granuloma stability, perhaps through induction of tumor necrosis factor alpha (TNF-α). SecA2 also is required for normal bacterial cell envelope function and appearance, suggesting that this may be the mechanism through which it modulates host immune responses.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Wild-type M. marinum strain M and subsequent mutant strains used in this study were derived from a human clinical isolate (ATCC BAA-535). The ΔkasB strain has been described previously (13). The ΔsecA2 strain, an M. marinum secA2::Kanr mutant with an insertion mutation in the MMAR_2698 gene, was constructed using a previously published double-selection strategy (12). Flanking upstream sequence was amplified using PCR with genomic DNA template and primers SecA2U5 (5′-TAATACTAGTTGAACAGCACATTCAGTC-3′) and SecA2U3 (5′-TATAGATATCTAGGCCAGGTTTGATCGG-3′), where genomic sequences are italicized. Likewise, downstream sequence was amplified using primers SecA2D5 (5′-TAATGATATCTGGCACCGATATCCGGTT-3′) and SecA2D3 (5′-TTAATGCATACTAGTACAGACCCCAGATCAGAAACG-3′). These two flanking sequences were sequentially cloned into pCR 2.1-TOPO (Invitrogen). A kanamycin resistance gene derived from pUC4k (GE Healthcare) was then inserted between the upstream and downstream segments using EcoRV. This entire piece containing the upstream-Kanr-downstream segment was finally cloned into pLYG304 (12). The mutant was then isolated after double selection, and the mutated genomic region was PCR amplified and tested with restriction digestion (see Fig. S1 in the supplemental material) and confirmed by DNA sequencing. The ΔsecA2 strain was complemented with secA2 (MMAR_2698) to produce the SA2comp strain, by integration of this gene and the mycobacterial hsp60 promoter into the ΔsecA2 chromosomal attB site using the pMV306.hyg shuttle vector (35). For experiments, strains were grown to mid-log phase at 30°C and 105 rpm in Middlebrook 7H9 broth (Difco) supplemented with 0.2% glycerol, 0.05% Tween 80, and 10% Middlebrook albumin-dextrose-catalase enrichment (BBL) or at 32°C on Middlebrook 7H10 agar plates (Difco) supplemented with 0.5% glycerol and 10% Middlebrook oleic acid-albumin-dextrose-catalase enrichment (BBL). Medium was supplemented with 10% sucrose, kanamycin (30 μg/ml), zeocin (50 μg/ml), or hygromycin (50 μg/ml) as appropriate.
Macrophage infections.
Murine BMDMs were cultured from C57BL/6 wild-type mice and infected with M. marinum as described previously (24). For all experiments, 4 × 105 BMDMs were seeded in 12-well plates overnight and then infected at a multiplicity of infection (MOI) of 1. For activated-macrophage experiments, BMDMs were incubated with recombinant mouse gamma interferon (IFN-γ; 30 ng/ml) for 24 h before infection and included in the medium for 4 h postinfection. The TNF-α concentration was measured by enzyme-linked immunosorbent assay (eBioscience) at 24 h postinfection.
Zebrafish Infections.
Wild-type zebrafish (AB strain) were purchased from the Zebrafish International Resource Center (Eugene, OR) and maintained in static tanks under conditions and standards specified by the IACUC at Genentech, Inc., and outlined previously (8). For infections, fish were anesthetized with 0.015% tricaine methanesulfonate (MS-222) for 3 to 5 min and then injected intraperitoneally with 104 M. marinum bacteria in 50 μl of phosphate-buffered saline (PBS) or 50 μl of PBS alone for controls. To assay the bacterial load in the whole fish, fish were euthanized in 0.05% MS-222 for 20 min, bathed in 70% ethanol, and then homogenized in 0.1% Triton X-100 (1.5 ml) using disposable tissue grinders (VWR 47732-450). Each fish homogenate (250 μl) was decontaminated of normal flora using BBL Mycoprep reagent (BD). Tenfold serial dilutions were plated on 7H10 plates for enumeration of colony forming units (CFU). Viability of the M. marinum strains was unaffected by incubation with 0.1% Triton X-100 (1 h) or Mycoprep reagent (25 min). For histopathological analysis, fish were euthanized at 7 days postinfection, fixed in 10% formalin for 7 days, and then processed whole for paraffin-embedded histology. Serial pairs of parasagittal sections with maximal representation of pancreatic and liver tissue were stained by hematoxylin-eosin (H&E) and Ziehl-Neelsen stains. Total pancreatic foci of three or more clustered acid-fast bacteria were enumerated, as were those clusters specifically associated with recognizable granulomas.
Mouse infections.
