Abstract
The proinflammatory cytokine interleukin-17 (IL-17) is involved in neutrophilic tissue infiltration, contributing to both microbial clearance as well as inflammation-associated tissue damage. Its role during bacterial corneal infections is unknown. We hypothesized that IL-17 responses would be detrimental in this setting and tested the impact of IL-17 receptor deficiency or antibody-mediated neutralization of IL-17 in a murine model of Pseudomonas aeruginosa ulcerative keratitis after scratch injury. We found that, compared with infected corneas from wild-type mice, those from IL-17 receptor (IL-17R)-deficient mice had significantly lower corneal pathology scores, neutrophil influx, and intracellular bacterial levels. Infected IL-17R-deficient corneas had low intercellular adhesion molecule 1 (ICAM-1) expression, and ICAM-1-deficient mice were similarly resistant to infection. Topical treatment with polyclonal antibodies to IL-17 resulted in significant reductions in corneal pathology and also lowered bacterial counts after infection with six different laboratory or clinical P. aeruginosa strains, including both invasive and cytotoxic strains. Thus, neutralization of IL-17 during P. aeruginosa corneal infection reduces neutrophil influx and pathology without compromising bacterial clearance and offers a promising new avenue for therapy of these potentially sight-threatening infections.
INTRODUCTION
While it is widely appreciated that inflammation in response to infection has both positive, protective effects as well as negative, destructive effects on tissues, it is less well appreciated that interventions that modulate inflammation can have different effects on outcomes in different tissues. The eye is a notable tissue to evaluate such effects, not only due to its basal immune-privileged status but also because inflammatory-mediated damage to tissues like the cornea that result in clearance of infecting microbes also leads to significant damage (35) that severely compromises eyesight, whereas comparable scarring in other tissues is often of little consequence. Pseudomonas aeruginosa is a leading cause of bacterial eye infections in humans (2, 13, 29, 33), and interventions are needed that promote bacterial clearance while limiting tissue-associated pathology due to a rapid and extensive influx of neutrophils.
Neutrophil recruitment to infected tissues, particularly tissues infected with extracellular bacteria and fungi (7, 25), is often highly dependent on the proinflammatory cytokine interleukin-17 (IL-17) produced by helper T cells called Th17 cells (11, 21), although the importance of IL-17 varies by pathogen, tissue, and type of infection. For example, antibody-mediated depletion of IL-17 or deficiency of the IL-17 receptor (IL-17R) had no effect on the course of P. aeruginosa lung infection, but these interventions did diminish the protective efficacy of a live-attenuated P. aeruginosa vaccine against lethal pneumonia (27). Intercellular adhesion molecule 1 (ICAM-1), a receptor for the neutrophil β2-integrins LFA-1 and Mac-1, is expressed on epithelial and endothelial cells during infection and facilitates neutrophil recruitment to infected tissues, including the eye (17), but the relative contributions of IL-17 and ICAM-1 to neutrophil influx and host defense during bacterial keratitis are unknown.
Neutralization of IL-17 activity in the cornea during bacterial keratitis could have potential therapeutic use due to the need to limit neutrophil-associated pathology in this setting, but decreasing neutrophil influx might also interfere with bacterial clearance. In this study, we show that the absence of the IL-17 receptor is associated with diminished ICAM-1 expression after P. aeruginosa corneal infection. We also demonstrate that absence of the IL-17 receptor or ICAM-1 or antibody-mediated neutralization of IL-17 leads to lower corneal pathology scores, diminished neutrophil infiltration, and decreased bacterial levels.
MATERIALS AND METHODS
Bacterial strains and mice.
We utilized clinical isolates of P. aeruginosa originally obtained from infected corneal ulcers: strains 6294 and 6354 (both are ExoS-producing invasive strains of serogroup O6), 6077 and 6206 (both are ExoU-producing cytotoxic strains of serogroup O11) (39), and the laboratory strain PAO1 as well as its cytotoxic variant, denoted ExoU+ PAO1, which contains a plasmid with the genes for the type III secretion toxin ExoU and its chaperone, SpcU (3). C57BL/6 mice were purchased from Taconic for experiments that included IL-17R knockout (KO) mice and from Jackson Laboratory for experiments that included ICAM-1 KO mice. IL-17R KO mice were provided by Amgen, and ICAM-1 KO mice (full gene deletion) were provided by Daniel Bullard of the University of Alabama at Birmingham (5). Animal experiments complied with institutional and federal guidelines regarding the use of animals in research.
Murine corneal infection model.
