Abstract
During infection, Staphylococcus aureus secretes two coagulases (Coa and von Willebrand factor binding protein [vWbp]), which, following an association with host prothrombin and fibrinogen, form fibrin clots and enable the establishment of staphylococcal disease. Within the genomes of different S. aureus isolates, coagulase gene sequences are variable, and this has been exploited for a classification of types. We show here that antibodies directed against the variable prothrombin binding portion of coagulases confer type-specific immunity through the neutralization of S. aureus clotting activity and protection from staphylococcal disease in mice. By combining variable portions of coagulases from North American isolates into hybrid Coa and vWbp proteins, a subunit vaccine that provided protection against challenge with different coagulase-type S. aureus strains in mice was derived.
INTRODUCTION
Staphylococcus aureus, a Gram-positive microbe that colonizes the human skin and nares, also causes invasive diseases such as skin and soft tissue infections, bacteremia, sepsis, and endocarditis (27). The emergence of methicillin-resistant strains in the community (community-acquired methicillin-resistant S. aureus [CA-MRSA]) and the increase in rates of hospital-acquired methicillin-resistant S. aureus (HA-MRSA) present a formidable therapeutic challenge (18). Although several vaccine development efforts have been launched, an FDA-licensed S. aureus vaccine is not yet available (8).
A hallmark of S. aureus isolates is their ability to form clots when inoculated into human citrate-plasma or blood (31). This phenotype has been linked to the secretion of coagulase (Coa) (6), which binds prothrombin and alters the enzyme's active site through the insertion of its N-terminal residues into the activation pocket, thereby providing for the cleavage of fibrinogen to fibrin (12). The mature form of Coa is comprised of N-terminal D1 and D2 domains, which enable the association with and activation of prothrombin (33) (Fig. 1). A linker (L) domain connects D12 and the repeat (R) region, comprised of tandem repeats of a 27-residue peptide that bind fibrinogen (34) (Fig. 1). The prothrombin·Coa complex (staphylocoagulase) converts soluble fibrinogen to insoluble fibrin, forming the mesh network of a clot (12, 21).
Fig 1.
Immune responses to coagulase. (A) Drawing to illustrate the primary structure of coagulase from S. aureus Newman (CoaNM), which was purified from E. coli via an N-terminal His6 tag. CoaNM encompasses the D1 and D2 domains involved in prothrombin (PT) binding, the linker (L) domain, and the repeat (R) domain, which is comprised of tandem repeats of a 27-residue peptide sequence that binds to fibrinogen (Fg). In addition to CoaNM, the D1Coa, D2Coa, D12Coa, LCoa, and RCoa domains were purified. (B) Rabbits were immunized with purified CoaNM, and immune sera were examined by an ELISA for serum IgG reactive with CoaNM, D1Coa, D2Coa, D12Coa, LCoa, or CTCoa. Statistical analysis was performed with the Student two-tailed t test (*, P < 0.05). (C) The association of D12Coa with human prothrombin was measured by an ELISA and perturbed with increasing concentrations of rabbit IgG directed against CoaNM (0 μM, 0.012 μM, 0.12 μM, or 1.2 μM) or the plague vaccine antigen V10 (1.2 μM) as a control. The association of CTCoa with fibrinogen was measured by an ELISA and perturbed with increasing concentrations of rabbit IgG directed against CoaNM (0 μM, 0.017 μM, 0.17 μM, or 1.7 μM) or the plague vaccine antigen V10 (1.7 μM) as a control. (D) Affinity-purified rabbit IgG specific for CoaNM (α-CoaNM), D12Coa (α-D12Coa), or CTCoa (α-CTCoa) was added to citrate-treated mouse blood and inoculated with S. aureus Newman to monitor the inhibition of staphylococcal coagulation.
When injected into animals, purified Coa clots blood in vivo, and this is thought to promote staphylococcal escape from phagocytic killing (13, 16). Coagulase typing, i.e., the neutralization of S. aureus coagulation of citrate-plasma with specific antiserum, has been used to distinguish 10 different serological Coa types (17). Coa types have also been analyzed by DNA sequencing, which revealed significant variation within coa sequences for the D12 domain and little variation for the linker and repeat regions, respectively (44). Is sequence variation within S. aureus coa genes the result of negative selection, as might occur when infected individuals develop antibody responses against secreted Coa? To begin to address this question, Watanabe and colleagues sequenced the coa genes from 126 S. aureus isolates, simultaneously analyzing them for coagulase serotype and clonal cluster (CC) type (44). The latter is accomplished via multilocus sequence typing (MLST), which examines sequences from seven different genes (arc, aro, glp, gmk, pta, tpi, and yqi) (11). With the exception of CC1 and CC8 strains, which frequently harbor different coa types, most of the isolates categorized as a single CC type by MLST also harbor a single coa sequence type (43). The variation of coa sequences is likely generated via horizontal gene transfer (for example, phage transduction or DNA transformation), because coa genes of the same sequence type are found scattered across the MLST tree (43). Together with the observation that pooled human immunoglobulin neutralizes most, but not all, coagulase types (40), these analyses suggest that coa gene diversification may enable S. aureus to circumvent the humoral immune responses of hosts with prior exposure to the pathogen (43). If so, Coa may represent a protective antigen of S. aureus and should be analyzed for its possible use as a vaccine antigen.
Nearly a century after the first description of staphylococcal coagulase, Bjerketorp and colleagues discovered von Willebrand factor (vWF) binding protein (vWbp) (3). vWbp is a secreted protein that, in addition to binding vWF, also associates with prothrombin to convert fibrinogen to fibrin (2, 12, 21). vWbp displays sequence homology to the Coa D12 domains (2, 44). However, its C-terminal domain lacks the L and R domains of Coa, which are replaced by unique vWF and fibrinogen binding sites (3, 6). Genome sequencing of prominent clinical strains discovered two distinct vwb alleles with variation in the predicted D12 domains (44). The immunization of mice with purified recombinant Coa and vWbp provides an additive level of protection against challenge with the same coagulase-type S. aureus strain in animal models of abscess formation and lethal bacteremia (6). S. aureus Newman mutants lacking coa and vwb, but not variants with single-gene deletions, displayed significant defects in mouse models of abscess formation or lethal bacteremia (6). The secretion of Coa and vWbp enables S. aureus to agglutinate in the presence of plasma, resulting in thromboembolic lesions as well as endocarditis and promoting the lethal outcome of staphylococcal bacteremia (28, 35). The blocking of coagulases with univalent direct thrombin inhibitors delays the time to death associated with lethal S. aureus challenge, further highlighting the importance of coagulases in staphylococcal disease (28).
Thus, Coa and vWbp promote the pathogenesis of S. aureus abscess formation and lethal bacteremia in mice by promoting staphylococcal coagulation and agglutination. If so, the antibody-mediated neutralization of Coa and vWbp may provide protection from staphylococcal disease, which could be exploited for the development of vaccines. Here we examined immune responses to coagulases and report that antibodies against the D12 domain neutralize staphylococcal coagulation in a type-specific manner. By injecting mice with a vaccine composed of four Coa-type D12 domains and two vWbp-type D12 domains (Coa4/vWbp2) that harbors antigenic determinants from the major North American isolates (CC1 [USA400], CC5 [USA100], CC8 [USA300], CC30, and CC45) (19, 36), mice could be protected against challenge with several different S. aureus strains.
MATERIALS AND METHODS
Ethics statement.
Experiments with blood from human volunteers involved a protocol that was reviewed, approved, and performed under the regulatory supervision of the University of Chicago's Institutional Review Board (IRB). Written, informed consent was provided by all volunteers. Animal experiments involving S. aureus challenge followed protocols that were reviewed, approved, and performed under the regulatory supervision of the University of Chicago's Institutional Biosafety Committee (IBC) and the Institutional Animal Care and Use Committee (IACUC). Animals were managed by the University of Chicago Animal Resource Center, which is accredited by the American Association for Accreditation of Laboratory Animal Care and the Department of Health and Human Services (DHHS) (protocol A3523-01). Animals were maintained in accordance with the applicable portions of the Animal Welfare Act and the DHHS Guide for the Care and Use of Laboratory Animals (31a). Veterinary Care was under the direction of full-time resident veterinarians boarded by the American College of Laboratory Animal Medicine. BALB/c mice and New Zealand White rabbits were purchased from Charles River Laboratories and Harlan Sprague-Dawley, respectively. The statistical analysis of staphylococcal sepsis was performed by using the two-tailed log rank test. The results of all animal experiments were examined for reproducibility.
Bacterial strains and growth of cultures.
S. aureus strains were cultured on tryptic soy agar or broth at 37°C. Escherichia coli strains DH5α and BL21(DE3) were cultured on Luria-Bertani agar or broth at 37°C. Ampicillin (100 μg/ml) was used for pET15b and pGEX2tk selection. Primers used for the amplification of staphylococcal DNA are found in Table S1 in the supplemental material.
Coa4 and vWbp2.