All studies were compliant with the ILAR guidelines and were approved by the IACUC at Genentech, Inc. Twelve-week-old, female C57BL/6 mice (Jackson Laboratory-West) and Rag2-knockout mice (B6.129S6-Rag2tm1Fwa N12; Taconic) were inoculated with 107 M. marinum bacteria in 100 μl of PBS via injection into the tail vein. For analysis of visible tail lesions, the lengths of individual lesions were measured and combined for each tail. For analysis of bacterial load in the tail, tails were severed at the tail base, weighed, cut into 5-mm pieces, and homogenized in 3 ml 0.1% Triton X-100 in Dulbecco's modified Eagle's medium, using an AHS 200 homogenizer (VWR) with sawtooth adaptors (10 × 105 mm; Troemner). Tenfold serial dilutions of homogenates were plated on 7H10 plates for enumeration of CFU, which were calculated as the number of CFU per gram of tissue. For cytokine analysis, tails were severed from the tail base and immediately placed on dry ice. While on dry ice, tails were cut into 5-mm pieces, frozen in liquid nitrogen, and pulverized with a biopulverizer (Biospec Products) that was chilled in liquid nitrogen. The tissue was incubated on ice for 1.5 h in 1 ml of PBS supplemented with complete, EDTA-free, protease inhibitor cocktail (Roche) and then centrifuged twice at 20,000 × g and 4°C for 20 min. Supernatants were collected for Bradford analysis (Bio-Rad) to determine total protein content for normalization of each tail sample and Luminex analysis (Bio-Rad) to quantify cytokine amounts in each tail. For histology, tails were fixed in 10% formalin, minimally decalcified in Immunocal decalcifier (Decal Chemical Corp.), trimmed into at least five 3-μm cross sections, embedded in paraffin, sectioned, and stained by H&E and Ziehl-Neelsen stains. Additionally, immunohistochemical staining for F4/80 (macrophages), CD3 (T cells), and B220 (B cells) was performed.
Quantitative RT-PCR analysis.
For analysis of secA2 expression levels in M. marinum, cultures were grown to mid-log phase in 7H9 broth. Cultures were incubated for 5 min with lysozyme and RNA was isolated using an RNeasy kit (74104; Qiagen). A second DNase treatment was performed per the manufacturer's instructions (M0303S; New England BioLabs). Quantitative reverse transcription-PCR (RT-PCR) was performed using a QuantiFast SYBR Green PCR kit (Qiagen 204054), the gene-specific primers listed in Table 1, and an ABI 7500 RT-PCR system. The fold induction in the ΔsecA2 and SA2comp strains was calculated relative to that of the wild-type bacteria, using the ΔΔCT (CT is threshold cycle) model with SodA as the reference gene and no template and no reverse transcriptase reactions for controls. For zebrafish cytokine analysis, previously published primer sets were used (17, 32, 42, 43). Whole fish were euthanized in 0.05% MS-222 for 20 min and then homogenized in 1 ml TRIzol (Invitrogen) per 50 mg of tissue. RNA was extracted from tissues according to product instructions and stored at −80°C. cDNA was synthesized using Anchored Oligo(dT)20 primer (12577-011; Invitrogen) and Superscript III reverse transcriptase (18080-044; Invitrogen). Quantitative RT-PCR was performed as stated above. Fold induction of experimental groups was calculated relative to that of the PBS control group, using the ΔΔCT model with β-actin as the reference gene and no template and no reverse transcriptase reactions for controls.
Table 1.
Primer sets used to quantitate gene expression by quantitative RT-PCRa
| Host and cytokine or gene | Forward primer (5′–3′) | Reverse primer (5′–3′) | Reference or source |
|---|---|---|---|
| Zebrafish | |||
| β-Actin | TGCTGTTTTCCCCTCCATTG | TTCTGTCCCATGCCAACCA | Rojo et al. (32) |
| IL-1β | TGGACTTCGCAGCACAAAATG | GTTCACTTCACGCTCTTGGATG | Watzke et al. (43) |
| IL-12 | TCTAACTTCAGCGCAGTGGA | TGCGGTGGTGTAGTGAGTG | Ito et al. (17) |
| IFN-γ | CTTTCCAGGCAAGAGTGCAGA | TCAGCTCAAACAAAGCCTTTCGCT | Vojtech et al. (42) |
| TNF-α | GATGGTGTCCAGGAGGAAAG | CAGAGTTGTATCCACCTGTT | Ito et al. (17) |
| M. marinum | |||
| secA2 | GTGGGTCAACTCGTCAAA | CTCTTCGGAGTACAGATCGA | This work |
| sodA | ACAAGCTGCTGATCTTCCAGGTCT | TGGCGAAGTCGACCTTCACATTCT | This work |
These primers were used for quantitative RT-PCR using QuantiFast SYBR Green. Sequences for all zebrafish cytokine primers were obtained from published studies, while those for M. marinum were developed here.
SDS sensitivity assay.
M. marinum (2.5 × 105 ml−1) grown to mid-log phase in 7H9 broth was inoculated into 7H9 containing sodium dodecyl sulfate (SDS; 0.01, 0.05, 0.1, and 0.25%, vol/vol) and incubated for 20 h at 30°C and 105 rpm. CFU were enumerated on 7H10 agar plates.
Cryo-electron tomography.
M. marinum grown to mid-log phase in 7H9 broth was prepared for microscopy as described previously (5).
Statistical analyses.
Significance was determined using one-way analysis of variance (ANOVA) testing across all the samples analyzed in a given experiment. When calculated F values were greater than critical F values in these analyses, Fisher's least-significant-difference post hoc tests were applied to determine pairwise significant differences. Significance is denoted in the figures (*, P < 0.05; **, P < 0.01).
RESULTS
SecA2 is needed for virulence and growth in zebrafish.
