Abstract
Nocardia brasiliensis is a Gram-positive facultative intracellular bacterium frequently isolated from human actinomycetoma. However, the pathogenesis of this infection remains unknown. Here, we used a model of bacterial delipidation with benzine to investigate the role of N. brasiliensis cell wall-associated lipids in experimental actinomycetoma. Delipidation of N. brasiliensis with benzine resulted in complete abolition of actinomycetoma without affecting bacterial viability. Chemical analyses revealed that trehalose dimycolate and an unidentified hydrophobic compound were the principal compounds extracted from N. brasiliensis with benzine. By electron microscopy, the extracted lipids were found to be located in the outermost membrane layer of the N. brasiliensis cell wall. They also appeared to confer acid-fastness. In vitro, the extractable lipids from the N. brasiliensis cell wall induced the production of the proinflammatory cytokines interleukin-1β (IL-1β), IL-6, and CCL-2 in macrophages. The N. brasiliensis cell wall extractable lipids inhibited important macrophage microbicidal effects, such as tumor necrosis factor alpha (TNF-α) and nitric oxide (NO) production, phagocytosis, bacterial killing, and major histocompatibility complex class II (MHC-II) expression in response to gamma interferon (IFN-γ). In dendritic cells (DCs), N. brasiliensis cell wall-associated extractable lipids suppressed MHC-II, CD80, and CD40 expression while inducing tumor growth factor β (TGF-β) production. Immunization with delipidated N. brasiliensis induced partial protection preventing actinomycetoma. These findings suggest that N. brasiliensis cell wall-associated lipids are important for actinomycetoma development by inducing inflammation and modulating the responses of macrophages and DCs to N. brasiliensis.
INTRODUCTION
Actinomycetoma is a chronic, infectious disease that is characterized by excessive tumefaction and deformation of affected tissues. Nocardia brasiliensis is the primary causative agent of actinomycetoma in the Western Hemisphere. The highest incidence of N. brasiliensis-induced actinomycetoma is observed in Mexico and Venezuela (27, 46). Diagnosis of actinomycetoma is based on clinical findings such as multiple inflammatory granulomatous nodules and subcutaneous fistulas that drain a purulent exudate, containing granules consisting of microcolonies of the causal agent, N. brasiliensis (46), and confirmed by an enzyme-linked immunosorbent assay (ELISA) (39). This bacterium is a Gram-positive, acid-fast microorganism that belongs to the Corynebacterineae suborder. Several microorganisms of clinical relevance, such as Mycobacterium, Corynebacterium, Actinomadura, and Gordonia, among others, belong to the Corynebacterineae suborder. The most distinctive characteristic that classifies species within this suborder of microorganisms is the presence of a chemotype IV cell envelope (11, 26). This type of cell envelope is characterized by an abundance of lipids with unusual chemical structures that can constitute up to 40% of the dry weight of the microorganism (11). These lipids are distributed in the following three major cell wall components: the cell membrane, the mycoloyl-arabinogalactan-peptidoglycan complex (MAPc), and the outer membrane layer (11, 12).
The cell membrane present in chemotype IV cell envelope-bearing Corynebacterineae spp. is chemically similar to the cell membranes present in microorganisms of any other species. In contrast, the MAPc is the true hallmark structure that defines and classifies microorganisms into this group. The MAPc present in chemotype IV cell envelopes is composed of a meso-diaminopimelic acid-containing peptidoglycan skeleton covalently linked to a linear d-galactan chain (34). The galactan chain is covalently bound to highly branched, d-arabinofuranosyl residues that form penta-arabinofuranosyl terminal motifs. Arabinose residues are further sterified by tightly packed mycolic acids that are 40 to 60 carbons long and characteristically extend perpendicularly to the plane of the cell envelope (1).
The mycolic acids contribute to the formation of the outer membrane layer of the cell envelope of Corynebacterineae, which is a zipper-like, asymmetric bilayer membrane. The inner leaflet is composed of the mycolic acids that extend from the MAPc. The outermost leaflet is composed of a variety of cell wall-associated glycolipids that are noncovalently bound to the inner leaflet (11, 20, 30, 32, 49). Because of the noncovalent association between the outer leaflet and the mycolic acids, it is possible to extract the wall-associated lipids from the outer membrane layer with organic solvents, thus disrupting the integrity of the cell envelope without affecting bacterial viability (9, 10, 44).
The nature and composition of the cell wall extractable lipids that are associated with the outer membrane layer vary between species. In general, for Mycobacterium spp. the main components are phthiocerol-containing lipids, phosphatidylinositol mannosides (PIM), lipomannan (LM), lipoarabinomannan (LAM), trehalose dimycolates (TDM or cord factor), trehalose monomycolates, glycopeptidolipids (GPL), and sulfolipids (11, 15, 16). For Nocardia spp., trehalose-containing lipids, glycolipids, diethyl ether-soluble lipids, tuberculostearic acid, nocobactin, and nocardones have been identified (2, 33, 35, 48).