To generate the hybrid proteins, coa and vwb from strain USA300 were PCR amplified. The 5′ primer included the restriction site (NcoI) to insert onto the vector (pET15b) as well as an additional restriction enzyme (AvrII) for future use. The 3′ primer included the restriction site (BamHI) for vector insertion. The inserts were cloned into E. coli strain DH5α. In each subsequent round of cloning, the D12 region from the next allele was added 5′ to the previous insert. In each case, the 5′ primer included the vector site (NcoI) and an additional restriction enzyme site for future use. The 3′ primer for each sequential insert contained the restriction site (AvrII for N315) included in the 5′ primer for the previous insert. The promoter region and His tag were restored in a subsequent round of cloning, and a C-terminal Strep tag was added in another round of cloning. The entire vector was sequenced to verify the DNA sequence. Finally, each vector was transformed into E. coli strain BL21 for protein expression and purification.
Protein purification.
E. coli BL21(DE3) cells harboring expression vectors (containing coa from S. aureus Newman; vwb from S. aureus strains Newman, USA300 and N315; or subdomains of coa and vwb as well as expression vectors containing the genetic sequence for the hybrid proteins Coa4 and vWbp2) were grown at 37°C and induced with 100 mM isopropyl-β-d-thiogalactopyranoside (IPTG) overnight at room temperature. Because of degradation during the purification of Coa, pGEX2tk expression vectors in E. coli DH5α were used to express coa from USA300, N315, MW2, MRSA252, 85/2082, and WIS as glutathione S-transferase (GST)-tagged constructs. At 3 h following induction, cells were centrifuged at 7,000 × g, suspended in 1× column buffer (0.1 M Tris-HCl [pH 7.5], 0.5 M NaCl), and lysed in a French pressure cell at 14,000 lb/in2. Lysates were subjected to ultracentrifugation at 40,000 × g for 30 min. The supernatant of pET15b constructs was subjected to Ni-nitrilotriacetic acid (NTA) chromatography, washed with column buffer and 10 mM imidazole, and eluted with 500 mM imidazole. For Strep-tagged proteins, lysate supernatants were subjected to chromatography over StrepTactin-Sepharose (GE), washed in 1× Strep wash buffer (0.1 M Tris-HCl [pH 8.0], 0.150 M NaCl, 0.1 M EDTA), and eluted in 1× Strep wash buffer containing 2.5 mM desthiobiotin. For GST-tagged proteins, the supernatant of cleared lysates was subjected to glutathione-Sepharose chromatography. To remove the GST tag, following washing with column buffer, the column buffer was switched to PreScission protease cleavage buffer containing 10 mM dithiothreitol (DTT), and the column was incubated with PreScission protease (GE Healthcare) overnight at the unit definition provided by GE. Liberated protein lacking the GST tag was then collected with additional protease cleavage buffer. Eluates were dialyzed against phosphate-buffered saline (PBS). To remove endotoxin, a 1:100 dilution of Triton X-114 was added, and the solution was chilled for 10 min, incubated at 37°C for 10 min, and centrifuged at 13,000 × g. This was repeated twice. The supernatant was loaded onto a HiTrap desalting column to remove remnants of Triton X-114.
Rabbit antibodies.
The protein concentration was determined by using a bicinchoninic acid (BCA) kit (Pierce). Purity was verified by SDS-PAGE analysis and Coomassie brilliant blue staining. Six-month-old New Zealand White female rabbits were immunized with 500 μg protein emulsified in complete Freund's adjuvant (CFA) (Difco) for initial immunization or incomplete Freund's adjuvant (IFA) for booster immunizations on days 24 and 48. On day 60, rabbits were bled, and serum was recovered for immunoblotting or passive-transfer experiments. For antibody purification, recombinant His6-Coa, His6-vWbp, or His6-ClfA (5 mg) was covalently linked to HiTrap N-hydroxysuccinimide (NHS)-activated high-performance (HP) columns (GE Healthcare). This antigen matrix was then used for affinity chromatography of 10 to 20 ml of rabbit serum at 4°C. The charged matrix was washed with 50 column volumes of PBS, and antibodies were eluted with elution buffer (1 M glycine [pH 2.5], 0.5 M NaCl) and immediately neutralized with 1 M Tris-HCl (pH 8.5). Purified antibodies were dialyzed overnight against PBS–0.5 M NaCl at 4°C.
Coagulation assay.
Cultures of staphylococcal strains grown overnight were diluted 1:100 into fresh tryptic soy broth (TSB) and grown at 37°C until they reached an optical density at 600 nm (OD600) of 0.4. One milliliter of culture was centrifuged, and staphylococci were washed and suspended in 1 ml of sterile PBS to generate a suspension of 1 × 108 CFU/ml. Whole blood from naïve BALB/c mice was collected, and sodium citrate was added to a final concentration of 1% (wt/vol). To assess bacterial blood-coagulating activity in the presence of antibodies, 10 μl of the stock bacterial culture was mixed with 10 μl of PBS containing a 30 μM anti-Coa and anti-vWbp mixture in a sterile plastic test tube (BD Falcon) and incubated for 15 min. To each tube, 80 μl of anti-coagulated mouse blood was added in a sterile plastic test tube (BD falcon) to achieve a final concentration of 1 × 107 CFU/ml. Test tubes were incubated at 37°C, and blood coagulation was verified by tipping the tubes to 45° angles at timed intervals. All experiments were performed twice for reproducibility.
Active immunization.
Three-week-old BALB/c mice (n = 10) were injected with 50 μg protein emulsified in incomplete Freund's adjuvant and complete Freund's adjuvant (3:2). At 11 days postvaccination, these mice were boosted with 50 μg protein, each emulsified in incomplete Freund's adjuvant. On day 21, mice were anesthetized with ketamine-xylazine, and blood was collected by retro-orbital bleeding using microhematocrit capillary tubes (Fisher) in Z-Gel microtubes (Sarstedt) for determining half-maximal titers. Tubes were centrifuged at 10,000 × g for 3 min, and serum was collected. Half-maximal antibody titers were measured by an enzyme-linked immunosorbent assay (ELISA).
Passive transfer of antibodies.
Six hours prior to infection, 6-week-old BALB/c mice (n = 10) were injected intraperitoneally with affinity-purified antibodies against full-length or subdomain constructs of Coa or vWbp or against V10 (control IgG specific for the LcrV plague antigen) at a dose of 5 mg/kg of body weight.
Sepsis.
Cultures of staphylococcal strains grown overnight were diluted 1:100 into fresh TSB and grown until they reached an OD600 of 0.4. Bacteria were centrifuged at 7,000 × g, washed, and suspended in a 1/10 volume of PBS. Six-week-old female BALB/c mice (n = 10) (Charles River) were injected retro-orbitally with suspensions containing 1 × 108 CFU (S. aureus Newman, N315, and WIS), 5 × 107 CFU (S. aureus USA300), or 2 × 108 CFU (S. aureus MW2 and CowanI) in 100 μl of PBS. Mice were monitored for survival over 10 days.
Renal abscess.
S. aureus strains were prepared as described above for sepsis, but following washing, bacterial pellets were suspended in an equal volume, resulting in 1-log-fewer CFU than for sepsis. To enumerate the staphylococcal load in kidney tissue at 5 days postinfection, mice were euthanized by CO2 asphyxiation, and kidneys were removed during necropsy. One kidney per mouse was homogenized in PBS–1% Triton X-100. Serial dilutions of homogenate were spread onto tryptic soy agar (TSA) and incubated for colony formation. The bacterial load in tissue was analyzed by pairwise comparisons between wild-type and mutant strains with the unpaired two-tailed Student t test. For histopathology, the alternate kidney was fixed in 10% formalin for 24 h at room temperature. Tissues were embedded in paraffin, thin sectioned, stained with hematoxylin and eosin, and examined by light microscopy to enumerate pathological lesions per organ. Data were analyzed in pairwise comparisons between wild-type and mutant strains with the unpaired two-tailed Student t test.
Coagulase activity.
Purified recombinant Coa or vWbp (100 nM) was mixed with human prothrombin (Innovative Research) in 1% sodium citrate–PBS. After an initial reading, fibrinogen (3 μM) (Sigma) was added, and the conversion of fibrinogen to fibrin was measured as the increase in the turbidity at 450 nm in a plate reader (BioTek) at 2.5-min intervals. As controls, the enzymatic activity of human alpha-thrombin (Innovative Research) or prothrombin alone was measured.
RESULTS
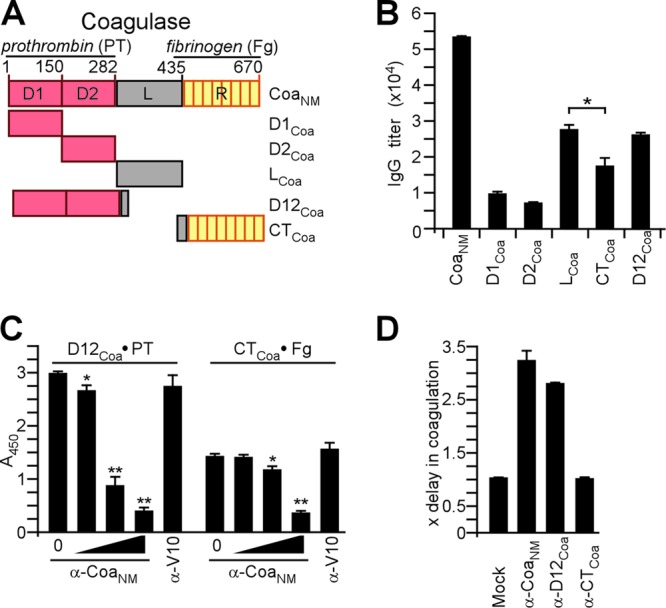
Antibodies against coagulase domains.