Corneal infection was initiated on scratch-injured eyes of anesthetized mice, as previously described (26, 38). Bacteria were grown overnight at 37°C on Trypticase soy agar (TSA) and resuspended in 1% proteose peptone, and the optical density was adjusted to achieve inoculum sizes of ∼1 × 104 CFU/eye for cytotoxic strains or 5 × 105 CFU/eye for invasive strains. Higher doses of bacteria (∼1 × 107 CFU/eye) were used for histopathology experiments. Mice were anesthetized with ketamine and xylazine, and then eyes were scratched and inoculated with the bacterial inoculum suspended in a 5-μl volume. Following infection, eyes were assigned a pathology score every 24 h by two individuals (T. S. Zaidi and T. Zaidi) unaware of the group assignments, using the following scheme (4): 0, eye macroscopically identical to the uninfected contralateral control eye; 1, faint opacity partially covering the pupil; 2, dense opacity covering the pupil; 3, dense opacity covering the entire anterior segment; 4, perforation of the cornea, phthisis bulbi (shrinkage of the globe after inflammatory disease), or both. In general, maximal pathology scores were observed 48 h after infection, so this time point is shown in all figures depicting corneal pathology scores. IL-17 production in mouse corneas was determined 24 h after P. aeruginosa 6294 and 6077 infection in an IL-17 enzyme-linked immunosorbent assay (ELISA; R&D Systems). Myeloperoxidase assays were performed using an ELISA kit from Enzo Life Sciences (Farmingdale, NY) according to the manufacturer's instructions.
Determinations of viable bacterial counts.
To determine the viable counts of adherent and invading P. aeruginosa strains, gentamicin exclusion assays were performed as previously described (26, 38). Briefly, animals were euthanized by carbon dioxide overdose, eyes were removed, and the corneas were harvested. The corneas were then rinsed in Dulbecco's modified Eagle's medium (DMEM) to remove nonadherent bacteria and placed in 1 ml of DMEM. The corneas were then vortexed vigorously for 1 min to remove adherent bacteria. These samples were then diluted and plated for bacterial enumeration. The corneas were then placed in 500 μg gentamicin/ml for 1 h to kill remaining external bacteria, washed three times in DMEM to remove the antibiotic, and then homogenized. The homogenate was then diluted and plated for bacterial enumeration. When no growth was detected, a result of 5 CFU was arbitrarily assigned, since the lower limit of detection was 10 CFU. This method of measuring adherent bacteria by vortexing yields results comparable to those obtained by subtracting the number of internalized bacteria from total bacteria (adherent plus internalized) in separate eyes (37), but the vortexing method has the advantage of determining bacterial levels in one eye sample.
Administration of polyclonal antibodies.
Rabbit polyclonal antibody to IL-17 (200 μg/ml; Santa Cruz Biotechnology) or control rabbit IgG (Sigma) in a 5-μl volume was applied topically to the eyes of mice while they were lightly anesthetized by isoflurane inhalation. Antibodies were administered at 0, 24, and 32 h postinfection. For a subset of experiments (those with strains 6294, 6206, and PAO1 in Fig. 4, below), rabbit antiserum to IL-17 (27) or normal rabbit serum was used. While this antiserum could contain antibodies that cross-react with P. aeruginosa lipopolysaccharide, such antibodies are unlikely to be protective, since our prior work (27) showed that this very same antiserum diminished vaccine-induced protection in a P. aeruginosa pneumonia model. Furthermore, in the current experiments, the antiserum yielded similar results as the commercially available anti-IL-17 polyclonal IgG.
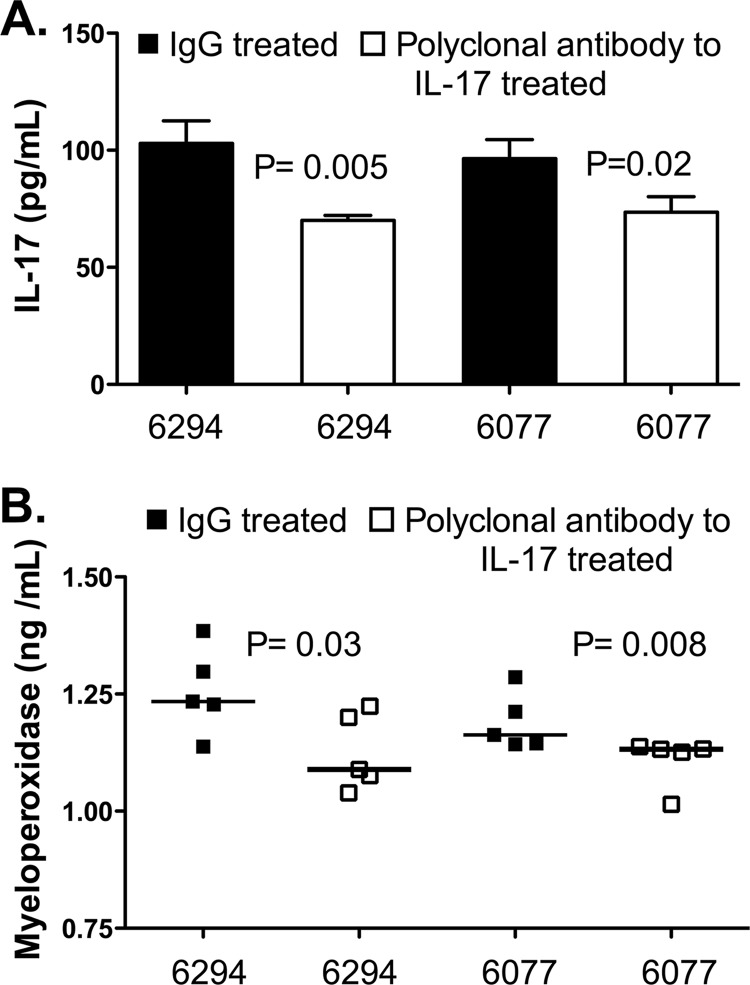
Fig 4.