Previous studies have shown that M. tuberculosis lacking SecA2 is partially attenuated for growth and virulence in a mouse model of infection (2, 21). To investigate the role of SecA2 in modulating the host immune response, we created an M. marinum strain with secA2 disrupted by a kanamycin-resistance cassette (ΔsecA2) via homologous recombination (12, 29) (Fig. 1A). We verified that the ΔsecA2 strain contained the expected gene-disrupted secA2 by PCR amplifying the mutated region with primers annealing outside the regions found in the knockout construct, diagnostically checking the product with restriction enzyme digestion (see Fig. S1 in the supplemental material), and sequencing this product. To complement the mutant, secA2 driven by the mycobacterial hsp60 promoter was stably inserted into the ΔsecA2 strain's genomic DNA at the attB site (35). The ΔsecA2 plus secA2 complemented strain (SA2comp) grown in 7H9 broth expressed 2.25-fold more secA2 mRNA than wild-type M. marinum, whereas secA2 was not detected in the ΔsecA2 strain by quantitative RT-PCR (Fig. 1B), indicating that the SecA2 protein indeed is not present in the mutant strain and is reconstituted in the complemented strain. When grown in liquid medium, the ΔsecA2 strain grew similarly to the wild-type and SA2comp strains (Fig. 1C), consistent with the reported in vitro growth of the M. tuberculosis ΔsecA2 strain (2). ΔsecA2 also grew similarly to the wild-type and SA2comp strains within isolated BMDMs, regardless of whether the macrophages were in an activated state (Fig. 1D). This lack of defects in vitro differs from mouse macrophage infections with the M. tuberculosis ΔsecA2 strain (21, 38) and likely reflects differences in bacterial physiology between M. marinum and M. tuberculosis. For instance, in the 96-hour time course of our macrophage infections with M. marinum here (Fig. 1D) and previously (18, 24), we measure 4 to 10 doublings of M. marinum, whereas 2 doublings have usually been monitored in the M. tuberculosis ΔsecA2 studies (21, 38).
Fig 1.
The ΔsecA2 strain is attenuated for virulence and growth in zebrafish. (A) Schematic diagram of construction of the M. marinum ΔsecA2 strain. Approximately 1.6 kb of secA2 was replaced with a kanamycin-resistance cassette by homologous recombination. (B) Quantitative RT-PCR analyses of secA2 mRNA expression in the M. marinum ΔsecA2 and SA2comp strains relative to wild type. 7H9 broth cultures grown to mid-log phase were analyzed. (C) Growth of the M. marinum cultures in 7H9 broth. The optical density (OD) at 600 nm was measured over time. The ratio of the generation time of the ΔsecA2 and SA2comp strains to that of wild type averaged 0.96 ± 0.09 (range, 0.92 to 1.09) and 1.06 ± 0.1 (range, 0.96 to 1.16), respectively, in four independent experiments. SEM values are given. (D) Unactivated (top) or activated (bottom) BMDMs from C57BL/6 mice were infected with M. marinum wild type, ΔsecA2, or SA2comp at an MOI of 1, and intracellular bacterial growth was determined by enumeration of CFU from macrophage lysates at various times postinfection. Shown are data from one representative experiment, in which duplicate samples were analyzed. (E) Zebrafish were infected with various M. marinum strains via intraperitoneal injection in three independent experiments, and their survival was observed over time (wild type, n = 10; ΔsecA2 strain, n = 12; and SA2comp strain, n = 11). (F) Bacterial burden in the whole fish was quantified by enumeration of CFU at multiple times postinfection. The values represent the mean ± SEM (n = 3 per time point for wild type; n = 6 per time point for the ΔsecA2 and SA2comp strains). Data represent two independent experiments with similar results for the ΔsecA2 and SA2comp strains. One of these experiments also analyzed wild type. The results for the ΔsecA2 and SA2comp strains are significantly different (*, P < 0.03).
To assess whether SecA2 is required for virulence of M. marinum in vivo, we infected adult zebrafish. All zebrafish infected with 104 wild-type bacilli died at between 12 and 25 days postinfection, as did fish infected with the SA2comp strain (Fig. 1E). At the time of death, the abdomens of the zebrafish were visibly hemorrhaging, indicating an inflammatory response. However, no hemorrhaging was detected in zebrafish infected with 104 ΔsecA2 bacilli, and all fish survived until 38 weeks postinfection, at which point they were euthanized. Thus, SecA2 in M. marinum is required for virulence in zebrafish. To determine whether the ΔsecA2 strain fails to kill the fish because it is more easily cleared by the host, CFU were enumerated from the whole fish at multiple times postinfection (Fig. 1F). The wild-type and SA2comp strains showed steady growth throughout the first 11 days of infection. In contrast, the ΔsecA2 strain was comparable to the wild-type and SA2comp strains in growth until 7 days postinfection but was cleared thereafter. These data suggest that SecA2 is required for continued growth and survival of mycobacteria in vivo, and this requirement is perhaps related to the onset of acquired immunity. These kinetics are consistent with the findings of a previous study in which adaptive immunity effects began to appear at between 8 and 11 days postinfection, when a similar dose of M. marinum was used to infect adult zebrafish (39).
SecA2 markedly enhances the inflammatory response in zebrafish.
Histopathological analyses of the liver and pancreas were performed at 7 days postinfection, the time point at which the bacterial burdens were indistinguishable (Fig. 1F). While zebrafish infected with both the SA2comp and wild-type strains had equivalent numbers of granulomas in the liver and pancreas per zebrafish (6.6 ± 3.23 [standard error of the mean {SEM}] for the SA2comp strain and 6 ± 0.89 for wild type), granulomas were rarely detected in ΔsecA2 mutant-infected zebrafish. In fact, granulomas were found in only 1 of 10 ΔsecA2 mutant-infected fish. In addition, ΔsecA2 mutant-infected fish had less overall inflammation, although the quality of the inflammation was similar (Fig. 2). Thus, the ΔsecA2 strain induced less inflammation and fewer granulomas.