Rabbits were immunized with affinity-purified His-tagged Coa derived from the coagulase gene of S. aureus Newman (CoaNM). CoaNM-specific immune serum was examined by an ELISA, which revealed serum IgG antibody responses to antigen (Fig. 1A and B). To analyze the antibody responses against specific subdomains, affinity-purified recombinant proteins (D1Coa, D2Coa, D12Coa, LCoa, and CTCoa) were subjected to an ELISA (Fig. 1B). Immune serum harbored antibodies against each of the domains tested (Fig. 1B). Of note, antibodies against LCoa were more abundant than antibodies that recognized the repeat domain (CTCoa) (P < 0.05 for LCoa versus CTCoa). Antibodies against D12Coa were more abundant than those that recognized the repeat domain, but this difference did not achieve statistical significance. To probe the biological function of antibodies in rabbit immune serum, we used variable amounts of affinity-purified CoaNM antibodies to perturb the association of D12Coa with human prothrombin or the association of CTCoa with fibrinogen (Fig. 1C). We calculated that 120 nM anti-Coa IgG blocked D12Coa binding to prothrombin, whereas 1.7 μM anti-Coa IgG blocked the association of CTCoa with fibrinogen (Fig. 1C).
Rabbit CoaNM immune serum was subjected to affinity chromatography using either full-length CoaNM (anti-CoaNM antibody), D12Coa (anti-D12Coa antibody), or CTCoa (anti-CTCoa antibody). Equimolar amounts of affinity-purified IgG were added to citrate-blood samples obtained from naïve BALB/c mice, which were subsequently inoculated with S. aureus CC8 strain Newman (1). Compared to control samples without antibody, the addition of either anti-CoaNM or anti-D12Coa IgG caused a significant delay in the clotting time, whereas the anti-CTCoa antibody did not (Fig. 1D). Thus, rabbits respond to immunization with CoaNM by generating antigen-specific IgG molecules that are directed predominantly against D12Coa and LCoa and interfere with the clotting activity of secreted Coa. In contrast, antibodies against CTCoa are generated in a lesser abundance and do not interfere with S. aureus Newman in vitro blood coagulation.
Type-specific and cross-protective inhibition of S. aureus coagulation.
To examine the ability of the anti-CoaNM antibody to block the coagulation of other strains isolated from human infections, antigen-specific IgG was added to citrate-blood samples from naïve mice that were subsequently inoculated with S. aureus N315 (CC5), Mu50 (CC5), Newman (CC8), USA300 (CC8), MRSA252 (CC30), CowanI (CC30), MW2 (CC1), or MSSA476 (CC1) (Table 1). CoaNM-specific IgG delayed clotting by S. aureus Newman (CC8), USA300 (CC8), and N315 (CC5) but not clotting by MW2 (CC1), MSSA476 (CC1), Mu50 (CC5), MRSA252 (CC30), or CowanI (CC3) (Table 1). A 1.5-fold or greater delay in staphylococcal coagulation was considered a significant reduction in the clotting time. These results suggested that antibodies against CoaNM not only interfere with the coagulation of S. aureus strains from the same CC type (or Coa type) but also may interfere with the coagulation of strains of other types (N315). To examine the generality of type-specific and cross-protective inhibition, CoaN315, CoaMW2, and CoaMRSA252 were purified, and rabbit immune sera were generated (Table 1). CoaN315-specific IgG inhibited the coagulation of S. aureus N315 (CC5) and Newman (CC8). Antibodies directed against CoaMW2 inhibited the clotting of S. aureus MW2 (CC1), N315 (CC5), Newman (CC8), and USA300 (CC8). Antibodies against CoaMRSA252 had very little activity in this assay. The coagulation of mouse blood by S. aureus strains was inhibited by antibodies raised against the corresponding Coa, with the exception of the CC30 antibody (CC8, CC5, and CC1 isolates). Cross-neutralization of coagulation was observed for antibodies directed against the coagulase from the CC8 strain and for the coagulases of CC1 and CC5 strains. Finally, antibodies directed against Coa from the CC1, CC5, CC8, CC30, and CC45 strains did not neutralize the clotting of S. aureus strain Mu50 (CC5), CowanI (CC30), or MSSA476 (CC30). We presume that blood clotting in these isolates may depend predominantly on another factor, for example, vWbp (see below).
Table 1.
Type-specific or cross-protective inhibition of staphylococcal coagulation by coagulase antibodiesa
| Coa type | CC type | Strain | Mean fold delay in time to clotting with antibody (SEM) |
||||
|---|---|---|---|---|---|---|---|
| Mock | Anti-Newman | Anti-N315 | Anti-MW2 | Anti-MRSA252 | |||
| IIa | 5 | N315 | 1.0 (0.0) | 1.8 (0.7) | 1.6 (0.4) | 1.7 (0.5) | 1.3 (0.2) |
| IIa | 5 | Mu50 | 1.0 (0.0) | 1.0 (0.0) | 1.2 (0.2) | 1.2 (0.2) | 1.1 (0.3) |
| IIIa | 8 | Newman | 1.0 (0.0) | 2.2 (0.5) | 1.6 (0.4) | 2.3 (0.6) | 1.2 (0.1) |
| IIIa | 8 | USA300 | 1.0 (0.0) | 1.5 (0.6) | 1.3 (0.3) | 1.6 (0.7) | 1.4 (0.4) |
| IVa | 30 | MRSA252 | 1.0 (0.0) | 0.9 (0.1) | 1.3 (0.0) | 1.1 (0.1) | 0.9 (0.1) |
| IVa | 30 | CowanI | 1.0 (0.0) | 0.9 (0.1) | 1.0 (0.0) | 1.0 (0.1) | 0.8 (0.3) |
| VIIa | 1 | MW2 | 1.0 (0.0) | 1.2 (0.3) | 0.9 (0.1) | 1.5 (0.3) | 1.1 (0.1) |
| VIIa | 1 | MSSA476 | 1.0 (0.0) | 1.0 (0.1) | 0.9 (0.1) | 1.3 (0.1) | 1.1 (0.4) |
Calcium-chelated mouse blood was inoculated with 1 × 106 CFU of the indicated strain of S. aureus mixed with PBS or anti-Coa antibody from strain Newman, MW2, N315, or MRSA252 (3 μM final concentration); incubated at 37°C; and monitored over time. Values represent fold delays in the time to clotting, calculated as the length of time for the clot to form in the presence of the antibody divided by the absence of antibody for that strain. Values in parentheses reflect the standard errors of the mean from three or four independent experiments. A 1.5-fold or greater delay in staphylococcal coagulation was considered a significant reduction in the clotting time.
Coagulase antibodies and their protective effect against staphylococcal disease.
Purified CoaNM, D12Coa, or CTCoa was emulsified and injected as a prime-boost regimen into BALB/c mice (n = 10). Sera of mock (PBS)-, CoaNM-, D12Coa-, or CTCoa-immunized animals were examined by an ELISA for IgG responses to antigen, which were detected in vaccinated animals but not in control mice (Fig. 2A and B). The immunization of mice with CoaNM raised antibodies directed predominantly against D12Coa and, to a lesser degree, against CTCoa (Fig. 2A). D12Coa immunization raised high-titer antibodies that reacted with full-length CoaNM (Fig. 2A). In contrast, CTCoa immunization generated weak antibody responses (Fig. 2A). Mice were challenged by intravenous injection with S. aureus Newman and observed for 10 days to assess protection against lethal sepsis (Fig. 2B). Compared to mock-immunized animals, vaccination with CoaNM, D12Coa, or CTCoa resulted in an increased time to death (P < 0.001 for CoaNM versus PBS, P < 0.01 for D12Coa versus PBS, and P < 0.05 for CTCoa versus PBS). Immune responses against CoaNM did not generate increased protection compared to either D12Coa or CTCoa vaccination (P > 0.05 for CoaNM versus CTCoa and P > 0.05 for D12Coa versus CTCoa).
Fig 2.