Cell wall extractable lipids associated with the outer membrane layer (or simply cell wall-associated lipids) have important implications in the pathogenesis of microorganisms of the Corynebacterineae spp., including Nocardia spp. For instance, the diethyl ether-soluble lipids of Nocardia asteroides are highly toxic to mice. They induce a cachectic state that ultimately leads to death when systemically administered (23). In addition to the diethyl ether-soluble lipids, other wall-associated lipids of N. asteroides are known to induce inflammatory responses when systemically administered. The most notable of these compounds is 6,6′-trehalose dimycolate, or TDM, which induces a strong and deadly cachectic state when systemically administered to mice (42). When administered subdermally, TDM can also induce a strong, local inflammatory response that mimics important histopathological aspects of the disease caused by N. asteroides, including the induction of granuloma formation (19). These findings suggest that wall-associated lipids modulate important aspects of the immune responses elicited against the infecting microorganism and that these lipids are important determinants of N. asteroides virulence.
It is unknown whether the observations about the importance of cell wall-associated lipids in bacterial pathogenesis from studies with N. asteroides are also valid for other members of the Nocardia genus, including N. brasiliensis. It is not known whether the cell wall-associated lipids are virulence factors implicated in N. brasiliensis pathogenicity. It is also not known whether the wall-associated lipids are implicated in the development of actinomycetoma induced by N. brasiliensis. Here, we report that N. brasiliensis cell wall-associated lipids are implicated in the development of experimental actinomycetoma and act principally by inducing a strong inflammatory response and inhibiting several of the microbicidal effects of macrophages, including the inhibition of tumor necrosis factor alpha (TNF-α) production, phagocytosis, production of nitric oxide (NO), and bacterial killing. In addition, we demonstrate that the N. brasiliensis wall-associated lipids suppressed the expression of major histocompatibility complex class II (MHC-II), CD80, and CD40 by dendritic cells (DCs) and strongly induced the production of tumor growth factor β (TGF-β) by these cells.
MATERIALS AND METHODS
Media, cells, and culture conditions.
The L929 cell line (designation number CCL-1, obtained from ATCC, Manassas, VA) was maintained in L929 medium, consisting of high-glucose Dulbecco's modified Eagle medium (DMEM) (Gibco, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS) (HyClone; Thermo Fisher, Waltham, MA), GlutaMax (2 mM), penicillin (100 U/ml), streptomycin (100 μg/ml), and amphotericin (0.25 μg/ml) (all from Gibco). The cells were incubated at 37°C in a 5% CO2 atmosphere.
The L929 cell line was used to produce the L-conditioned medium. Briefly, after removing the medium from confluent L929 cell cultures, the cell monolayers were washed once with Hanks' balanced salt solution (HBSS) and incubated in fresh L929 medium for 7 days without changing the medium. Subsequently, the conditioned medium was collected, filtered using a 0.2-μm syringe filter, and used to differentiate macrophages from the bone marrow cell cultures as described below.
N. brasiliensis culture and delipidation with benzine.
N. brasiliensis (ATCC 700358) was cultured statically at 37°C in brain heart infusion broth (BHI) (Difco, BD Diagnostics, Sparks, MD) for 72 h before processing. Under these conditions, N. brasiliensis grows as a thick biofilm on the surface of the media. To obtain a single cell suspension, the biomass was collected in 50-ml centrifuge tubes, washed 3 times in sterile saline, and dissociated using a Dounce tissue grinder. After another wash step, the residual cellular clumps were eliminated by centrifuging the suspensions three times at 200 × g. The supernatants containing the unicellular cells in suspension were collected after each centrifugation step. After the third centrifugation, Kinyoun staining was performed to confirm the unicellularity of the cells in suspension. Only unicellular cell suspensions of N. brasiliensis were used throughout the study.
Unicellular cell suspensions were delipidated using benzine (boiling points, 35 to 60°C; Thermo Fisher Scientific, Inc., Waltham, MA). Briefly, after adding 30 ml of benzine per 500 mg of bacteria, the suspensions were vortexed vigorously for 5 min, and the bacteria were then allowed to settle for 5 min. Then, the suspensions were centrifuged at 500 × g for 5 min. The supernatant, containing the extracted lipid material, was collected in a glass container for further chemical analysis (see below). The pellet, consisting of the bacteria, was subjected to delipidation for two or four additional delipidation processes to generate N. brasiliensis delipidated three times (Tx3) or five times (Tx5), respectively.
After the last extraction, the suspensions were washed extensively using sterile saline, suspended in 20% glycerol in saline, and stored in 1-ml vials at −70°C. At least three vials were used in a plate-counting assay on BHI agar dishes to determine the number of CFU per vial.
Viability determination.
The bacterial viability after the benzine treatment was determined by two different approaches. First, 10 mg (wet weight) of delipidated or control bacteria was suspended in sterile saline. From this suspension, 100 μl, which contained 1 mg of bacteria, was serially diluted and plated onto blood agar plates for CFU determination. Viability was determined by comparing the total CFU present in 1 mg of the delipidated bacteria to the CFU of untreated, control bacteria. Additionally, the viability of the N. brasiliensis cells after benzine treatment was tested using the BacLight live/dead kit (Invitrogen, Molecular Probes, Eugene, OR) by following the manufacturer's instructions.
Isolation and analysis of the lipids in benzine extract.