(A) IL-17 levels in corneal homogenates 48 h after topical administration of control IgG or rabbit polyclonal antibody to IL-17 during infection with P. aeruginosa. Bars indicate means, and error bars show standard deviations. P values were determined in unpaired t tests. (B) Myeloperoxidase levels in corneal homogenates 48 h after topical administration of control IgG or rabbit polyclonal antibody to IL-17 during infection with P. aeruginosa. Bars indicate medians. P values were determined with the Mann-Whitney U test.
Histopathology and confocal microscopy.
After euthanasia, eyes were enucleated and fixed in 4% paraformaldehyde. Tissues were embedded in paraffin, and anterior sections of the corneal epithelium were prepared and stained with hematoxylin and eosin. Sections were analyzed by a veterinary pathologist. Images shown correspond to areas of maximal pathology in each cornea. If not shown, the anterior chamber was free of inflammatory cells. For confocal microscopy, paraffin-embedded sections were heated at 65°C for 1 h and then deparaffinized with xylene, followed by rehydration with an ethanol-water mixture. After blocking at 37°C for 2 h in blocking buffer (2% normal goat serum and 0.2% bovine serum albumin in phosphate-buffered saline [PBS]), sections were incubated at 37°C for 2 h with goat anti-mouse ICAM-1 antibody (R&D Systems) at a 1:25 dilution in blocking buffer. The secondary antibody was donkey anti-goat IgG (Invitrogen), used at a 1:500 dilution in blocking buffer, with incubation at 37°C for 1 h. After washing in PBS, sections were mounted using Aqua-Poly/Mount (Polysciences) and then analyzed by confocal microscopy. Control sections from infected wild-type mice were incubated with secondary antibody only and demonstrated no ICAM-1 staining (data not shown).
Statistical analysis.
Parametric data were compared using an unpaired t test,, and nonparametric data were compared by using the Mann-Whitney U test. All analyses were performed using GraphPad's Prism software.
RESULTS
IL-17 is produced in the cornea during P. aeruginosa keratitis.
A prominent feature of P. aeruginosa corneal infection is that the majority of the bacteria reside within the epithelial cells (9, 10), and prevention of bacterial entry into cells diminishes infection levels and tissue pathology (37). Antibody- and complement-mediated, neutrophil-dependent phagocytic killing of extracellular bacteria that interrupts cell-to-cell transmission can protect against infection (40), with entry of neutrophils (31) and resolution of inflammation, both of which are critical to successful control of infection and preservation of tissue integrity. To evaluate the role of IL-17 in this process, an ELISA was used to detect IL-17 in homogenized mouse corneas harvested 24 h after P. aeruginosa scratch injury-induced infection. We detected 73 ± 8 pg/ml (mean ± standard deviation) after infection with strain 6294 and 33 ± 13 pg/ml after infection with strain 6077. The higher level of IL-17 after infection with strain 6294 was likely related to the 50-fold-higher inoculum needed to cause infection with this strain.
IL-17R-deficient mice are resistant to P. aeruginosa corneal infection.