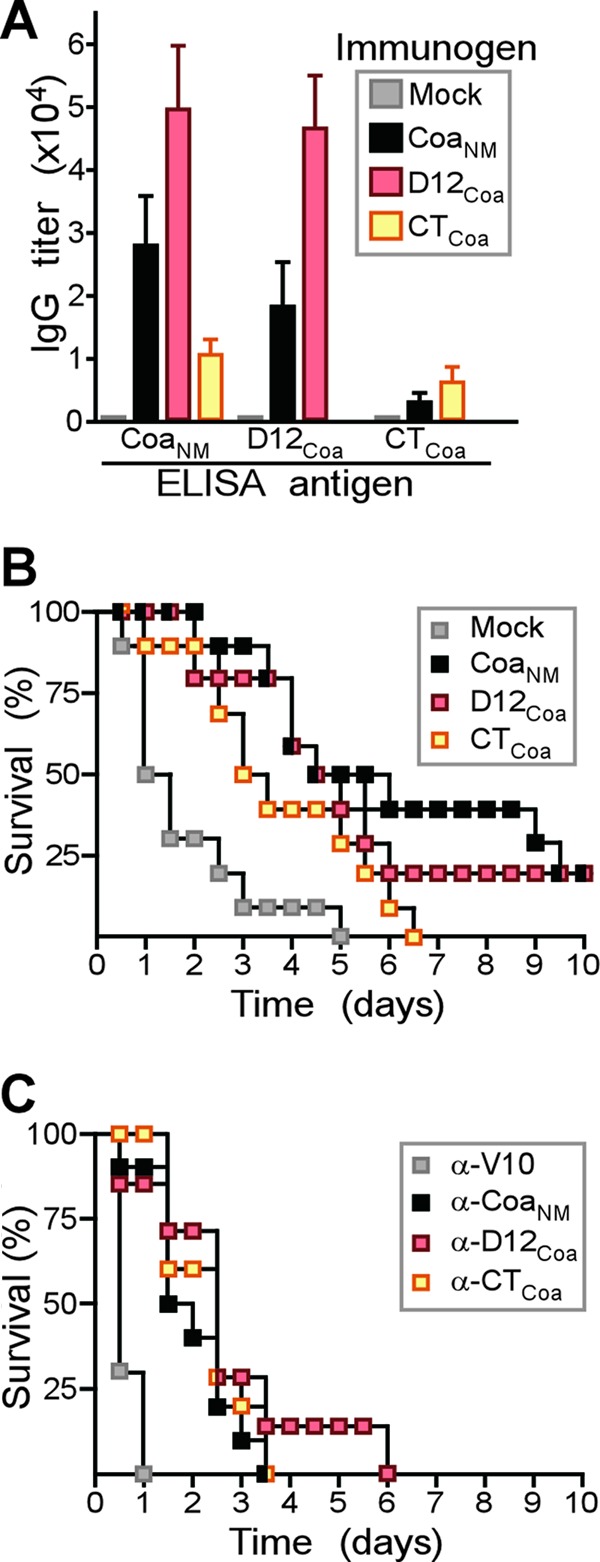
Coagulase domains as vaccine antigens. (A) Recombinant purified CoaNM, D12Coa, and CTCoa were used to immunize BALB/c mice (n = 5) with a prime-boost regimen, and immune sera were analyzed by an ELISA for the reactivity of mouse serum IgG toward purified CoaNM, D12Coa, or CTCoa. (B) Cohorts of BALB/c mice (n = 10) were immunized with a prime-boost regimen of purified CoaNM, D12Coa, and CTCoa and challenged by intravenous injection with S. aureus Newman (1 × 108 CFU). The survival of animals was monitored over 10 days. (C) Affinity-purified rabbit IgG specific for CoaNM (α-CoaNM), D12Coa (α-D12Coa), CTCoa (α-CTCoa), or V10 (α-V10) was injected at a concentration of 5 mg/kg of body weight into the peritoneal cavity of naïve BALB/c mice. Passively immunized mice were challenged by intravenous injection with S. aureus Newman (1 × 108 CFU), and the survival of animals was monitored over 10 days.
We asked whether antibodies directed against D12Coa or CTCoa provide protection against a lethal S. aureus challenge. Affinity-purified rabbit IgG was injected into the peritoneal cavity of naïve BALB/c mice at a concentration of 5 mg/kg of body weight (Fig. 2C). Four hours later, animals were challenged by the intravenous injection of S. aureus Newman (Fig. 2C). Compared to control antibodies specific for the V10 plague protective antigen (7) (anti-V10 antibody), IgGs directed against CoaNM, D12Coa, or CTCoa each caused a delay in the time to death for the corresponding cohort of challenged animals (P < 0.05 for all vaccines versus PBS) (Fig. 2C). No significant differences in disease protection were detected among antibodies directed against D12Coa, CTCoa, and full-length CoaNM (Fig. 2C). Thus, compared to D12Coa immunization, the CTCoa domain elicits low antibody responses. However, the passive transfer of antibodies against D12Coa and CTCoa provided similar levels of protection against a lethal S. aureus Newman challenge. These data suggest that the antibody-mediated neutralization of S. aureus Newman coagulase activity may not be an absolute prerequisite for disease protection. Following exposure to full-length CoaNM, BALB/c mice mount robust immune responses against D12Coa and LCoa but generate few antibodies against CTCoa.
Antibodies against von Willebrand factor binding protein domains.
Rabbits were immunized with affinity-purified His-tagged vWbp derived from the vwb gene of S. aureus Newman (vWbpNM). Immune serum was examined by an ELISA, which revealed serum IgG antibody responses to antigen (Fig. 3A and B). To analyze antibody responses against specific subdomains, affinity-purified D1vWbp, D2vWbp, D12vWbp, CT1vWbp, and CTvWbp were subjected to an ELISA (Fig. 3B). Immune serum harbored antibodies against each subdomain tested (Fig. 3B). Of note, antibodies against D1vWbp and D2vWbp were less abundant than antibodies that recognized both domains together (D12vWbp). Compared to immune responses against D12vWbp, antibodies directed against CTvWbp were 30% less abundant (P > 0.05 for D12vWbp versus CTvWbp). To probe the biological function of antibodies in the immune serum, we used variable amounts of vWbpNM-specific IgG to perturb the association of D12vWbp with human prothrombin and the association of CTvWbp with fibrinogen (Fig. 3C and D). A minimal concentration of 1.3 μM anti-vWbp IgG blocked D12vWbp binding to prothrombin as well as the CTvWbp association with fibrinogen (Fig. 3D).
Fig 3.
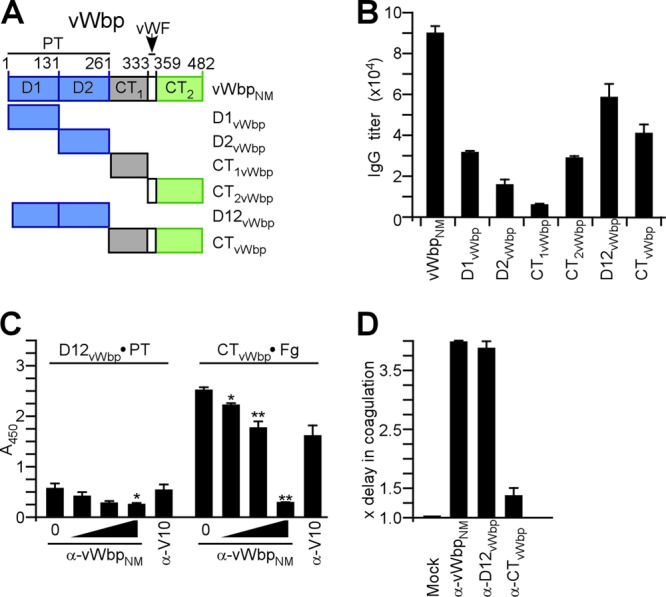
Immune responses to von Willebrand factor binding protein (vWbp). (A) Drawing to illustrate the primary structure of vWbp from S. aureus Newman (vWbpNM), which was purified from E. coli via an N-terminal His6 tag. vWbpNM encompasses the D1 and D2 domains, involved in prothrombin binding, as well as the von Willebrand factor (vWF) binding domain. In addition to vWbpNM, the D1vWbp, D2vWbp, D12vWbp, CT1vWbp, CT2vWbp, and CTvWbp domains were purified. (B) Rabbits were immunized with purified vWbpNM, and immune sera were examined by an ELISA for serum IgG reactive with vWbpNM, D1vWbp, D2vWbp, D12vWbp, CT1vWbp, CT2vWbp, and CTvWbp. (C) The association of D12vWbp with human prothrombin or the binding of CTvWbp to fibrinogen was measured by an ELISA and perturbed with increasing concentrations of rabbit IgG directed against vWbpNM (0 μM, 0.013 μM, 0.13 μM, or 1.3 μM) or the plague vaccine antigen V10 (1.3 μM) as a control. (D) Affinity-purified rabbit IgG specific for vWbpNM (α-vWbpNM), D12vWbp (α-D12vWbp), or CTvWbp (α-CTvWbp) was added to citrate-treated mouse blood and inoculated with S. aureus Newman to monitor the inhibition of staphylococcal coagulation.
Equimolar amounts of affinity-purified IgG were added to citrate-blood samples obtained from naïve BALB/c mice and subsequently inoculated with a coa mutant derived from S. aureus Newman (6). Compared to control samples without antibody, both anti-vWbp and anti-D12vWbp antibodies caused small delays in the clotting time, whereas anti-CTvWbp antibody did not delay the clotting time (Fig. 3D). Thus, rabbits respond to immunization with vWbpNM by generating antigen-specific IgG directed against D12vWbp, CT1vWbp, and CTvWbp. Antibodies against D12vWbp interfere with the vWbp-mediated coagulation of mouse blood in vitro.
Antibodies against vWbp domains and their protective effect on staphylococcal disease.