Fig 2.
SecA2 is needed for normal granuloma numbers in zebrafish. At 7 days postinfection of zebrafish with different M. marinum strains in two independent experiments, histopathological analysis was performed by H&E and Ziehl-Neelsen (acid-fast) staining. The granuloma burden per fish was enumerated from liver and pancreas sections.
SecA2 is important for disease and granuloma stability in mice.
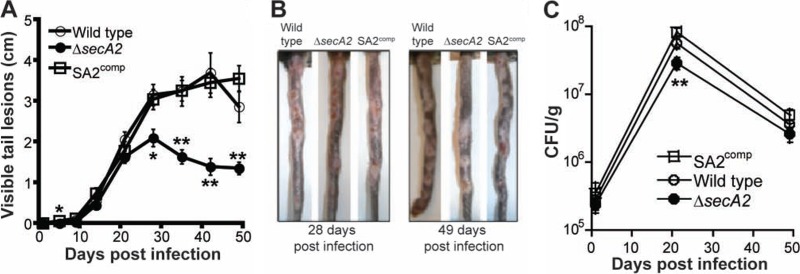
To determine whether SecA2 had a similar role in a distant host, mice were infected with M. marinum as previously described (3). When C57BL/6 mice were infected with 107 wild-type, ΔsecA2, or SA2comp bacteria via the tail vein, disease was localized to cooler areas of the mouse, like the tail, ears, paws, and nose, presumably because the optimal growth temperature of M. marinum is ∼32°C. The burden of visible granulomatous tail lesions in each mouse was quantified over 7 weeks by measuring and combining the length of all lesions on each tail (Fig. 3A). Lesions began to appear at about 9 days postinfection in all infected mice. For the first 3 weeks, the disease caused by the ΔsecA2 strain was indistinguishable from that caused by the wild-type and SA2comp strains (Fig. 3A). However, after 3 weeks, ΔsecA2 mutant-infected mice had less disease. In fact, lesions caused by the ΔsecA2 strain but not the wild-type or SA2comp strain seemed to resolve (Fig. 3B). Hence, the ΔsecA2 strain is able to cause disease early in infection but is attenuated for virulence after 3 weeks. Histopathological analyses of individual tail lesions were performed at 2 and 7 weeks postinfection to analyze the granuloma architecture (data not shown and Fig. 4). There was no difference at either time point in the character of the inflammation of the lesions induced by the wild-type, ΔsecA2, or SA2comp strain. Granulomatous structures rich in macrophages and T cells, with occasional B cells, were observed at both time points with H&E staining (data not shown and Fig. 4) as well as immunohistochemical staining (data not shown), regardless of the infecting bacterial genotype. Thus, in mice as well as zebrafish, SecA2 appeared to control the extent rather than the quality of the host inflammatory response.
Fig 3.
SecA2 is needed for virulence and stable granulomas in mice. (A to C) C57BL/6 mice were infected via injection of different M. marinum strains into the tail vein in two independent experiments. (A) The lengths of all visible lesions on each tail were combined at each time point, and the averaged values represent the mean ± SEM (n = 18 per group). Significant differences between the ΔsecA2 and SA2comp strains are noted (*, P < 0.05; **, P < 0.01). (B) Representative images of tails from infected mice at 4 and 7 weeks postinfection. (C) Bacterial burden in the whole tail was quantified by enumeration of CFU at multiple time points postinfection. Growth of the ΔsecA2 strain is significantly different (**, P < 0.003) from growth of SA2comp at 21 days.
Fig 4.
Granulomas in mice are histopathologically similar. Mice were infected via injection of 107 M. marinum wild-type, ΔsecA2, or SA2comp bacteria into the tail vein. Cross sections of three infected mouse tails per experimental group were analyzed at 7 weeks postinfection by H&E and Ziehl-Neelsen (acid-fast) staining.
The growth of the mycobacterial strains in the mouse tail was measured by determination of the numbers of CFU over a 7-week period (Fig. 3C). Growth of the M. marinum strains peaked at 21 days. The bacterial numbers then declined, likely due to the onset of adaptive immunity (3). Although the ΔsecA2 strain's bacterial load was significantly less than that of both the wild type (P < 0.04) and the complemented strain (P = 0.0001) at the peak of infection (Fig. 3C), the difference was small. While there was significantly more sustained inflammation in the lesions caused by the wild-type and SA2comp strains (Fig. 3B), the ΔsecA2 bacteria were not cleared significantly more than wild type after 4 more weeks of infection. These data suggest that in mice, M. marinum SecA2 is primarily required for maintenance of inflammatory lesions rather than for bacterial growth.
SecA2 is required for robust induction of TNF-α in both zebrafish and mice.