Benzine extracts were pooled, centrifuged at 20,000 × g for 15 min, and evaporated using a rotavapor. The sediment was suspended in 3 ml of benzine, centrifuged one more time, and evaporated using nitrogen. After being weighed, the extracted material was redissolved in isopropanol (Sigma-Aldrich, St. Louis, MO) at a final concentration of 1 mg/ml and stored at −70°C.
The extracted lipids were initially examined by thin-layer chromatography (TLC) (10- by 10-cm plates, cut from an aluminum-backed 20- by 20-cm silica gel F254 sheet; Merck & Co, Inc., Whitehouse Station, NJ) using chloroform-methanol (9:1, vol/vol) as the solvent. Plates were developed using molybdophosphoric acid (10%, wt/vol, in 70% ethanol for all lipids) or orcinol (0.1%, wt/vol, in 40% H2SO4 for sugars) followed by heating at 140°C. Lipids were fractionated using column chromatography (silica gel, 0.040 to 0.063 mm; Merck & Co, Inc., Whitehouse Station, NJ) with an increasing concentration of methanol (0%, 2%, 5%, 10%, 15%, and 30%) in chloroform. The identity of some of the compounds was investigated using spectroscopic techniques. Thus, the structures of the TDM and the monomycolate of trehalose (MMT) were obtained at 10% and 15% methanol in chloroform, respectively, and they were confirmed by 1H nuclear magnetic resonance (NMR) and 1H-1H homonuclear correlated spectroscopy (1H-1H-COSY). The mycolic acids from TDM were liberated by saponification (15% KOH in methanol, 90°C, overnight) and studied by ion trap mass spectrometry in negative mode using an MSDIonTrap-VL (Agilent) instrument. Acylglycerols and other apolar compounds, eluted with chloroform and chloroform-methanol (5%), were identified only tentatively. Among the lipids that were eluted with chloroform, a major, apolar compound was purified using silica gel 60 (70-230 mesh; Merck & Co, Inc., Whitehouse Station, NJ), liquid chromatography, and benzine (boiling point, 60°C to 80°C) as the eluent. This compound was further examined by 1H NMR (400 MHz, 298 K; solvent, deuterochloroform) and by ESI-TOF-MS (electrospray ionization–time of flight-mass spectrometry) using an ESI-TOF 6220 apparatus (Agilent Technology, Santa Clara, CA).
Electron microscopy of N. brasiliensis.
Unicellular suspensions of N. brasiliensis were fixed in 1.5% KMnO4 for 45 min at room temperature. After being washed two times with distilled water, the cells were suspended in 1% (wt/vol) uranyl nitrate in water and were incubated at room temperature for 1 h. The cells were then incubated with acetone followed by an overnight incubation in Epon-acetone. After the incubation, the cells were incubated in Epon for an additional 72 h at 60°C. The resulting blocks were sectioned and visualized by transmission electron microscopy.
Infection of mice with N. brasiliensis.
Ten- to 12-week-old BALB/c mice were infected with 10 mg (wet weight) of N. brasiliensis suspended in 100 μl of sterile saline solution in the rear footpad, as previously reported (38). Using the ellipsoid formula, inflammation was measured with a scientific caliper and recorded as cm3 of inflammation. All animal experiments were performed according to approved animal protocols by following regulations on experimental animal use as specified by our institution.
Isolation of bone marrow-derived macrophages (BMDM).
Six- to eight-week-old naïve BALB/c mice were euthanized by cervical dislocation. The limbs were removed, and after dissection, the femurs and tibias were perfused with macrophage-differentiating medium consisting of high-glucose DMEM (Gibco) supplemented with 20% fetal calf serum (FCS), 30% L-conditioned medium, glutamine (2 mM), and penicillin/gentamicin. Bone marrow cells were washed in HBSS, adjusted to 5 × 106 cells per ml, and cultured in macrophage-differentiating medium in non-tissue-culture-treated petri dishes at 37°C in a 5% CO2 atmosphere. After 2 days of culture, the nonadherent cells were removed by gently washing the plates with HBSS, and fresh macrophage differentiation medium was added to the adherent cell population. After an additional 4 days of incubation, the cells were harvested, analyzed for macrophage differentiation, and used for experiments. Over 95% of the cells that were differentiated with this protocol were CD11b+/F4/80+ macrophages as determined by flow cytometry (data not shown).
Isolation of bone marrow-derived myeloid DCs.
Bone marrow cells were harvested as previously described. The bone marrow cells were then adjusted to a concentration of 5 × 106 per ml and seeded in polystyrene tissue culture-treated petri dishes in RPMI 1640 (Gibco) that was supplemented with 10% FBS, glutamine (2 mM), antibiotics, and 40 ng/ml of recombinant granulocyte-macrophage colony-stimulating factor (GM-CSF) (Peprotech, Rocky Hill, NJ). The medium was changed and replaced with fresh medium after 2 and 4 days. At day 6, the cells were harvested, analyzed by flow cytometry, and used for experiments. Over 90% of the cells that were obtained using this protocol were CD11c+/CD11b− DCs (data not shown).
In vitro macrophage and DC infection with wild-type or delipidated N. brasiliensis.