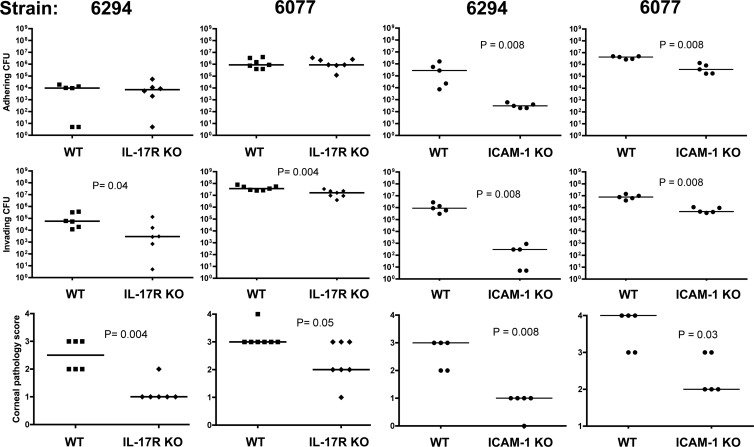
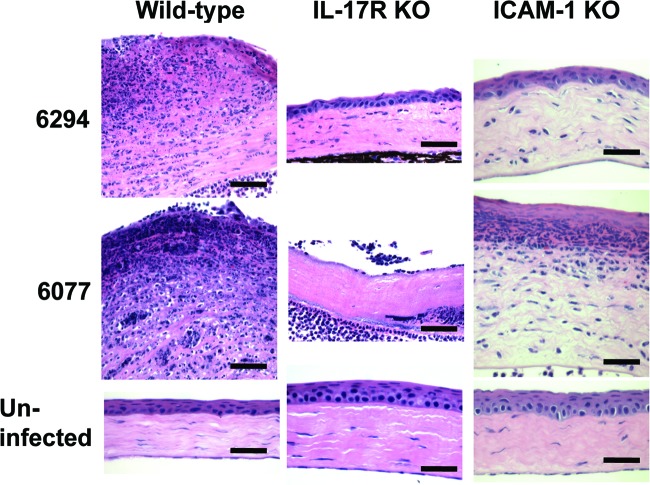
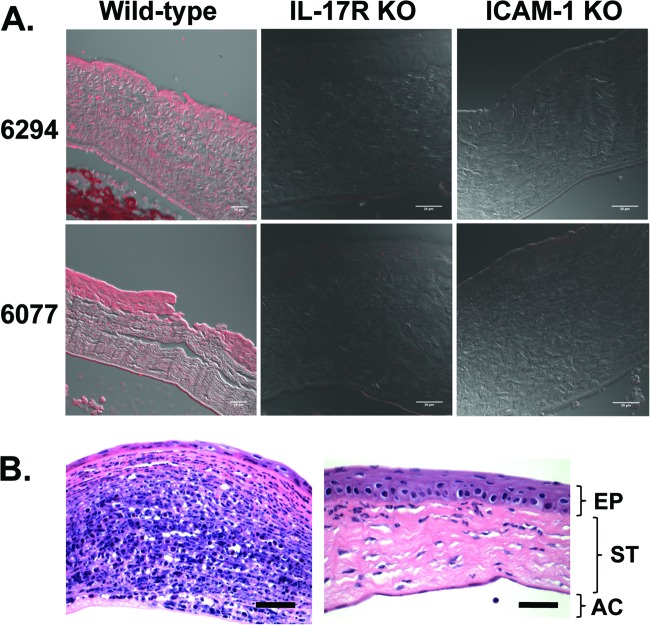
IL-17R-deficient mice, which have been shown to be highly susceptible to Klebsiella pneumoniae lung infection (36) and to have diminished vaccine-induced protection from P. aeruginosa pneumonia (27), were unexpectedly more resistant to P. aeruginosa corneal infection. These mice had significantly lower corneal pathology scores and intracellular bacterial levels 48 h after infection with either strain 6294 or strain 6077 (Fig. 1). Histopathologic evaluation of infected corneas showed less neutrophilic infiltration in the IL-17R KO mice (Fig. 2), consistent with the known role of IL-17 in stimulating the production of CXC chemokines KC and macrophage inflammatory protein 2 as well as granulocyte colony-stimulating factor at sites of infection (36). Corneas of uninfected IL-17R KO mice were histologically normal, although, for unclear reasons, they had slightly thickened stromal and epithelial layers compared to wild-type mice (Fig. 2). Because IL-17 has also been shown to regulate ICAM-1 expression and neutrophil adhesion in nonocular tissues (1, 28), we next tested the susceptibility of ICAM-1 KO mice to P. aeruginosa corneal infection, and we found that the ICAM-1 KO mice had similarly reduced corneal pathologies (Fig. 2). Corneas from uninfected ICAM-1 KO mice were, as observed for IL-17R KO mice, slightly thicker than those from wild-type mice. Notably, corneas from infected IL-17R KO mice had undetectable ICAM-1 expression (Fig. 3A), far less than that seen in corneas from infected wild-type mice. In addition to the expected epithelial ICAM-1 staining, the infected wild-type mice demonstrated a low level of non-cell-associated ICAM-1 staining in the corneal stroma, which was possibly due to release of soluble ICAM-1.
Fig 1.
Levels of extracellular adhering bacteria (top panels), intracellular invading bacteria (middle panels), and corneal pathology scores (bottom panels) 48 h after infection of wild-type (WT), IL-17R KO, or ICAM-1 KO mice with P. aeruginosa strains 6294 and 6077. Points indicate values for an individual mouse; bars indicate medians. P values were calculated with the Mann-Whitney U test.
Fig 2.
Corneal histopathology 48 h after infection of wild-type, IL-17R KO, or ICAM-1 KO mice with P. aeruginosa strains 6294 and 6077 (as in Fig. 1). Sections were stained with hematoxylin and eosin. Magnification, ×60. Bar, 30 μm.
Fig 3.
(A) Corneal ICAM-1 expression 48 h after infection of IL-17R KO or ICAM-1 KO mice with P. aeruginosa strains 6294 and 6077. Subpanels depict the overlay of the red fluorescence channel and a phase-contrast image. Bar, 20 μm. (B) Corneal histopathology 48 h after topical administration of control IgG (left panel) or rabbit polyclonal antibody to IL-17 (right panel) during infection with P. aeruginosa strain 6077. Sections were stained with hematoxylin and eosin. Magnification, ×60. Bar, 30 μm. EP, epithelium; ST, stroma; AC, anterior chamber.