Studying the immune response in zebrafish in detail is challenging due to the limited number of reagents available to detect proteins important in immunity and inflammation. Fortunately, many of the key cytokines and immune cells found in mammalian systems are genetically conserved (25, 37), allowing us to analyze the expression of various target genes in response to infection. Using quantitative RT-PCR, expression of the mRNA of several cytokines was quantified from infected fish at 3, 7, and 11 days postinfection (Fig. 5). While there were no differences in cytokine induction at 3 days postinfection, the ΔsecA2 strain induced less TNF-α and interleukin-1β (IL-1β) at 7 days postinfection, even though the bacterial load was similar at this point. By 11 days postinfection, the wild-type and SA2comp strains induced greater than 4-fold more TNF-α and IL-1β than the ΔsecA2 strain, while they induced equivalent amounts of IFN-γ and IL-12. There was a marked increase in IFN-γ between 7 and 11 days postinfection, consistent with the onset of adaptive immunity, but this was unaffected by the presence of SecA2. Thus, SecA2 makes an important contribution to the increase in inflammatory macrophage cytokines (TNF-α, IL-1β) during M. marinum infection in zebrafish that is not paralleled by an increase in IFN-γ or IL-12, cytokines primarily involved in adaptive immunity (6).
Fig 5.
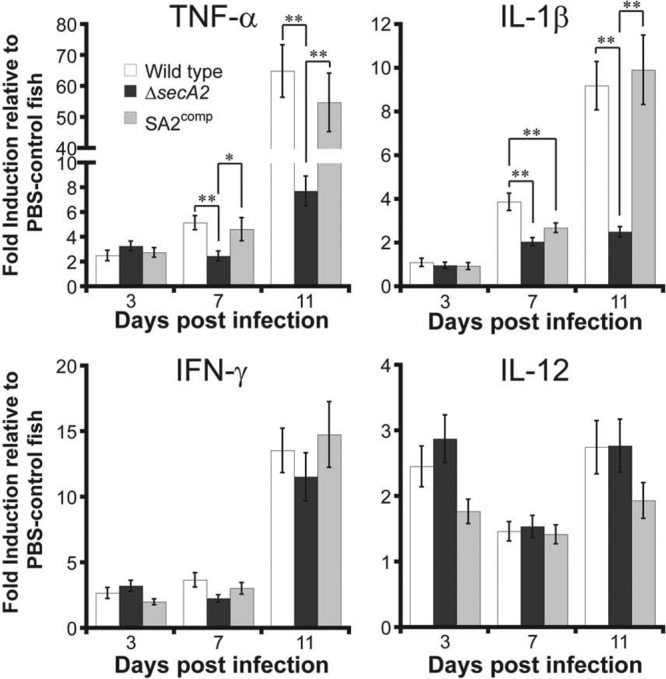
SecA2 promotes induction of TNF-α and IL-1β in zebrafish. Cytokine mRNA expression from whole fish infected with different M. marinum strains or a buffer control was measured by quantitative RT-PCR using the gene-specific primers listed in Table 1. Values represent average fold induction relative to the PBS-treated control fish ± SEM (n = 8 per group per time point for all groups, except n = 6 for the PBS-treated control set). Data averaged from two independent experiments are shown. ANOVA analyses found statistically significant differences between groups for TNF-α and IL-1β (*, P < 0.05; **, P < 0.01).
At 3 and 6 weeks postinfection, cytokine protein profiles were obtained from the tails of uninfected naïve mice and mice infected with wild-type, ΔsecA2, or SA2comp bacteria (Fig. 6). At 3 weeks postinfection, the ΔsecA2 strain induced less TNF-α, IFN-γ, and IL-17 and more IL-10 than wild-type or SA2comp M. marinum. Thus, in both fish and mice, the infection with the ΔsecA2 strain induced less TNF-α at a point of infection where the bacterial burden was comparable to that of wild-type-infected animals. IFN-γ production was diminished in mice but not in fish, whereas IL-1β production was diminished in fish but not in mice, suggesting that these two hosts have both unique and overlapping cytokine responses to M. marinum infection. TNF-α secretion by mouse macrophages infected with ΔsecA2 or wild-type strains was similar (see Fig. S2 in the supplemental material), suggesting that the SecA2-dependent difference in TNF-α seen in vivo is not the result of differences in direct interaction with macrophages but reflects more complex immune cell interactions during infection.
Fig 6.
SecA2 is needed for early induction of TNF-α, IFN-γ, and IL-17 in mice. At 3 and 6 weeks postinfection, cytokine levels from the whole tail of each experimental or naïve uninfected animal were quantified by Luminex analysis. Values represent the average amount of cytokine detected (pg/ml) divided by the total amount of protein present in each tail suspension (mg/ml) ± SEM (n = 6 per experimental group per time point). Data averaged from two independent experiments are shown. Significant differences in TNF-α, IFN-γ, and IL-17 were found at 3 weeks between infection with the M. marinum ΔsecA2 strain and infection with either the wild-type or SA2comp strain (*, P < 0.05; **, P < 0.01). No cytokines were detected in uninfected mice.
Rag2-knockout mice are susceptible to ΔsecA2 infection.
The kinetics of disease in both zebrafish (Fig. 1F) and mice (Fig. 3A) following infection by the wild-type or ΔsecA2 strain suggested that the role for secA2 is more prominent after the onset of adaptive immunity. To better examine only the innate immune response, Rag2-knockout mice (34), which lack mature B and T cells, were infected with the ΔsecA2 or the SA2comp strain. Infection of these immunodeficient mice led to more severe disease, and the infected Rag2-knockout mice began to die at 3 weeks postinfection (see Fig. S3 in the supplemental material). However, there was no difference in mortality rate between mice infected with the ΔsecA2 and SA2comp strains. Diagnostic necropsy with H&E and Ziehl-Neelsen stains of the limbs, tail, and nasal tissue of these mice revealed cellulitis, osteomyelitis, and numerous acid-fast-positive bacilli associated with severe inflammation (data not shown). As expected, no granulomatous structures were found. Therefore, adaptive immunity is required for control of M. marinum infection in mice, and the ΔsecA2 strain can be as pathogenic as wild type if mature B and T cells are absent.