Purified vWbpNM, D12vWbp, or CTvWbp was emulsified and injected into BALB/c mice (n = 10) as a prime-boost regimen. Sera of mock (PBS)-immunized or vWbpNM-, D12vWbp-, and CTvWbp-immunized animals were examined by an ELISA for IgG responses to antigen, revealing specific immune responses in vaccinated animals but not in control mice (Fig. 4A and B). Of note, the immunization of mice with vWbpNM raised antibodies predominantly against D12vWbp and, to a lesser degree, antibodies that were directed against CTvWbp (Fig. 4A). D12vWbp immunization raised high-titer antibodies that reacted with full-length vWbpNM (Fig. 4A). In contrast, CTvWbp immunization generated weak antibody responses (Fig. 4A). Mice were challenged by intravenous injection with S. aureus Newman, and a 10-day observation period was used to assess protection against lethal sepsis (Fig. 4B). Compared to mock-immunized animals, vaccination with vWbpNM, D12vWbp, or CTvWbp resulted in an increased time to death (P < 0.01 for vWbpNM versus PBS, P < 0.05 for D12vWbp versus PBS, and P < 0.05 for CTvWbp versus PBS). Immune responses against vWbpNM outperformed vaccination with D12vWbp but not CTvWbp in generating protection against lethal S. aureus challenge (P < 0.05 for vWbpNM versus D12vWbp and P > 0.05 for vWbpNM versus CTvWbp) (Fig. 4B).
Fig 4.
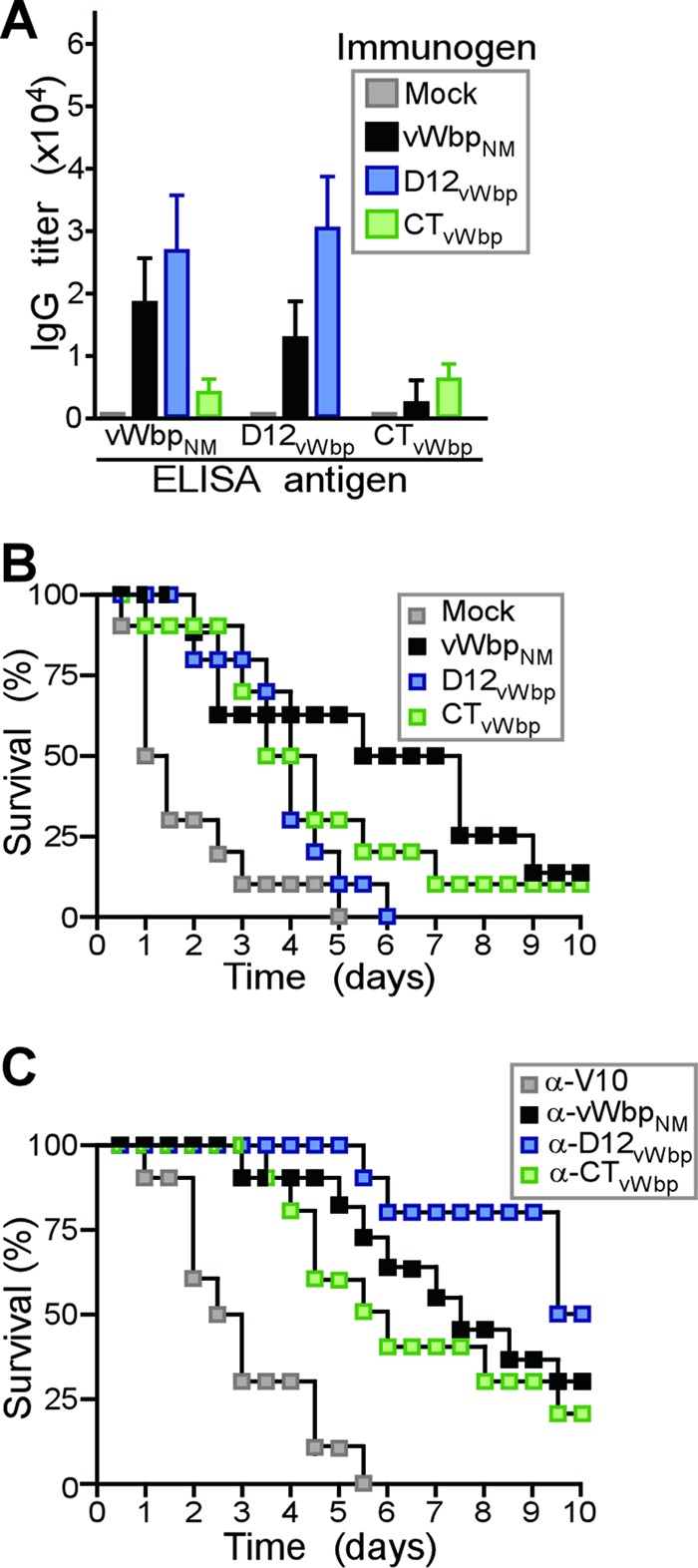
von Willebrand factor binding protein (vWbp) domains as vaccine antigens. (A) Recombinant purified vWbpNM, D12vWbp, and CTvWbp were used to immunize BALB/c mice (n = 5) with a prime-boost regimen, and immune sera were analyzed by an ELISA for the reactivity of mouse serum IgG toward purified vWbpNM, D12vWbp, and CTvWbp. (B) Cohorts of BALB/c mice (n = 10) were immunized with a prime-boost regimen of purified vWbpNM, D12vWbp, and CTvWbp and challenged by intravenous injection with S. aureus Newman (1 × 108 CFU). The survival of animals was monitored over 10 days. (C) Affinity-purified rabbit IgG specific for vWbpNM (α-vWbpNM), D12vWbp (α-D12vWbp), CTvWbp (α-CTvWbp), or V10 (α-V10) was injected at a concentration of 5 mg/kg of body weight into the peritoneal cavity of naïve BALB/c mice. Passively immunized mice were challenged by intravenous injection with S. aureus Newman (1 × 108 CFU), and the survival of animals was monitored over 10 days.
We asked whether antibodies directed against D12vWbp or CTvWbp provide protection against a lethal S. aureus challenge. Affinity-purified rabbit IgG was injected into the peritoneal cavity of naïve BALB/c mice at a concentration of 5 mg/kg of body weight (Fig. 4C). Twenty-four hours later, animals were challenged by the intravenous injection of S. aureus Newman (Fig. 4C). Compared to control antibodies (anti-V10 antibody), IgGs directed against vWbpNM, D12vWbp, or CTvWbp each caused a delay in the time to death for the corresponding cohort of challenged animals (P < 0.05 for all vaccines versus anti-V10 antibody) (Fig. 4C). No significant differences in disease protection were detected among antibodies directed against D12vWbp, CTvWbp, and full-length vWbpNM (Fig. 4C). Thus, in contrast to D12vWbp, immunization with the CTvWbp domain elicits low antibody responses. The passive transfer of antibodies against D12vWbp and CTvWbp provides similar levels of protection against a lethal S. aureus Newman challenge. These data suggest that the antibody-mediated neutralization of S. aureus Newman vWbp via antibodies directed against either D12vWbp or CTvWbp correlates with disease protection. Following exposure to full-length vWbpNM, BALB/c mice mounted robust immune responses against D12vWbp and CT1vWbp but generated few antibodies against CTvWbp.
Cross-protective attributes of the CoaNM/vWbpNM vaccine.
Purified recombinant CoaNM and vWbpNM were emulsified and injected into BALB/c mice (n = 10) as a prime-boost immunization regimen. Sera of mock (PBS)- and CoaNM/vWbpNM-immunized animals were examined by an ELISA for IgG responses to each antigen (Fig. 5A). Mice were challenged by the intravenous injection of S. aureus and monitored for 10 days (Fig. 5). CoaNM/vWbpNM immunization raised protection against S. aureus USA300 (CC8, the same type as S. aureus Newman) but not against MW2 (CC1) or N315 (CC5) (Fig. 5B to D). Nevertheless, CoaNM/vWbpNM immunization generated protection against challenge with S. aureus CowanI (CC30) and WIS (CC45) (Fig. 5E and F). Taken together, these data indicate that the CoaNM/vWbpNM vaccine provided type-specific immunity (CC8 strains Newman and USA300) as well as cross-protection against some (CC30 and CC45), but not all, coagulase-type strains (CC1 and CC5).
Fig 5.
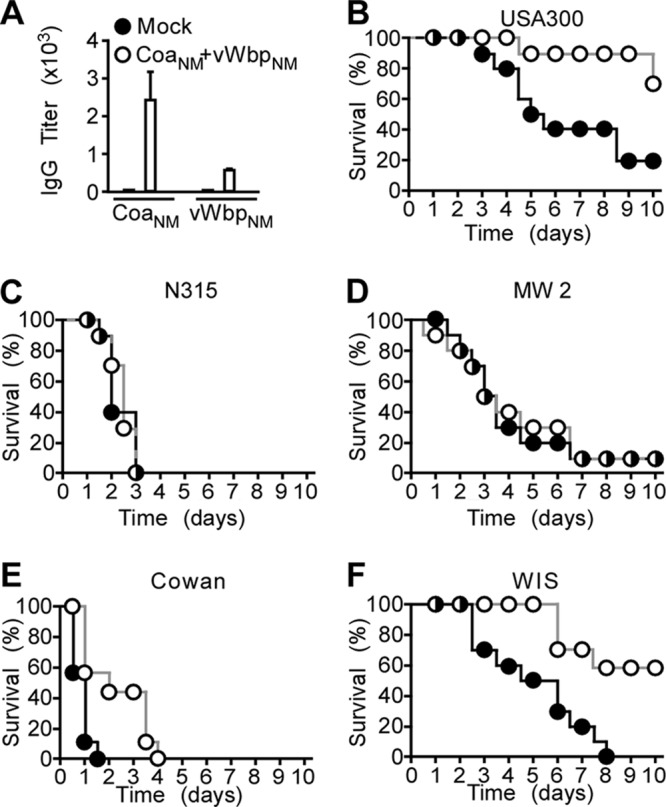
Immunization of mice with the CoaNM/vWbpNM vaccine and spectrum of disease protection against different S. aureus isolates. (A) Recombinant CoaNM/vWbpNM or mock (PBS) vaccine was used to immunize BALB/c mice (n = 5) with a prime-boost regimen. Immune sera were analyzed by an ELISA for the reactivity of mouse serum IgG toward purified CoaNM and vWbpNM. (B to F) Cohorts of BALB/c mice (n = 10) were immunized with a prime-boost regimen of purified CoaNM/vWbpNM or mock vaccine and challenged by intravenous injection with S. aureus USA300 (B), N315 (C), MW2 (D), CowanI (E), or WIS (F). The survival of animals was monitored over 10 days.