Topical IL-17 neutralization reduces corneal pathology and bacterial burden.
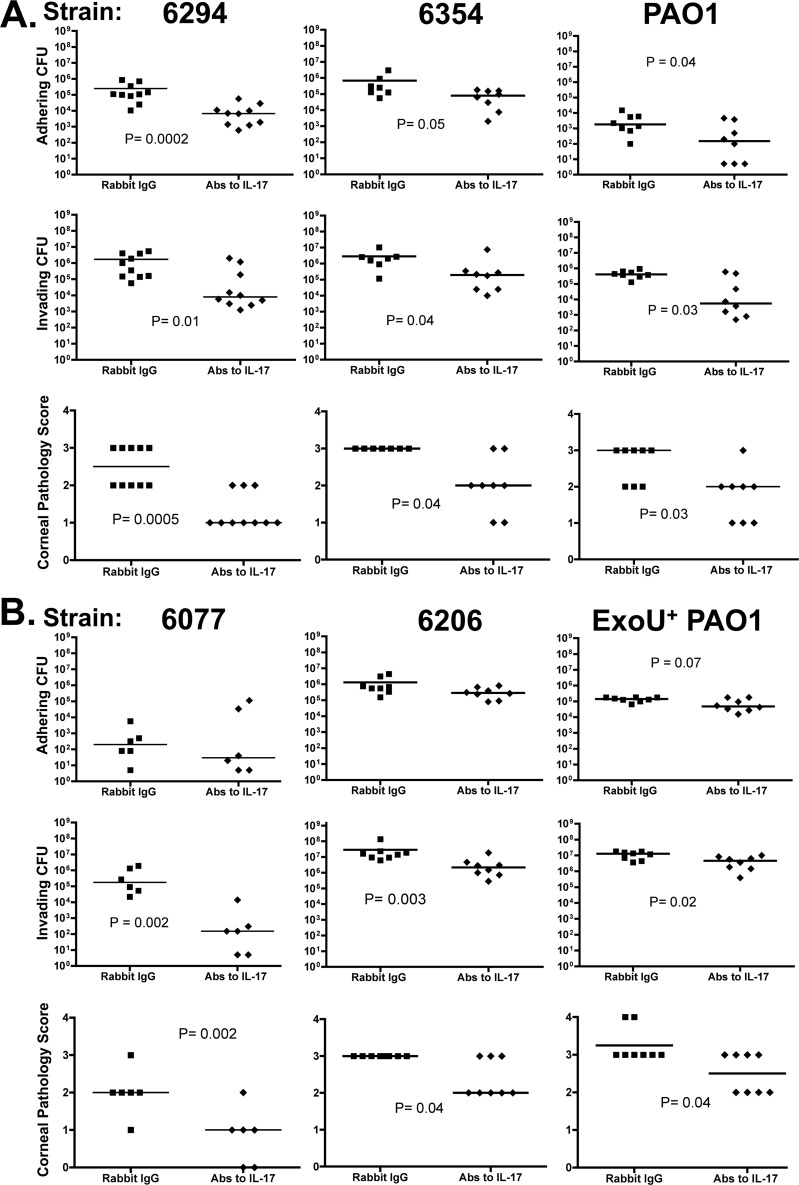
Based on these results, we predicted that antibody-mediated neutralization of IL-17 might have beneficial effects during P. aeruginosa corneal infection. Here we use the term neutralization broadly, as it may result from inhibition of ligand-receptor binding or from enhanced clearance of the cytokine via antigen-antibody complexes. As shown in Fig. 3B, neutrophil infiltration after infection with strain 6077 was dramatically reduced by topical therapy with antibody to IL-17, which was also associated with significantly lower levels of IL-17 in the cornea (Fig. 4A). The decreased tissue levels of neutrophils seen on histopathology were verified by measurements of myeloperoxidase in corneal homogenates, which showed significantly lower levels of myeloperoxidase in the anti-IL-17-treated groups (Fig. 4B). We next evaluated if IL-17 neutralization had a comparable effect in a number of clinical and laboratory isolates of P. aeruginosa (Fig. 5), one of which was from a pair of isogenic strains, of which one member overexpressed the type III secretion toxin ExoU. For all the strains tested (invasive strains in Fig. 5A and cytotoxic strains in Fig. 5B), the corneal pathology scores and intracellular (invading) bacterial levels were significantly lower in mice treated topically with antibodies to IL-17 than in those treated with control antibodies. The decrease in intracellular bacterial CFU was substantial (about 2 log10), particularly after infection with ExoS-producing strains, which also showed significantly lower levels of extracellular bacteria (Fig. 4A).
Fig 5.