BMDM were cultured in macrophage medium consisting of high-glucose DMEM (Gibco) supplemented with 10% FBS and l-glutamine (2 mM), without antibiotics. DCs were cultured in DC medium consisting of RPMI 1640 (Gibco) supplemented with 10% FBS and glutamine (2 mM) and without antibiotics. Prior to infection, both the BMDM and DCs were seeded at a final concentration of 5 × 104 cells per well and cultured for 18 h at 37°C in a 5% CO2 atmosphere. Before the incubation was complete, a frozen vial of wild-type, Tx3, and Tx5 N. brasiliensis was thawed, and the cells were washed, suspended, and adjusted to 1.2 × 106 CFU/ml in either macrophage medium (containing 2% FBS for the infection of macrophages) or DC medium (containing 2% FBS for the infection of DCs). After 18 h of incubation, the medium from the macrophage or DC cultures was removed and changed with fresh medium containing wild-type, Tx3, or Tx5 N. brasiliensis. The bacteria were suspended in culture to yield a final multiplicity of infection (MOI) of 3:1 (3 bacteria per macrophage or DC). After an initial 2-h infection, the medium that contained the bacteria in suspension was removed. After washing the cellular monolayer with HBSS to removed nonphagocytosed bacteria, fresh medium was added (this moment was considered 0 h of infection). Cells were then incubated for an additional 12, 24, or 48 h before being processed further for different experiments such as measurement of cytokine levels, NO detection, and determination of MHC molecule expression or macrophage intracellular killing.
Determination of the macrophage phagocytic index.
BMDM were suspended in macrophage medium (supplemented with 2% FBS), seeded on glass chamber slides (Lab-Tek; Nalge Nunc, Naperville, IL) at a concentration of 4 × 104 cells per chamber, and incubated for 18 h at 37°C in a 5% CO2 atmosphere. Then, the monolayers were washed with HBSS. Fresh medium containing wild-type, Tx3, or Tx5 N. brasiliensis at a final concentration of 2.5 × 105 cells per ml was then added. After 60 min of incubation at 37°C, the macrophages were extensively washed with HBSS to remove the nonphagocytosed bacteria, fixed with 10% buffered formalin, Kinyoun stained, and then analyzed using oil immersion microscopy (100×). At least 200 cells per well in triplicates were examined by counting the number of internalized bacteria per cell. The phagocytic index was calculated by multiplying the percentage of cells that contained at least one bacterium by the mean number of bacteria per positive cell.
Stimulation of macrophages and DCs with N. brasiliensis wall-associated extractable lipids.
Lipids (stored in isopropanol at a concentration of 1 mg/ml) were thawed, heated at 60°C for 10 min to completely suspend the lipidic extract, sonicated, and adjusted to 100 μg, 10 μg, or 1 μg per ml in isopropanol. Lipidic suspensions were layered onto 24-well tissue culture plates (Corning Inc., Corning, NY) and were incubated at 37°C under sterile conditions until the solvent was completely evaporated (usually after an overnight incubation). A well containing the solvent without lipids was evaporated and used as a control. Once the solvent had evaporated completely, the plates were seeded with BMDM or DCs as previously described (see above).
Macrophage intracellular killing assay with alamarBlue.
Macrophages were infected with wild-type, Tx3, or Tx5 N. brasiliensis as previously described. After 12, 24, and 48 h of infection, the media from the macrophage cultures that contained extracellular bacteria in suspension were collected and saved. The macrophage monolayers were lysed with 0.05% SDS to release the intracellular bacteria. After extensive washing with HBSS to remove the SDS, the intracellular and extracellular bacteria were pooled to determine the total CFU of N. brasiliensis using an alamarBlue assay (13, 40). Briefly, a standard curve was generated by incubating 0.2 × 106, 0.4 × 106, 0.8 × 106, 1.0 × 106, 1.2 × 106, 1.4 × 106, 1.6 × 106, 1.8 × 106, and 2 × 106 CFU of N. brasiliensis in LB medium (Difco, BD Diagnostics, Sparks, MD) containing 10% alamarBlue (Invitrogen, Carlsbad, CA). In parallel, the samples that contained the total bacteria from the infected macrophage cultures were also incubated in 10% LB alamarBlue solution. After 24 h of incubation at 37°C in constant rotation, the reduction of the alamarBlue by the bacteria was measured using a fluorometer (excitation, 530 nm; emission, 590 nm). The fluorescence values for each group of infected macrophages were extrapolated against the fluorescence values of the standard curve to determine the number of CFU that were present in each macrophage sample.
Macrophage activation in response to IFN-γ stimulation.
In some experiments, the effects of gamma interferon (IFN-γ) on modulating the microbicidal effects of macrophages against N. brasiliensis were investigated. Macrophages were seeded for 18 h (as previously described) in macrophage medium containing 2% FBS with 100 U/ml of IFN-γ (eBioscience, San Diego, CA). After incubation, macrophages were washed to remove IFN-γ and infected with wild-type, Tx3, or Tx5 N. brasiliensis as described above. After 12, 24, and 48 h, macrophages were either analyzed for MHC-II or T cell costimulatory molecule expression by flow cytometry or assessed for the number of N. brasiliensis CFU using the alamarBlue assay. In addition, NO levels in culture supernatant were determined using the Griess reaction (Invitrogen, Carlsbad, CA) by following the manufacturer's instructions.