Immune responses elicited by the Coa4/vWbp2 vaccine.
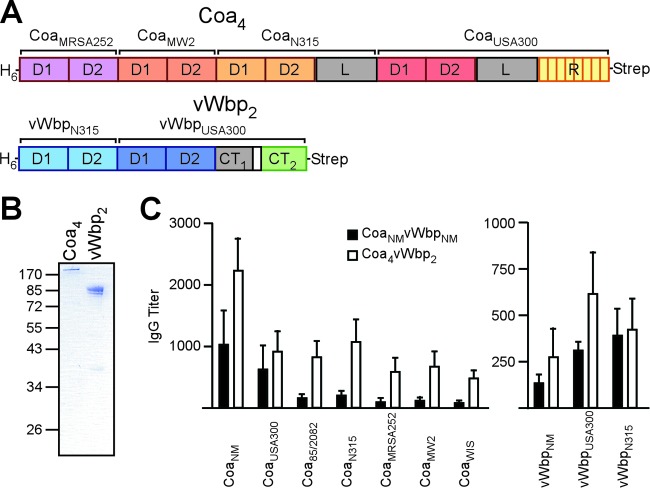
A polypeptide that harbors the D12 domains of CoaMRSA252, CoaMW2, and CoaN315 and full-length CoaUSA300 in addition to N-terminal His6 and C-terminal Strep tags (Coa4) was engineered (Fig. 6A). Coa4 was purified by affinity chromatography on StrepTactin-Sepharose and Ni-NTA Sepharose (Fig. 6B). When analyzed by Coomassie-stained SDS-PAGE gels, affinity-purified Coa4 was revealed as a 190-kDa polypeptide (Fig. 6B). Coa4 encompasses the D12 domains from four of the most frequently detected coagulase-type S. aureus isolates from North American patients (CC1, CC5, CC8, and CC30) (9). The vWbp2 polypeptide encompasses the D12 domain of vWbpN315 and full-length vWbpUSA300 in addition to N-terminal His6 and C-terminal Strep tags (Fig. 6A). vWbp2 was purified by affinity chromatography and migrated at the expected mass of 85 kDa on Coomassie-stained SDS-PAGE gels (Fig. 6B). Mice (n = 5) were immunized with a prime-boost regimen of CoaNM/vWbpNM or Coa4/vWbp2, and immune responses to various coagulase and von Willebrand factor binding protein types were examined by an ELISA (Fig. 6C and D). The CoaNM/vWbpNM vaccine raised antibodies in mice that bound to the coagulases from CC8 strains but displayed little cross-reactivity toward CoaN315, CoaMRSA252, CoaMW2, or CoaWIS. In comparison, Coa4 immunization raised high-titer antibodies not only against CC8 type coagulases but also against the coagulases from CC1, CC5, CC30, and CC45 strains. Compared to vWbpNM, vWbp2 raised high-titer antibodies against vWbp of the CC5 and CC8 strains (Fig. 6D).
Fig 6.
Immunogenicity of the Coa4/vWbp2 vaccine. (A) Drawing to illustrate the design of the Coa4 and vWbp2 vaccine components. Coa4 is comprised of an N-terminal six-histidyl tag (H6); the Coa D12 domains of S. aureus strains MRSA252, MW2, and N315; and the full-length mature sequence of Coa from strain USA300 in addition to a C-terminal Strep tag. vWbp2 is comprised of an N-terminal six-histidyl tag, the vWbp D12 domains of S. aureus N315, and the full-length mature sequence of vWbp from strain USA300 in addition to a C-terminal Strep tag. (B) Coa4 and vWbp2 were purified from E. coli via streptavidin affinity chromatography and analyzed by Coomassie-stained SDS-PAGE gels. (C) BALB/c mice (n = 5) were immunized with either CoaNM/vWbpNM or Coa4/vWbp2 by using a prime-boost regimen. Immune sera were analyzed by an ELISA for the reactivity of mouse serum IgG toward purified recombinant coagulase from S. aureus Newman (CoaNM), USA300 (CoaNM), 85/2082 (Coa85/2082), N315 (CoaN315), MRSA252 (CoaMRSA252), MW2 (CoaMW2), or WIS (CoaWIS) as well as purified recombinant vWbp from S. aureus Newman (vWbpNM), USA300 (vWbpNM), or N315 (vWbpN315).
Cross-protective attributes of the Coa4/vWbp2 vaccine.
Purified recombinant Coa4/vWbp2 was emulsified and injected into BALB/c mice (n = 10) by using a prime-boost immunization regimen. Sera of mock (PBS)- and Coa4/vWbp2-immunized animals were examined by an ELISA for IgG responses to Coa4 as well as vWbp2, which revealed antigen-specific immune responses in vaccinated but not in control mice (Fig. 7A). The intravenous injection of mice with S. aureus and a 10-day observation period were used to assess vaccine protection against lethal challenge with various strains (Fig. 7). As expected, Coa4/vWbp2 immunization raised protection against S. aureus CC8 strain USA300 (6). Similar to CoaNM/vWbpNM immunization, the Coa4/vWbp2 vaccine raised protection against S. aureus CowanI (CC30) and WIS (CC45) challenges. Unlike CoaNM/vWbpNM, Coa4/vWbp2 protected mice against lethal challenge with either S. aureus N315 (CC5) or MW2 (CC1) (Fig. 7B to F) (P < 0.05 for mock versus Coa4/vWbp2). Taken together, these data indicate that the CoaNM/vWbpNM vaccine provided type-specific immunity as well as cross-protection against some, but not all, coagulase-type strains (Fig. 5E and F). Furthermore, the Coa4/vWbp2 vaccine protected animals against a challenge with the relevant S. aureus CC types isolated from North American patients with staphylococcal disease (Fig. 7).
Fig 7.
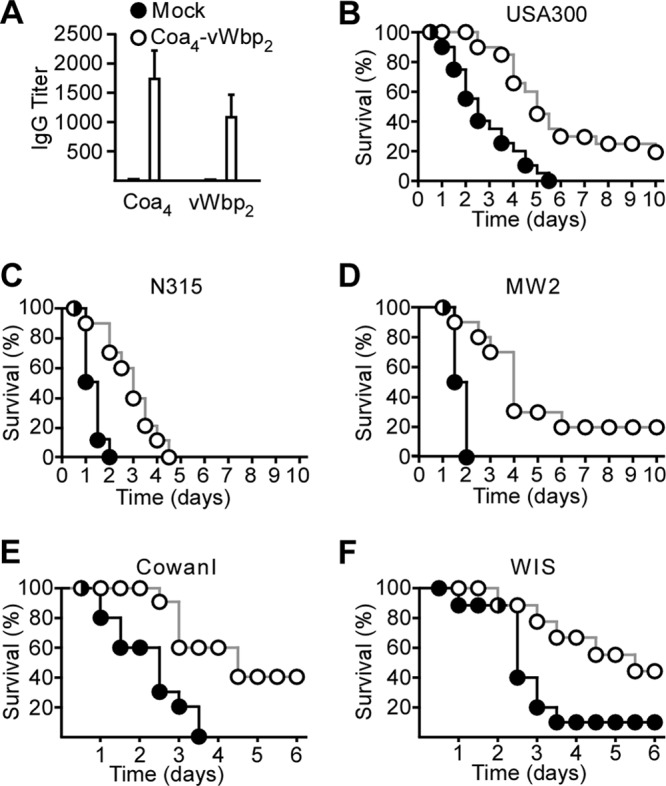
Immunization of mice with the Coa4/vWbp2 vaccine and spectrum of disease protection against different S. aureus isolates. (A) Coa4/vWbp2 or mock (PBS) vaccine was used to immunize BALB/c mice (n = 5) with a prime-boost regimen. Immune sera were analyzed by an ELISA for the reactivity of mouse serum IgG toward purified Coa4 and vWbp2. (B to F) Cohorts of BALB/c mice (n = 20 in panel B and n = 10 in panels C to F) were immunized with a prime-boost regimen of purified Coa4/vWbp2 or mock vaccine (B) and challenged by intravenous injection with S. aureus USA300 (B), N315 (C), MW2 (D), CowanI (E), or WIS (F). The survival of animals was monitored over 10 days.