Levels of extracellular adhering bacteria (top panels), intracellular invading bacteria (middle panels), and corneal pathology scores (bottom panels) 48 h after infection with the indicated P. aeruginosa strains (invasive strains [A] and cytotoxic strains [B]) treated topically with anti-IL-17 antibody or rabbit IgG control. Treatments were administered 0, 24, and 32 h after infection. Points indicate values for an individual mouse; bars indicate medians. P values were calculated with the Mann-Whitney U test.
DISCUSSION
In this study, we show that IL-17 is critically involved in the pathogenesis of P. aeruginosa corneal infection. Both IL-17R KO mice and ICAM-1 KO mice were relatively resistant to P. aeruginosa corneal infection, with lower corneal pathology scores and lower levels of invading bacteria. Furthermore, neutralization of IL-17 by using topical antibodies reduced corneal pathology and bacterial invasion and, to a variable degree, the extracellular bacteria adhering to the corneal tissue.
The diminished leukocyte influx into the infected corneal tissue of IL-17R KO mice that we observed is likely related to the low ICAM-1 expression in this tissue. Indeed, IL-17 has been shown to increase endothelial ICAM-1 expression and neutrophil recruitment in a p38 mitogen-activated protein kinase-dependent manner (28). In studies using keratinocytes, IL-17 combined with gamma interferon (but not IL-17 alone) increased ICAM-1 expression (1), highlighting the cell-specific and inflammatory milieu-specific nature of IL-17 effects.
A number of studies have shown that ICAM-1 is involved in neutrophilic infiltration of the cornea. After scratch injury alone (without infection), neutrophils recruited from the limbal vessels into the corneal stroma make ICAM-1-mediated surface contact with stromal keratinocytes, and ICAM-1 KO mice demonstrate decreased stromal infiltration (12). In P. aeruginosa keratitis after scratch injury, Hazlett and coworkers reported that ICAM-1-deficient mice had less-severe corneal opacities (as we observed) but similar neutrophil numbers and inflammatory cell infiltration on histopathology (17), which is different from our findings. These differences might be related to the fact that the mice used in those studies had an exon 4 deletion and could theoretically express alternatively spliced variants of ICAM-1, which has been reported for the ICAM-1 exon 5 deletion KO mice (19). The entire coding region of the ICAM-1 gene is absent in the ICAM-1 KO mice utilized in our studies (5), so alternative splicing is not possible. Notably, other experimental methods that diminish corneal neutrophil infiltration, specifically, neutropenia or the absence of MyD88 signaling, result in a similar low corneal pathology in this P. aeruginosa infection model (40). However, in striking contrast to the IL-17R KO and ICAM-1 KO mice, neutropenic or MyD88 KO mice show uncontrolled infection with bacterial dissemination to the brain and systemic organs (40). We speculate that the IL-17/ICAM-1 pathway of neutrophil tissue infiltration of the eye leads to enhanced tissue destruction, possibly by neutrophil-derived proteases, and such destruction actually enhances bacterial colonization.
Neutrophils migrate into the corneal stroma early in bacterial infection (16, 18) and are thought to be required for bacterial killing (31). However, their presence also contributes to corneal damage (35). T cells, particularly T helper type 1 (Th1) cells, have also been shown to play a role in the pathogenesis of bacterial corneal infections related to the recruitment of neutrophils (14, 15). In a mouse model of herpes simplex virus (HSV) stromal keratitis, Rouse and coworkers recently reported that after a transient burst of IL-17 production from innate γδT cells, Th1 cells infiltrated the cornea early while Th17 cells appeared later (days 15 to 21), at the peak of corneal pathology, and that IL-17R KO mice and wild-type mice administered anti-IL-17 antibody (intraperitoneal or subconjunctival) had less severe disease (32). Other workers have shown diminished early neutrophil infiltration and corneal opacities in IL-17R KO mice 2 days after HSV corneal infection, but they did not evaluate animals beyond day 11 (24). Although these findings are somewhat similar to those reported here, the pathogenesis of HSV stromal keratitis, a chronic disorder, is very different from bacterial keratitis. The initial acute viral infection is thought to be controlled by the Th1 response, which initiates an inflammatory environment in the cornea that is hypothesized to damage the cornea, uncovering self-antigens that drive the subsequent Th17-mediated inflammatory reaction (32).
In our studies, we found that IL-17 contributes to pathology by promoting corneal opacification, but it is unknown if Th17 cells are a major source of this cytokine in the cornea during bacterial infection. Indeed, recent studies have shown that primary corneal cells from the murine corneal eye cup and corneal endothelial cell lines can impair the effector functions and activation of Th17 cells by a cell contact-dependent mechanism (30), suggesting that these corneal endothelial cells, which are actually specialized epithelial cells, contribute to the maintenance of the privileged immune status of the anterior chamber of the eye. Multiple innate immune cell types, mostly in nonocular tissues, have been described to have the capacity to secrete IL-17 (7), including neutrophils themselves (20) as well as γδ T cells (22, 32), NK1.1-negative invariant TC receptor NKT cells (23), Paneth cells (34), Thy1+ SCA1+ CD3− CD4− KIT− cells (6), and CD3− CD4+ LTi-like cells (8). While finding the sources of IL-17 in the cornea will be important, we have at a minimum determined that topical application of IL-17-neutralizing reagents might help improve outcomes for P. aeruginosa keratitis without compromising bacterial clearance and thus open potential new avenues of treatment for these sight-threatening infections.