Cytokine determination by ELISA.
Macrophages and DCs were infected with wild-type, Tx3, or Tx5 N. brasiliensis or stimulated with N. brasiliensis wall-associated extractable lipids as described above. After 12, 24, or 48 h of infection or stimulation with lipids, the supernatant was recovered, filtered through a 0.22-μm syringe filter, and stored at −80°C until further analysis. Analysis of interleukin-1β (IL-1β), IL-6, CCL-2, TNF-α, and TGF-β from the supernatants was performed using the commercial eBioscience ready-set ELISA kit according to the manufacturer's instructions.
Antibodies and flow-cytometric analysis.
The following antibodies (eBioscience) and concentrations were used: fluorescein isothiocyanate (FITC) rat anti-mouse MHC-II (clone NIMR-4, 1:200), PE rat anti-mouse CD80 (clone 16-10A1, 1:200), FITC rat anti-mouse CD86 (clone GL-1, 1:200), PE rat anti-mouse PDL-2 (clone 122, 1:100), PE rat anti-mouse CD40 (clone 1C10, 1:100), FITC rat anti-mouse F4/80 (clone BM8, 1:100), PE rat anti-mouse CD11b (clone M1/70, 1:250), and FITC rat anti-mouse CD11c (clone N418, 1:200).
For flow-cytometric analysis, BMDM and DCs were infected with wild-type, Tx3, or Tx5 N. brasiliensis or were stimulated with N. brasiliensis wall-associated extractable lipids as described above. After 12, 24, or 48 h, the macrophages were harvested by trypsinization (0.25% trypsin in phosphate-buffered saline [PBS] [Gibco]) and washed in PBS without Ca2+ or Mg2+ (DPBS). The cells were suspended in 100 μl of antibody binding buffer (2 mM EDTA, 0.3% bovine serum albumin [BSA] in DPBS), and 10% normal rat serum was added to block nonspecific Fc binding. After 10 min of incubation in the blocking buffer, the antibody diluted in antibody binding buffer was added. After 30 min of incubation at 4°C, the cells were washed in PBS, fixed in 10% buffered formalin, and processed using FACSCalibur (BD Biosciences) and Cellquest Pro version 5.1 for data analysis.
Protection experiment.
To investigate the protective effect of the delipidated N. brasiliensis, we used three groups of 10 mice. One group was immunized with 1 mg of heat-killed N. brasiliensis suspended in 100 μl of PBS. Another group received 1 mg of live Tx5 N. brasiliensis suspended in 100 μl of PBS for immunization. The third group was injected with the vehicle alone (100 μl of PBS) as a control.
Fifteen days after immunization, the mice were challenged with 1 mg of wild-type N. brasiliensis. This day was considered day 1 in our experiments. Inflammation was recorded until day 120 after challenge as previously described.
Statistics.
The individual significance value per group was calculated using a two-way analysis of variance (ANOVA) and a Bonferroni multiple test to ascertain significance. A P value of less than 0.05 was considered significant.
RESULTS
Benzine treatment does not affect the viability of N. brasiliensis, but it does abolish its ability to induce experimental actinomycetoma.
The effect of benzine treatment on N. brasiliensis viability was investigated, and no significant difference in the number of CFU obtained after each treatment was observed (Fig. 1A). In addition, we observed 92.5 ± 5.1%, 94.4 ± 4.8%, and 91.2 ± 6.7% viability in wild-type, Tx3, and Tx5 N. brasiliensis, respectively, when the BacLight live/dead viability kit was used. No statistical significant differences were found between groups, suggesting that benzine treatment does not affect N. brasiliensis viability.
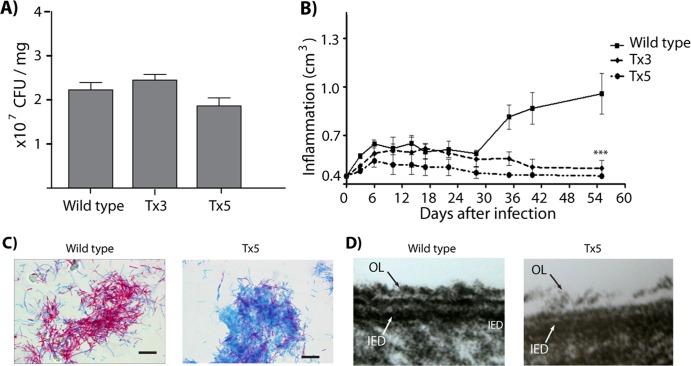
Fig 1.
(A) Viability of wild-type, Tx3, and Tx5 N. brasiliensis by plate counting. (B) Inflammation area (in cm3) in the footpad of mice infected with wild-type, Tx3, or Tx5 N. brasiliensis. (C) Kinyoun staining of wild-type and Tx5 N. brasiliensis. N. brasiliensis loses its acid-fastness after benzine treatment. (D) Transmission electron microscopy images of wild-type and Tx5 N. brasiliensis. A disruption in the integrity of the cell envelope is observed after benzine treatment. The outmost electrodense layer is missing from the Tx5 N. brasiliensis cell wall.