We asked whether Coa4/vWbp2 immunization protects mice against staphylococcal abscess formation. BALB/c mice were immunized with a prime-boost regimen of Coa4/vWbp2 or the mock control and challenged by the intravenous inoculation of a sublethal dose of S. aureus strain USA300, N315, MW2, or CowanI. Five days after challenge, animals were euthanized and necropsied, and kidneys were removed. One of the two kidneys from each mouse was fixed, thin sectioned, and stained with hematoxylin-eosin for subsequent histopathology analyses (Table 2). The other kidney was homogenized and spread onto agar plates to enumerate the staphylococcal load as CFU (Table 2). Coa4/vWbp2 immunization led to a significant reduction in the bacterial load for animals challenged with S. aureus MW2 and CowanI but not for mice challenged with USA300 and N315. Of note, Coa- or vWbp-specific antibodies do not promote the opsonophagocytic killing of bacteria but interfere with staphylococcal abscess formation by neutralizing coagulases, thereby reducing the ability of staphylococci to replicate within the protective environment of these lesions (10). Compared to mock-immunized animals, Coa4/vWbp2 immunization reduced staphylococcal abscess formation in renal tissues 5 days following challenge with S. aureus strain USA300, CowanI, MW2, or N315 (Table 2).
Table 2.
Active immunization of mice with Coa4/vWbp2 and protection against challenge with S. aureus strain USA300, N315, MW2, or CowanIf
| Vaccine | Staphylococcal load in renal tissue |
Abscess formation |
|||
|---|---|---|---|---|---|
| Mean log10 CFU · g−1 (SEM)a | P valueb | Reduction (log10 CFU · g−1)c | Mean no. of lesions (SEM)d | P valuee | |
| S. aureus USA300 | |||||
| Mock | 7.31 (0.37) | NAg | NA | 8.8 (1.72) | |
| Coa4/vWbp2 | 6.48 (0.41) | 0.150 | 0.835 | 4.3 (1.11) | 0.0434 |
| S. aureus N315 | |||||
| Mock | 7.25 (0.13) | NA | NA | 16.6 (1.49) | |
| Coa4/vWbp2 | 7.10 (0.24) | 0.805 | 0.151 | 11.3 (0.84) | 0.0205 |
| S. aureus MW2 | |||||
| Mock | 8.04 (0.25) | NA | NA | 66.5 (8.41) | |
| Coa4/vWbp2 | 7.25 (0.20) | 0.029 | 0.789 | 27.5 (4.39) | 0.0011 |
| S. aureus CowanI | |||||
| Mock | 6.94 (0.16) | NA | NA | 7.9 (1.27) | |
| Coa4/vWbp2 | 5.59 (0.51) | 0.028 | 1.35 | 4.6 (0.73) | 0.0279 |
Means of staphylococcal load calculated as log10 CFU · g−1 in homogenized renal tissues 5 days after infection of cohorts of 10 BALB/c mice per immunization. Standard errors of the means are indicated.
Statistical significance was calculated with the Mann-Whitney test, and P values were recorded; P values of <0.05 were deemed significant.
Reduction in bacterial load calculated as log10 CFU · g−1.
Histopathology of thin-sectioned, hematoxylin-eosin-stained kidneys from 8 to 10 animals. The average number of abscesses per kidney was recorded and averaged again for the final mean (and standard error of the mean).
Statistical significance was calculated with the nonparametric Mann-Whitney test, and P values were recorded. P values of <0.05 are significant.
BALB/c mice (n = 8 to 10) were challenged by the intravenous inoculation of 5 × 106 CFU S. aureus USA300, 1 × 107 CFU S. aureus N315, 2 × 107 CFU S. aureus MW2, or 1 × 108 CFU S. aureus CowanI. Five days later, animals were sacrificed by CO2 asphyxiation, and both kidneys were removed. One kidney was fixed in formaldehyde, embedded in paraffin, thin sectioned, and hematoxylin-eosin stained, and four sagittal sections per kidney were analyzed for abscess formation. The other kidney was homogenized in PBS containing 1% Triton X-100, the homogenate was spread onto agar medium for colony formation, and the staphylococcal load was enumerated as CFU. The experiment was conducted twice.
NA, not applicable.
DISCUSSION
Early work on coagulase demonstrated that following S. aureus infection, humans as well as animals generate Coa-specific antibodies (25, 41). When transferred to naïve rabbits, these antibodies may neutralize S. aureus coagulation and, at least in some cases, may confer immunity to challenge with S. aureus (24, 26). The active immunization of rabbits with preparations containing coagulase prolonged the life of rabbits that had been challenged by intravenous inoculation with lethal doses of S. aureus (4). A comparison of different (phage-typed) S. aureus isolates for the inhibition of plasma clotting by coagulase antiserum revealed both phage type-specific and nonspecific neutralization (10, 15, 25, 26, 37). These data supported a general concept for the existence of serological types of Coa, which are not strictly linked to S. aureus phage types (38).
Purified coagulase toxoid, encompassing purified Coa from S. aureus strains M1 and Newman adsorbed onto aluminum phosphate, was examined for the therapeutic immunization of 71 patients with chronic furunculosis (14). Compared to placebo, coagulase immunization generated an increase in coagulase-specific antibody titers but failed to improve the clinical outcome of chronic furunculosis (14). Of note, the development of neutralizing antibodies or the possibility of type-specific immunity was not examined (14). Thus, although early work revealed the preclinical efficacy of coagulase subunit vaccines, clinical studies failed to demonstrate efficacy in a human trial. As most of those studies were conducted from 1945 to 1965, one must consider the limited tools for the isolation of highly purified coagulases as well as the inability to type S. aureus strains or coagulase vaccine preparations on the basis of their nucleotide sequence. Furthermore, earlier studies were conducted without a knowledge of vWbp or of the molecular mechanisms of Coa- and vWbp-mediated prothrombin activation and fibrinogen cleavage (12, 21). We recently observed that both coagulases secreted by S. aureus Newman, CoaNM and vWbpNM, are sufficient for the ability of this strain to cause abscess formation and rapidly lethal bacteremia in mice (6). In active and passive immunization experiments, antibodies against both CoaNM and vWbpNM were required to confer protection against abscess formation or lethal bacteremia (6). On the basis of those observations, we hypothesize that coagulases may function as protective antigens that elicit antibody responses against Coa and vWbp, which protect animals and humans against S. aureus disease (6). In agreement with this model, the expression of coa and vwb is a universal trait of S. aureus strains (5). Of note, the coa gene of S. aureus isolates is variable (29), with a greater variation in amino acid sequence than even the tandem repeats of the protein A (spa) gene; the variation in spa is used for epidemiological typing experiments (20, 43). S. aureus mutants that are unable to express coa have not yet been isolated from humans with manifest staphylococcal disease. The vwb gene is less variable (29). By analyzing currently available S. aureus genome sequences for vwb homology, we identified three alleles. Two of the vwb alleles varied in their coding sequences for the D12 domain (S. aureus N315 and USA300 are representatives of these alleles), whereas the third allele harbored a nucleotide substitution positioning a premature stop codon at position 317 in the D12 domain (S. aureus MRSA252).
Enabled by these observations, we report here that the immunization of rabbits or mice with Coa and vWbp generated antibodies predominantly against the D12 domain of CoaNM or vWbpNM. D12-specific antibodies neutralized the coagulase activities of S. aureus Newman and, when transferred to naïve animals, conferred protection against lethal bacteremia. The neutralization and disease protection of CoaNM- and vWbpNM-specific antibodies occurred in a type-specific manner, not unlike the type-specific immunity reported previously for Streptococcus pyogenes M proteins (22, 23) or the pilus (T) antigens of S. pyogenes and Streptococcus agalactiae (30, 32). Informed by the structural vaccinology approach for pilus antigens (32, 39), we engineered two polypeptides that encompass the D12 domains of the major Coa and vWbp types from the North American S. aureus isolates: CC1, CC5, CC8, CC30, and CC45 strains (42). The purified products Coa4 and vWbp2 were used as antigens and elicited antibody responses against the D12 domains of every Coa and vWbp type examined. The immunization of mice with Coa4/vWbp2 provided protection against lethal bacteremia challenge with representative S. aureus CC1, CC5, CC8, CC30, and CC45 strains. Thus, the design criteria for the Coa4/vWbp2 vaccine, to generate broad-coverage immune responses to Coa and vWbp against clinically relevant S. aureus strains, have been met.
In addition to the type-specific neutralization of Coa and vWbp via antibodies directed against the D12 domain, antibodies against the R (Coa) and CT (vWbp) domains also provided protection against S. aureus disease. As antibodies against the R and CT domains do not affect the coagulation of fibrin via secreted Coa·prothrombin and vWbp·prothrombin complexes, we surmise that these adaptive immune mechanisms target coagulases via another mechanism. We currently do not appreciate how antibodies against the R domain of Coa or the CT domain of vWbp provide protection. It seems plausible that these antibodies may mediate the removal of Coa and vWbp from circulation via the binding of immune complexes to Fc receptors on macrophages. Until the molecular mechanism of protection is revealed, the overall value of a vaccine strategy that targets the R and CT domains of Coa and vWbp cannot be appreciated.
Supplementary Material
ACKNOWLEDGMENTS
We thank Philip Nussenzweig for technical assistance and members of our laboratory for discussion.
This work was supported by grants from the National Institute of Allergy and Infectious Diseases (NIAID) Infectious Diseases Branch (AI52474 and AI92711 to O.S. and AI52767 to D.M.M.). M.M. was a trainee of the Graduate Training in Growth and Development program at the University of Chicago (HD009007). D.M.M. and O.S. acknowledge membership within and support from the Region V Great Lakes Regional Center of Excellence in Biodefense and Emerging Infectious Diseases Consortium (NIH award 1-U54-AI-057153).