ACKNOWLEDGMENTS
We are grateful to F. W. Luscinskas for assistance in obtaining ICAM-1 KO mice.
This work was supported by NIH grants EY016144 to G.B.P. and HL092515 to G.P.P.
Footnotes
Published ahead of print 16 July 2012
REFERENCES
- 1. Albanesi C, Cavani A, Girolomoni G. 1999. IL-17 is produced by nickel-specific T lymphocytes and regulates ICAM-1 expression and chemokine production in human keratinocytes: synergistic or antagonist effects with IFN-gamma and TNF-alpha. J. Immunol. 162:494–502 [PubMed] [Google Scholar]
- 2. Alexandrakis G, Alfonso EC, Miller D. 2000. Shifting trends in bacterial keratitis in south Florida and emerging resistance to fluoroquinolones. Ophthalmology 107:1497–1502 [DOI] [PubMed] [Google Scholar]
- 3. Allewelt M, Coleman FT, Grout M, Priebe GP, Pier GB. 2000. Acquisition of expression of the P. aeruginosa ExoU cytotoxin leads to increased bacterial virulence in a murine model of acute pneumonia and systemic spread. Infect. Immun. 68:3998–4004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Berk RS, Hazlett LD, Beisel KW. 1987. Genetic studies on resistance and susceptibility genes controlling the mouse cornea to infection by Pseudomonas aeruginosa. Antibiot. Chemother. 39:83–91 [DOI] [PubMed] [Google Scholar]
- 5. Bullard DC, et al. 2007. Intercellular adhesion molecule-1 expression is required on multiple cell types for the development of experimental autoimmune encephalomyelitis. J. Immunol. 178:851–857 [DOI] [PubMed] [Google Scholar]
- 6. Buonocore S, et al. 2010. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature 464:1371–1375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cua DJ, Tato CM. 2010. Innate IL-17-producing cells: the sentinels of the immune system. Nat. Rev. Immunol. 10:479–489 [DOI] [PubMed] [Google Scholar]
- 8. Cupedo T, et al. 2009. Human fetal lymphoid tissue-inducer cells are interleukin 17-producing precursors to RORC+ CD127+ natural killer-like cells. Nat. Immunol. 10:66–74 [DOI] [PubMed] [Google Scholar]
- 9. Fleiszig SM, Zaidi TS, Fletcher EL, Preston MJ, Pier GB. 1994. Pseudomonas aeruginosa invades corneal epithelial cells during experimental infection. Infect. Immun. 62:3485–3493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fleiszig SM, Zaidi TS, Pier GB. 1995. Pseudomonas aeruginosa invasion of and multiplication within corneal epithelial cells in vitro. Infect. Immun. 63:4072–4077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Freitas A, et al. 2009. IL-17 receptor signaling is required to control polymicrobial sepsis. J. Immunol. 182:7846–7854 [DOI] [PubMed] [Google Scholar]
- 12. Gagen D, et al. 2010. ICAM-1 mediates surface contact between neutrophils and keratocytes following corneal epithelial abrasion in the mouse. Exp. Eye Res. 91:676–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Galentine PG, et al. 1984. Corneal ulcers associated with contact lens wear. Arch. Ophthalmol. 102:891–894 [DOI] [PubMed] [Google Scholar]
- 14. Hazlett LD. 2002. Pathogenic mechanisms of P. aeruginosa keratitis: a review of the role of T cells, Langerhans cells, PMN, and cytokines. DNA Cell. Biol. 21:383–390 [DOI] [PubMed] [Google Scholar]
- 15. Hazlett LD, McClellan S, Kwon B, Barrett R. 2000. Increased severity of Pseudomonas aeruginosa corneal infection in strains of mice designated as Th1 versus Th2 responsive. Invest. Ophthalmol. Vis. Sci. 41:805–810 [PubMed] [Google Scholar]
- 16. Hazlett LD, Rosen DD, Berk RS. 1977. Pseudomonas eye infections in cyclophosphamide-treated mice. Invest. Ophthalmol. Vis. Sci. 16:649–652 [PubMed] [Google Scholar]
- 17. Hobden JA, Masinick-McClellan S, Barrett RP, Bark KS, Hazlett LD. 1999. Pseudomonas aeruginosa keratitis in knockout mice deficient in intercellular adhesion molecule 1. Infect. Immun. 67:972–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kernacki KA, Berk RS. 1994. Characterization of the inflammatory response induced by corneal infection with Pseudomonas aeruginosa. J. Ocul. Pharmacol. 10:281–288 [DOI] [PubMed] [Google Scholar]
- 19. King PD, et al. 1995. Novel isoforms of murine intercellular adhesion molecule-1 generated by alternative RNA splicing. J. Immunol. 154:6080–6093 [PubMed] [Google Scholar]