The ΔsecA2 strain has an altered cell envelope.
SecA2 is needed for secretion of two lipoproteins to the cell envelope in M. smegmatis (14), so we subjected the ΔsecA2 strain to several stresses in vitro to test the functional integrity of its cell envelope. The ΔsecA2 strain was much more sensitive to SDS than the wild-type or SA2comp strain (Fig. 7). Compared to wild-type strains, SDS decreased the growth of the ΔsecA2 strain to levels similar to those for the ΔkasB strain (Fig. 7), a mutant that has a defect in mycolic acid synthesis and is known to have a defective permeability barrier (13). However, unlike the ΔkasB strain, the ΔsecA2 strain did not show increased sensitivity to hydrogen peroxide or rifampin (data not shown), suggesting that the cell envelope defects of the two mutants are distinct. To further test a role for SecA2 in cell envelope structure, we visualized whole bacilli by cryo-electron tomography. Unlike traditional electron microscopy, this technique preserves cellular architecture, including the cell envelope, and allows three-dimensional reconstruction of the intact bacterial cell (26). Twenty-five percent of the ΔsecA2 bacilli had large protrusions from the cell envelope that were not present in wild type (see Fig. S4 in the supplemental material). These protrusions differed from the ribbon-like loops observed in the ΔkasB strain. While the contents of these protrusions are unknown, these data suggest a role for SecA2 in maintaining the cell wall. Thus, SecA2 is required for the functional and structural integrity of the cell envelope.
Fig 7.

The ΔsecA2 strain has abnormal cell envelope function. M. marinum wild-type, ΔsecA2, SA2comp, and ΔkasB strains were incubated with various concentrations of SDS (0.01, 0.05, 0.1, and 0.25%, vol/vol) in 7H9 broth for 20 h at 30°C. Sensitivity was determined by enumerating CFU, and the assay detection limit is shown with a dashed line. Mean ± SD is shown, and is averaged from two independent experiments. Statistically significant differences between the SA2comp strain and both the ΔsecA2 and ΔkasB strains are noted (*, P < 0.05; **, P < 0.01). For simplicity, lower error bars are not shown for the ΔsecA2 and ΔkasB strains.
DISCUSSION
These studies demonstrate that SecA2 is required for full expression of disease during infection of both zebrafish and mice with M. marinum. A careful analysis of both pathology and host cytokines revealed that SecA2 is required for both TNF-α mRNA production and the stability of mature granulomas in both species. We hypothesize that SecA2-dependent secretion of TNF-α is responsible for the effects on granuloma numbers, since TNF-α is known to be required for granuloma formation and maintenance during mycobacterial infection (16). TNF-α has a well-described host-protective role during mycobacterial infection in mice, since either absence of TNF-α (1) or neutralization of TNF-α (27) enhances susceptibility to infection. Similarly, in zebrafish larvae infected with M. marinum, knockdown of TNF receptor 1 facilitated the formation of destabilized granulomas that disintegrated and increased mycobacterial growth, killing the zebrafish host more rapidly (4). Thus, it is likely that early induction of TNF-α is associated with initial host protection, while secretion later in the disease may be exploited by the bacteria through granuloma usage as a niche for survival and possibly growth (9). In this context, it is interesting that the primary effects of SecA2 on infection are seen only after infection has been established, likely at the onset of adaptive immunity. SecA2-mediated secretion apparently enhances this late TNF-α synthesis. We suggest that this is required for bacterial survival and pathology in a natural host (zebrafish) and for pathology in mice, which are incidental hosts. This model is consistent with our observation that loss of M. marinum SecA2 does not affect TNF-α secretion in isolated macrophages, since its effects are not apparent until after recruitment of additional mechanisms of adaptive immunity. Consistent with this, the ΔsecA2 strain is as virulent as a complemented strain during infection of Rag2-knockout mice, which lack mature T and B cells. Thus, we predict that SecA2-secreted products are likely to affect host cells other than the macrophage. It is instructive to compare these findings with those from studies on the role of SecA2 in M. tuberculosis infection of macrophages and mice (2, 21, 38). In these studies, SecA2-dependent effects on growth in isolated macrophages were discovered and correlated with an increase in TNF-α secretion by these macrophages. The difference between the requirement for SecA2 in M. marinum and M. tuberculosis for growth in isolated macrophages may result from the increased growth rate of M. marinum in macrophages, which likely increases other bacterial products in sufficient quantities to lead to TNF-α production in this circumstance. The normal growth of the M. marinum ΔsecA2 strain in vitro allowed us to uncover additional effects of SecA2 on host-pathogen interactions that occur only in the in vivo environment.