We next investigated the induction of actinomycetoma by benzine-delipidated N. brasiliensis. As previously reported (37, 38), wild-type N. brasiliensis induced an inflammatory process characterized by an initial inflammatory peak approximately 7 days postinfection; the inflammation persisted and became more severe for the duration of the actinomycetoma lesion (Fig. 1B). Clinically, mice inoculated with control N. brasiliensis presented a typical actinomycetoma characterized by tumefaction, deformation, multiple nodules, and fistulas that drained a purulent exudate onto the skin surface by day 45 postinfection. Mice inoculated with Tx3 and Tx5 N. brasiliensis failed to induce inflammation after day 30 postinfection. Consequently, no actinomycetoma was observed in these groups of mice (Fig. 1B). These results demonstrate that delipidation with benzine abolished the ability of N. brasiliensis to induce actinomycetoma. Because similar results were obtained using the Tx3 and Tx5 N. brasiliensis, we decided to compare the effects of the wild-type N. brasiliensis to those of the Tx5 N. brasiliensis group.
Loss of acid-fastness and bacterial aggregation after treatment with benzine are associated with modifications in the outermost, electrodense layer of the N. brasiliensis cell envelope.
Several cytochemical and morphological changes were observed in N. brasiliensis following benzine treatment. These included reduced bacterial aggregation in suspension and loss of bacterial acid-fastness after benzine treatment. In addition, transmission electron microscopy revealed a disruption of N. brasiliensis cell wall integrity, which was characterized by a lack of the outermost electrodense layer (Fig. 1D, OL), in N. brasiliensis with lipids extracted five times with benzine. These results indicate that the lipids extracted with benzine locate in the outermost layer of the N. brasiliensis cell wall envelope.
Analysis of the N. brasiliensis lipids in the benzine extract.
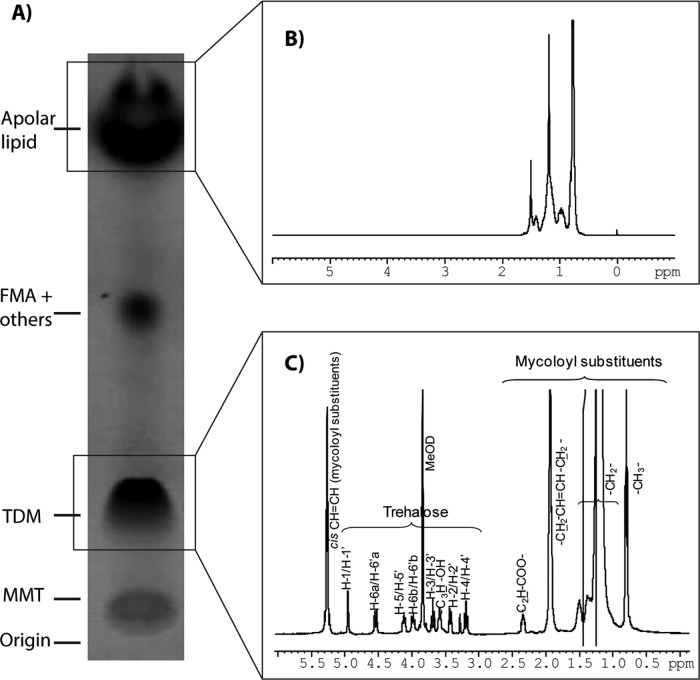
1H-1H-COSY and 1H NMR analyses of the different fractions obtained by column chromatography (chloroform-methanol) of the benzine extract revealed the presence of apolar lipids, free mycolic acids, and other compounds, such as TDM and MMT (Fig. 2). Among the different lipids identified in our chemical analysis, the predominant lipids were TDM and a highly apolar compound.
Fig 2.
Chemical analysis of lipids extracted from N. brasiliensis cell wall with benzine. (A) MMT, monomycolate of trehalose; TDM, 6,6′di-mycoloyl trehalose; FMA + others, free mycolic acid and other lipids. Apolar lipid refers to the main apolar lipid isolated, purified, and analyzed. (B) 1H NMR spectrum of the apolar compound (400 MHz, 298 K, deuterochloroform). (C) 1H NMR spectrum of cord factor, 400 MHz, 298 K, deuterochloroform-deuteromethanol (6:1, vol/vol). The resonances due to the protons of trehalose and mycoloyl substituents are indicated. C2H and C3H, correspond, respectively, to the protons located at positions C2 and C3 of the mycoloyl substituents. MeOD, deuteromethanol.
The structure of the N. brasiliensis TDM was resolved by correlating the compound structure to that previously reported for other Corynebacterineae TDM, in particular the Rhodococcus opacus TDM (33). Notably, the anomeric H-1/H-1′ protons resonated at 4.9 ppm (J1, 2 < 4 Hz), and the H-6s resonated at 4.7 ppm and 3.9 ppm. This indicated an α, α′ configuration for trehalose and that the two C-6-hydroxyl groups were esterified. The two anomeric protons of MMT (6-mono-mycoloyl trehalose) resonated at 5.3 ppm and at 4.9 ppm, and the H-6 protons resonated at 4.5 ppm, 4.0 ppm, 3.6 ppm (two of them), suggesting that only one C-6 hydroxyl was linked to a molecule of mycolic acid.