We declare a conflict of interests as inventors of patent applications that are related to the development of Staphylococcus aureus vaccines and are currently under commercial license.
Footnotes
Published ahead of print 23 July 2012
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1. Baba T, Bae T, Schneewind O, Takeuchi F, Hiramatsu K. 2008. Genome sequence of Staphylococcus aureus strain Newman and comparative analysis of staphylococcal genomes. J. Bacteriol. 190:300–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bjerketorp J, Jacobsson K, Frykberg L. 2004. The von Willebrand factor-binding protein (vWbp) of Staphylococcus aureus is a coagulase. FEMS Microbiol. Lett. 234:309–314 [DOI] [PubMed] [Google Scholar]
- 3. Bjerketorp J, et al. 2002. A novel von Willebrand factor binding protein expressed by Staphylococcus aureus. Microbiology 148:2037–2044 [DOI] [PubMed] [Google Scholar]
- 4. Boake WC. 1956. Antistaphylocoagulase in experimental staphylococcal infections. J. Immunol. 76:89–96 [PubMed] [Google Scholar]
- 5. Cheng AG, DeDent AC, Schneewind O, Missiakas DM. 2011. A play in four acts: Staphylococcus aureus abscess formation. Trends Microbiol. 19:225–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cheng AG, et al. 2010. Contribution of coagulases towards Staphylococcus aureus disease and protective immunity. PLoS Pathog. 6:e1001036 doi:10.1371/journal.ppat.1001036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. DeBord KL, et al. 2006. Immunogenicity and protective immunity against bubonic and pneumonic plague by immunization of mice with the recombinant V10 antigen, a variant of LcrV. Infect. Immun. 74:4910–4914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. DeDent A, Kim HK, Missiakas DM, Schneewind O. 2012. Exploring Staphylococcus aureus pathways to disease for vaccine development. Semin. Immunopathol. 34:317–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. DeLeo FR, Otto M, Kreiswirth BN, Chambers HF. 2010. Community-associated meticillin-resistant Staphylococcus aureus. Lancet 375:1557–1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Duthie ES, Lorenz LL. 1952. Staphylococcal coagulase: mode of action and antigenicity. J. Gen. Microbiol. 6:95–107 [DOI] [PubMed] [Google Scholar]
- 11. Enright MC, Day NPJ, Davies CE, Peacock SJ, Spratt BG. 2000. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J. Clin. Microbiol. 38:1008–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Friedrich R, et al. 2003. Staphylocoagulase is a prototype for the mechanism of cofactor-induced zymogen activation. Nature 425:535–539 [DOI] [PubMed] [Google Scholar]
- 13. Hale JH, Smith W. 1945. The influence of coagulase on the phagocytosis of staphylococci. Br. J. Exp. Pathol. 26:209–216 [Google Scholar]
- 14. Harrison KJ. 1963. Clinical trial of coagulase and alpha-hemolysin toxoids in chronic furunculosis. Br. Med. J. ii:149–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Harrison KJ. 1964. The protection of rabbits against infection with staphylococci by immunization with staphylocoagulase toxin or toxoid. J. Pathol. Bacteriol. 87:145–150 [PubMed] [Google Scholar]
- 16. Johnstone JM, Smith DD. 1956. Coagulase activity in vivo. Nature 178:982–983 [DOI] [PubMed] [Google Scholar]
- 17. Kanemitsu K, Yamamoto H, Takemura , Kaku M, Shimada J. 2001. Relatedness between the coagulase gene 3′-end region and coagulase serotypes among Staphylococcus aureus strains. Microbiol. Immunol. 45:23–27 [DOI] [PubMed] [Google Scholar]
- 18. Klevens RM, Edwards JR, Gaynes RP, and National Nosocomial Infections Surveillance System 2008. The impact of antimicrobial-resistant, health care-associated infections on mortality in the United States. Clin. Infect. Dis. 47:927–930 [DOI] [PubMed] [Google Scholar]
- 19. Klevens RM, et al. 2007. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 298:1763–1771 [DOI] [PubMed] [Google Scholar]
- 20. Koreen L, et al. 2004. spa typing method for discriminating among Staphylococcus aureus isolates: implications for use of a single marker to detect genetic micro- and macrovariation. J. Clin. Microbiol. 42:792–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kroh HK, Panizzi P, Bock PE. 2009. von Willebrand factor-binding protein is a hysteretic conformational activator of prothrombin. Proc. Natl. Acad. Sci. U. S. A. 106:7786–7791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lancefield R. 1962. Current knowledge of type-specific M antigens of group A streptococci. J. Immunol. 89:307–313 [PubMed] [Google Scholar]
- 23. Lancefield RC. 1928. The antigenic complex of Streptococcus hemolyticus. I. Demonstration of a type-specific substance in extracts of Streptococcus hemolyticus. J. Exp. Med. 47:91–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lominski I. 1949. Susceptibility and resistance to staphylococcal infection. J. Gen. Microbiol. 3:IX. [PubMed] [Google Scholar]
- 25. Lominski I, Roberts GBS. 1946. A substance in uman serum inhibiting staphylocoagulase. J. Pathol. Bacteriol. 58:187–197 [DOI] [PubMed] [Google Scholar]
- 26. Lominski I, et al. 1962. Immunisation against experimental staphylococcal infection with coagulase-rich preparations. Lancet i:1315–1318 [DOI] [PubMed] [Google Scholar]
- 27. Lowy FD. 1998. Staphylococcus aureus infections. N. Engl. J. Med. 339:520–532 [DOI] [PubMed] [Google Scholar]
- 28. McAdow M, et al. 2011. Preventing Staphylococcus aureus sepsis through the inhibition of its agglutination in blood. PLoS Pathog. 7:e1002307 doi:10.1371/journal.ppat.1002307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McCarthy AJ, Lindsay JA. 2010. Genetic variation in Staphylococcus aureus surface and immune evasion genes is lineage associated: implications for vaccine design and host-pathogen interactions. BMC Microbiol. 10:173 doi:10.1186/1471-2180-10-173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mora M, et al. 2005. Group A Streptococcus produce pilus-like structures containing protective antigens and Lancefield T antigens. Proc. Natl. Acad. Sci. U. S. A. 102:15641–15646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Much H. 1908. Über eine Vorstufe des Fibrinfermentes in Kulturen von Staphylokokkus aureus. Biochem. Z. 14:143–155 [Google Scholar]
- 31a. National Research Council 1996. Guide for the care and use of laboratory animals. National Academies Press, Washington, DC [Google Scholar]
- 32. Nuccitelli A, et al. 2011. A structure-based approach to rationally design a chimeric protein for an effective vaccine against group B Streptococcus infections. Proc. Natl. Acad. Sci. U. S. A. 108:10278–10283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Panizzi P, Friedrich R, Fuentes-Prior P, Bode W, Bock PE. 2004. The staphylocoagulase family of zymogen activator and adhesion proteins. Cell. Mol. Life Sci. 61:2793–2798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Panizzi P, et al. 2006. Fibrinogen substrate recognition by staphylocoagulase (pro)thrombin complexes. J. Biol. Chem. 281:1179–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Panizzi P, et al. 2011. In vivo detection of Staphylococcus aureus endocarditis by targeting pathogen-specific prothrombin activation. Nat. Med. 17:1142–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Patel G, et al. 2011. Clinical and molecular epidemiology of methicillin-resistant Staphylococcus aureus among patients in an ambulatory hemodialysis center. Hosp. Epidemiol. Am. 32:881–888 [DOI] [PubMed] [Google Scholar]
- 37. Rammelkamp CH, Hezebicks MM, Dingle JH. 1950. Specific coagulases of Staphylococcus aureus. J. Exp. Med. 91:295–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rammelkamp CH, Jr, Lebovitz JJ. 1956. Immunity, epidemiology and antimicrobial resistance. The role of coagulase in staphylococcal infections. Ann. N. Y. Acad. Sci. 65:144–151 [DOI] [PubMed] [Google Scholar]
- 39. Schneewind O, Missiakas D. 2011. Structural vaccinology to thwart antigenic variation in microbial pathogens. Proc. Natl. Acad. Sci. U. S. A. 108:10029–10030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Streitfeld MM, Sallman B, Shoelson SM. 1959. Staphylocoagulase inhibition by pooled human gamma-globulin. Nature 184:1665–1666 [DOI] [PubMed] [Google Scholar]
- 41. Tager M, Hales HB. 1948. The experimental production of antibodies to staphylocoagulase. J. Immunol. 60:475–485 [PubMed] [Google Scholar]
- 42. Tenover FC, et al. 2012. Characterization of nasal and blood culture isolates of methicillin-resistant Staphylococcus aureus from patients in the United States. Antimicrob. Agents Chemother. 56:1324–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Watanabe S, et al. 2009. Genetic diversity of staphylocoagulase genes (coa): insight into the evolution of variable chromosomal virulence factors in Staphylococcus aureus. PLoS One 4:e5714 doi:10.1371/journal.pone.0005714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Watanabe S, et al. 2005. Structural comparison of ten serotypes of staphylocoagulases in Staphylococcus aureus. J. Bacteriol. 187:3698–3707 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.