How SecA2 affects TNF-α mRNA expression in vivo is unknown. Previous work has identified a number of secreted proteins that depend on SecA2, but none have been convincingly tied to its role in pathogenesis. Interestingly, SecA2 is required for secretion of two lipoproteins to the cell envelope in nonpathogenic M. smegmatis (14). Our findings that M. marinum bacteria lacking SecA2 have increased sensitivity to SDS and have morphological abnormalities of the cell envelope are consistent with a role for SecA2 in cell wall synthesis in M. marinum as well. This could suggest a role for the cell wall in SecA2-dependent manipulation of host immunity. While much remains to be done to explore this hypothesis, roles for bacterial lipoproteins in triggering host responses through Toll-like receptors and other mechanisms are well-known (16, 20), as is the ability of cell envelope components to disseminate widely and regulate immune responses during mycobacterial infection (33).
Given that fish and mice are evolutionarily distant in their relatedness, it is not surprising that our studies also identified differences between them for cytokine induction in response to infection with M. marinum ΔsecA2. Measurement of mRNA in fish and protein in mice may have further contributed to the observed difference. Understanding the basis for these differences would require a comparison of immune responses in fish and mice more detailed than is currently possible. However, we believe that by focusing on the common responses between the distant hosts, we have found an important mechanism through which SecA2 modulates host immunity. In both fish and mice, SecA2 enhances TNF-α during the adaptive immune response to M. marinum infection. We suggest that this is important for establishment of well-formed granulomas, which in turn can act as a niche for bacterial survival and proliferation.
Supplementary Material
ACKNOWLEDGMENTS
All work was funded by internal funds at Genentech, Inc., where S. Park, J. Kim, C. D. Austin, A. Paler-Martinez, M. Xu, and E. J. Brown are fully paid employees.
We thank S. Nation II for assistance with the M. marinum/mouse infection experiments and S. Boyd and C. Wang for comments on the manuscript.
Footnotes
Published ahead of print 30 July 2012
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1. Bean AG, et al. 1999. Structural deficiencies in granuloma formation in TNF gene-targeted mice underlie the heightened susceptibility to aerosol Mycobacterium tuberculosis infection, which is not compensated for by lymphotoxin. J. Immunol. 162:3504–3511 [PubMed] [Google Scholar]
- 2. Braunstein M, Espinosa BJ, Chan J, Belisle JT, Jacobs WR., Jr 2003. SecA2 functions in the secretion of superoxide dismutase A and in the virulence of Mycobacterium tuberculosis. Mol. Microbiol. 48:453–464 [DOI] [PubMed] [Google Scholar]
- 3. Carlsson F, et al. 2010. Host-detrimental role of Esx-1-mediated inflammasome activation in mycobacterial infection. PLoS Pathog. 6:e1000895 doi:10.1371/journal.ppat.1000895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Clay H, Volkman HE, Ramakrishnan L. 2008. Tumor necrosis factor signaling mediates resistance to mycobacteria by inhibiting bacterial growth and macrophage death. Immunity 29:283–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Comolli LR, Kundmann M, Downing KH. 2006. Characterization of intact subcellular bodies in whole bacteria by cryo-electron tomography and spectroscopic imaging. J. Microsc. 223:40–52 [DOI] [PubMed] [Google Scholar]
- 6. Cooper AM, Khader SA. 2008. The role of cytokines in the initiation, expansion, and control of cellular immunity to tuberculosis. Immunol. Rev. 226:191–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cosma CL, Sherman DR, Ramakrishnan L. 2003. The secret lives of the pathogenic mycobacteria. Annu. Rev. Microbiol. 57:641–676 [DOI] [PubMed] [Google Scholar]
- 8. Cosma CL, Swaim LE, Volkman H, Ramakrishnan L, Davis JM. 2006. Zebrafish and frog models of Mycobacterium marinum infection. Curr. Protoc. Microbiol. Chapter 10:Unit 10B.2 [DOI] [PubMed] [Google Scholar]
- 9. Davis JM, Ramakrishnan L. 2009. The role of the granuloma in expansion and dissemination of early tuberculous infection. Cell 136:37–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. DiGiuseppe Champion PA, Cox JS. 2007. Protein secretion systems in Mycobacteria. Cell. Microbiol. 9:1376–1384 [DOI] [PubMed] [Google Scholar]
- 11. Egen JG, et al. 2008. Macrophage and T cell dynamics during the development and disintegration of mycobacterial granulomas. Immunity 28:271–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gao LY, et al. 2004. A mycobacterial virulence gene cluster extending RD1 is required for cytolysis, bacterial spreading and ESAT-6 secretion. Mol. Microbiol. 53:1677–1693 [DOI] [PubMed] [Google Scholar]
- 13. Gao LY, et al. 2003. Requirement for kasB in Mycobacterium mycolic acid biosynthesis, cell wall impermeability and intracellular survival: implications for therapy. Mol. Microbiol. 49:1547–1563 [DOI] [PubMed] [Google Scholar]
- 14. Gibbons HS, Wolschendorf F, Abshire M, Niederweis M, Braunstein M. 2007. Identification of two Mycobacterium smegmatis lipoproteins exported by a SecA2-dependent pathway. J. Bacteriol. 189:5090–5100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hinchey J, et al. 2007. Enhanced priming of adaptive immunity by a proapoptotic mutant of Mycobacterium tuberculosis. J. Clin. Invest. 117:2279–2288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huynh KK, Joshi SA, Brown EJ. 2011. A delicate dance: host response to mycobacteria. Curr. Opin. Immunol. 23:464–472 [DOI] [PubMed] [Google Scholar]