The mycolic acids of TDM varied from 52 to 62 carbon atoms, and the majority of the mycolic acids were C56 and C58 with two and three unsaturated carbons, respectively.
Among the apolar lipids, which according to 1H NMR were mostly triacylglycerols, a very apolar compound was purified (not shown). This apolar compound showed an Rf of approximately 0.9 in silica gel plates when benzine was used as the solvent. The compound showed an apparent monoisotopic mass (M+H)+ of 683.45, and in the 1H NMR analysis a resonance between 0.8 and 1.5 ppm was detected. The complete structure of this apolar compound could not be further resolved. It was identified as a possible isoprenoid-like compound.
Induction of proinflammatory cytokines and inhibition of TNF-α production by N. brasiliensis cell wall-associated lipids.
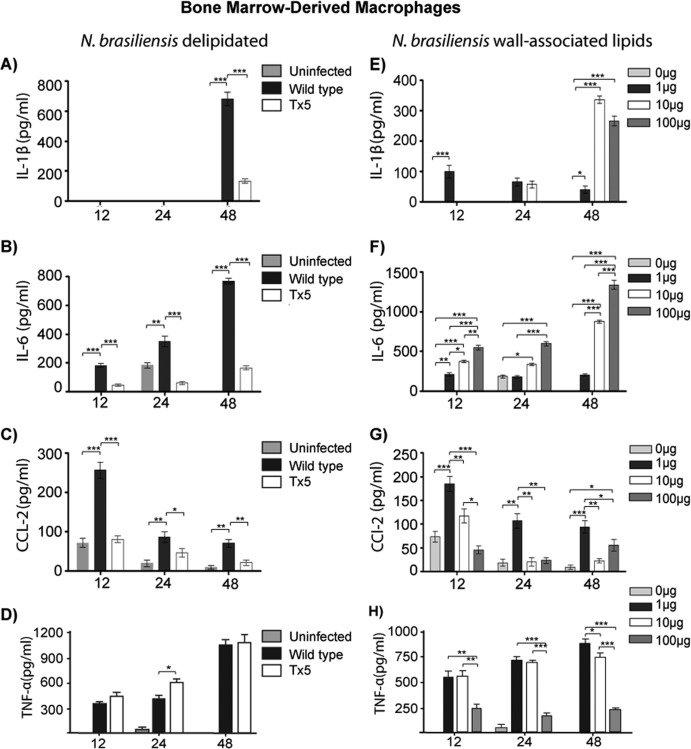
We investigated the induction of proinflammatory cytokines by delipidated and wild-type N. brasiliensis. Briefly, bone marrow-derived macrophages were infected with wild-type or Tx5 N. brasiliensis, and the concentrations of the proinflammatory cytokines IL-1β, IL-6, CCL-2, and TNF-α were analyzed at several time points.
BMDM infected with wild-type N. brasiliensis produced higher concentrations of IL-1β than did macrophages infected with Tx5 N. brasiliensis (P < 0.0001) (Fig. 3A).
Fig 3.
Production of cytokines at different time points by bone marrow-derived macrophages in response to wild-type and Tx5 N. brasiliensis (A through D) or to increasing concentrations of isolated wall-associated lipids from N. brasiliensis (E through H). Control BMDM were uninfected or unstimulated with lipids (0 μg in panels E through H). Data expressed as means ± standard deviations. *, P < 0.05; **, P < 0.001; ***, P < 0.0001 as assessed using a Bonferroni posttest.
Wild-type N. brasiliensis induced a progressive increase in the production of IL-6 by BMDM. The maximal production of this cytokine was observed after 48 h of infection (767.7 ± 29.4 pg/ml [Fig. 3B]). Although a similar pattern of IL-6 production was observed in macrophages infected with Tx5 N. brasiliensis, the concentrations of IL-6 were always lower than the levels induced by wild-type N. brasiliensis (P < 0.0001 in comparison with 48-h wild-type N. brasiliensis [Fig. 3B]).
Induction of CCL-2 expression by wild-type N. brasiliensis peaked after 12 h of infection (265.7 ± 63.9 pg/ml), and the expression decreased gradually afterwards (86.7 ± 28.6 pg/ml after 48 h postinfection [Fig. 3C]). In macrophages infected with Tx5 N. brasiliensis, a similar pattern of CCL-2 induction was observed (a higher peak of 91.9 ± 34.6 pg/ml at 12 h, decreasing gradually to 33.5 ± 18.9 pg/ml after 48 h). However, CCL-2 induction by Tx5 N. brasiliensis infection was always significantly lower than the levels observed in infection with wild-type N. brasiliensis (Fig. 3C).
After 24 h of infection, we observed a reduction in the production of TNF-α by macrophages infected with wild-type N. brasiliensis compared to Tx5 N. brasiliensis (489.2 ± 65.7 pg/ml in the wild type versus 597.7 ± 54.5 pg/ml in Tx5; P < 0.01 [Fig. 3D]).