- 20. Li L, et al. 2010. IL-17 produced by neutrophils regulates IFN-gamma-mediated neutrophil migration in mouse kidney ischemia-reperfusion injury. J. Clin. Invest. 120:331–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Linden A, Laan M, Anderson GP. 2005. Neutrophils, interleukin-17A and lung disease. Eur. Respir. J. 25:159–172 [DOI] [PubMed] [Google Scholar]
- 22. Martin B, Hirota K, Cua DJ, Stockinger B, Veldhoen M. 2009. Interleukin-17-producing gamma-delta T cells selectively expand in response to pathogen products and environmental signals. Immunity 31:321–330 [DOI] [PubMed] [Google Scholar]
- 23. Michel ML, et al. 2008. Critical role of ROR-gammat in a new thymic pathway leading to IL-17-producing invariant NKT cell differentiation. Proc. Nat. Acad. Sci. U. S. A. 105:19845–19850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Molesworth-Kenyon SJ, Yin R, Oakes JE, Lausch RN. 2008. IL-17 receptor signaling influences virus-induced corneal inflammation. J. Leukoc. Biol. 83:401–408 [DOI] [PubMed] [Google Scholar]
- 25. Peck A, Mellins ED. 2010. Precarious balance: Th17 cells in host defense. Infect. Immun. 78:32–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Preston MJ, et al. 1995. Rapid and sensitive method for evaluating Pseudomonas aeruginosa virulence factors during corneal infections in mice. Infect. Immun. 63:3497–3501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Priebe G, et al. 2008. IL-17 is a critical component of vaccine-induced protection against lung infection by LPS-heterologous strains of Pseudomonas aeruginosa. J. Immunol. 181:4965–4975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Roussel L, et al. 2010. IL-17 promotes p38 MAPK-dependent endothelial activation enhancing neutrophil recruitment to sites of inflammation. J. Immunol. 184:4531–4537 [DOI] [PubMed] [Google Scholar]
- 29. Schein OD, Glynn RJ, Seddon JM, Scardino VA, Kenyon KR. 1989. The relative risk of ulcerative keratitis among users of daily-wear and extended-wear soft contact lenses. N. Engl. J. Med. 321:773–778 [DOI] [PubMed] [Google Scholar]
- 30. Sugita S, et al. 2012. Inhibitory effect of corneal endothelial cells on IL-17-producing Th17 cells. Br. J. Ophthalmol. 96:293–299 [DOI] [PubMed] [Google Scholar]
- 31. Sun Y, Karmakar M, Taylor PR, Rietsch A, Pearlman E. 2012. ExoS and ExoT ADP ribosyltransferase activities mediate Pseudomonas aeruginosa keratitis by promoting neutrophil apoptosis and bacterial survival. J. Immunol. 188:1884–1895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Suryawanshi A, et al. 2011. Role of IL-17 and Th17 cells in herpes simplex virus-induced corneal immunopathology. J. Immunol. 187:1919–1930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tabbara KF, El-Sheikh HF, Aabed B. 2000. Extended wear contact lens related bacterial keratitis. Br. J. Ophthalmol. 84:327–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Takahashi N, et al. 2008. IL-17 produced by Paneth cells drives TNF-induced shock. J. Exp. Med. 205:1755–1761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Twining SS, Lohr KM, Moulder JM. 1986. The immune system in experimental Pseudomonas keratitis: model and early effects. Invest. Ophthalmol. Vis. Sci. 27:507–515 [PubMed] [Google Scholar]
- 36. Ye P, et al. 2001. Requirement of interleukin-17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J. Exp. Med. 194:519–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zaidi TS, Lyczak J, Preston M, Pier GB. 1999. Cystic fibrosis transmembrane conductance regulator-mediated corneal epithelial cell ingestion of Pseudomonas aeruginosa is a key component in the pathogenesis of experimental murine keratitis. Infect. Immun. 67:1481–1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zaidi TS, Preston MJ, Pier GB. 1997. Inhibition of bacterial adherence to host tissue does not markedly affect disease in the murine model of Pseudomonas aeruginosa corneal infection. Infect. Immun. 65:1370–1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zaidi TS, Priebe GP, Pier GB. 2006. A live-attenuated Pseudomonas aeruginosa vaccine elicits outer membrane protein-specific active and passive protection against corneal infection. Infect. Immun. 74:975–983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zaidi TS, Zaidi T, Pier GB. 2010. Role of neutrophils, MyD88-mediated neutrophil recruitment, and complement in antibody-mediated defense against Pseudomonas aeruginosa keratitis. Invest. Ophthalmol. Vis. Sci. 51:2085–2093 [DOI] [PMC free article] [PubMed] [Google Scholar]