- 17. Ito K, Takizawa F, Yoshiura Y, Ototake M, Nakanishi T. 2008. Expression profile of cytokine and transcription factor genes during embryonic development of zebrafish Danio rerio. Fish. Sci. 74:391–396 [Google Scholar]
- 18. Joshi SA, et al. 2012. EccA1, a component of the Mycobacterium marinum ESX-1 protein virulence factor secretion pathway, regulates mycolic acid lipid synthesis. Chem. Biol. 19:372–380 [DOI] [PubMed] [Google Scholar]
- 19. Koul A, Arnoult E, Lounis N, Guillemont J, Andries K. 2011. The challenge of new drug discovery for tuberculosis. Nature 469:483–490 [DOI] [PubMed] [Google Scholar]
- 20. Kovacs-Simon A, Titball RW, Michell SL. 2011. Lipoproteins of bacterial pathogens. Infect. Immun. 79:548–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kurtz S, McKinnon KP, Runge MS, Ting JP, Braunstein M. 2006. The SecA2 secretion factor of Mycobacterium tuberculosis promotes growth in macrophages and inhibits the host immune response. Infect. Immun. 74:6855–6864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Linell F, Norden A. 1954. Mycobacterium balnei, a new acid-fast bacillus occurring in swimming pools and capable of producing skin lesions in humans. Acta Tuberc. Scand. Suppl. 33:1–84 [PubMed] [Google Scholar]
- 23. Lonnroth K, et al. 2010. Tuberculosis control and elimination 2010-50: cure, care, and social development. Lancet 375:1814–1829 [DOI] [PubMed] [Google Scholar]
- 24. McLaughlin B, et al. 2007. A mycobacterium ESX-1-secreted virulence factor with unique requirements for export. PLoS Pathog. 3:e105 doi:10.1371/journal.ppat.0030105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Meeker ND, Trede NS. 2008. Immunology and zebrafish: spawning new models of human disease. Dev. Comp. Immunol. 32:745–757 [DOI] [PubMed] [Google Scholar]
- 26. Milne JL, Subramaniam S. 2009. Cryo-electron tomography of bacteria: progress, challenges and future prospects. Nat. Rev. Microbiol. 7:666–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mohan VP, et al. 2001. Effects of tumor necrosis factor alpha on host immune response in chronic persistent tuberculosis: possible role for limiting pathology. Infect. Immun. 69:1847–1855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Parent LJ, Salam MM, Appelbaum PC, Dossett JH. 1995. Disseminated Mycobacterium marinum infection and bacteremia in a child with severe combined immunodeficiency. Clin. Infect. Dis. 21:1325–1327 [DOI] [PubMed] [Google Scholar]
- 29. Pelicic V, et al. 1997. Efficient allelic exchange and transposon mutagenesis in Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. U. S. A. 94:10955–10960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ranganathan UD, et al. 2009. Recombinant pro-apoptotic Mycobacterium tuberculosis generates CD8+ T cell responses against human immunodeficiency virus type 1 Env and M. tuberculosis in neonatal mice. Vaccine 28:152–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rigel NW, Braunstein M. 2008. A new twist on an old pathway—accessory Sec [corrected] systems. Mol. Microbiol. 69:291–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rojo I, de Ilarduya OM, Estonba A, Pardo MA. 2007. Innate immune gene expression in individual zebrafish after Listonella anguillarum inoculation. Fish Shellfish Immunol. 23:1285–1293 [DOI] [PubMed] [Google Scholar]
- 33. Russell DG. 2011. Mycobacterium tuberculosis and the intimate discourse of a chronic infection. Immunol. Rev. 240:252–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shinkai Y, et al. 1992. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell 68:855–867 [DOI] [PubMed] [Google Scholar]
- 35. Stover CK, et al. 1991. New use of BCG for recombinant vaccines. Nature 351:456–460 [DOI] [PubMed] [Google Scholar]
- 36. Streit M, et al. 2006. Disseminated Mycobacterium marinum infection with extensive cutaneous eruption and bacteremia in an immunocompromised patient. Eur. J. Dermatol. 16:79–83 [PubMed] [Google Scholar]
- 37. Sullivan C, Kim CH. 2008. Zebrafish as a model for infectious disease and immune function. Fish Shellfish Immunol. 25:341–350 [DOI] [PubMed] [Google Scholar]
- 38. Sullivan JT, Young EF, McCann JR, Braunstein M. 2012. The Mycobacterium tuberculosis SecA2 system subverts phagosome maturation to promote growth in macrophages. Infect. Immun. 80:996–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Swaim LE, et al. 2006. Mycobacterium marinum infection of adult zebrafish causes caseating granulomatous tuberculosis and is moderated by adaptive immunity. Infect. Immun. 74:6108–6117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tobin DM, Ramakrishnan L. 2008. Comparative pathogenesis of Mycobacterium marinum and Mycobacterium tuberculosis. Cell. Microbiol. 10:1027–1039 [DOI] [PubMed] [Google Scholar]
- 41. van der Sar AM, et al. 2004. Mycobacterium marinum strains can be divided into two distinct types based on genetic diversity and virulence. Infect. Immun. 72:6306–6312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vojtech LN, Sanders GE, Conway C, Ostland V, Hansen JD. 2009. Host immune response and acute disease in a zebrafish model of Francisella pathogenesis. Infect. Immun. 77:914–925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Watzke J, Schirmer K, Scholz S. 2007. Bacterial lipopolysaccharides induce genes involved in the innate immune response in embryos of the zebrafish (Danio rerio). Fish Shellfish Immunol. 23:901–905 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.