The effects of the isolated N. brasiliensis cell wall-associated lipids on the induction of the proinflammatory cytokines by BMDM were also investigated. BMDM were incubated with increasing concentrations of the N. brasiliensis extracted lipids over several time points, and the production of IL-1β, IL-6, CCL-2, and TNF-α was analyzed. IL-1β production was maximal after 48 h of lipid stimulation, and the higher production of this cytokine was induced by the 10- and 100-μg concentrations (335 ± 33.5 pg/ml versus 272.1 ± 54.7 pg/ml, respectively [Fig. 3E]).
The N. brasiliensis extracted lipids induced a dose- and time-dependent production of IL-6 (Fig. 3F) that was similar to the time-dependent increment observed after infection with the wild-type microorganism (Fig. 3B and F). In addition, the induction of CCL-2 by the N. brasiliensis extracted lipids also resembled the pattern of production induced by the wild-type microorganism. Induction of CCL-2 by the N. brasiliensis lipids resulted in a maximal peak after 12 h of stimulation that decreased gradually afterward (Fig. 3C and G). However, the production of CCL-2 by BMDM was elevated at all time points when stimulated with 1 μg of extracted lipids (176.8 ± 59.8 pg/ml after 12 h, 121.3 ± 34.4 pg/ml after 24 h, and 109.8 ± 31.2 pg/ml after 48 h of stimulation [Fig. 3G]).
Production of TNF-α by BMDM was inversely proportional to the concentration of lipids used to stimulate the macrophages (Fig. 3H). After 48 h of lipid stimulation, we observed the production of TNF-α to be 864.3 ± 78.9 pg/ml, 705.7 ± 76.4 pg/ml, and 231.7 ± 45.5 pg/ml with 1 μg, 10 μg, and 100 μg of N. brasiliensis lipids, respectively (P < 0.001 between 1 μg and 100 μg and between 10 μg and 100 μg [Fig. 3H]). Importantly, we did not observe lipid-induced macrophage toxicity at any lipid concentration as determined by trypan blue viability staining (data not shown).
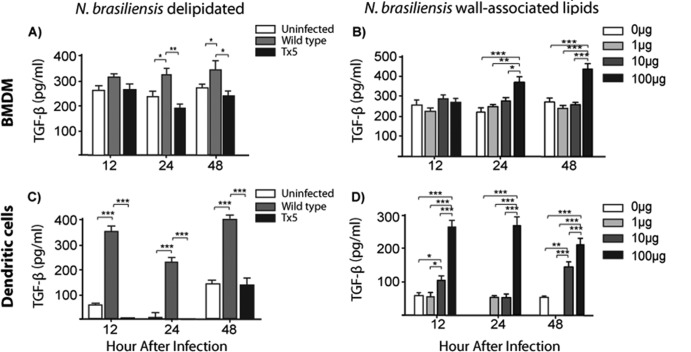
N. brasiliensis lipids induce the production of TGF-β in BMDM and dendritic cells.
To further understand the response of macrophages to the delipidated N. brasiliensis, we analyzed the production of TGF-β in BMDM that were infected with wild-type or Tx5 N. brasiliensis. Macrophages infected with wild-type N. brasiliensis produced significantly higher levels of TGF-β after 24 and 48 h of infection than did macrophages infected with Tx5 N. brasiliensis (after 24 h, 322.5 ± 39.8 pg/ml with the wild type and 187.9 ± 27.6 pg/ml with Tx5 N. brasiliensis; P < 0.001; after 48 h, 341.3 ± 76.3 pg/ml with the wild type and 222.9 ± 31.4 pg/ml with Tx5 N. brasiliensis; P < 0.01 [Fig. 4A]).
Fig 4.
Production of TGF-β at different time points by bone marrow-derived macrophages (BMDM) in response to wild-type and Tx5 N. brasiliensis (A) or to increasing concentrations of isolated wall-associated lipids from N. brasiliensis (B). Production of TGF-β at different time points by dendritic cells in response to wild-type and Tx5 N. brasiliensis (C) or to increasing concentrations of isolated wall-associated lipids from N. brasiliensis (D). As controls, cells were uninfected or unstimulated with lipids (0 μg in panels B and D). Data expressed as means ± standard deviations. *, P < 0.05; **, P < 0.001; ***, P < 0.0001 as assessed using a Bonferroni posttest.
We next investigated the production of TGF-β by macrophages in response to different concentrations of the isolated N. brasiliensis wall-associated lipids. We observed a dose-dependent increase in the production of TGF-β in response to N. brasiliensis lipids (223.4 ± 21.6 pg/ml, 234.5 ± 17.9 pg/ml, and 412.8 ± 39.9 pg/ml with 1 μg, 10 μg, and 100 μg of N. brasiliensis lipids, respectively; P < 0.0001 [Fig. 4B]).
We also investigated the production of TGF-β by DCs infected with wild-type or Tx5 N. brasiliensis. We observed an increased production of TGF-β in response to wild-type N. brasiliensis at all time points tested compared to infection with Tx5 N. brasiliensis (P < 0.0001 between wild-type and Tx5 N. brasiliensis at all analyzed times [Fig. 4C]). The effect of the N. brasiliensis lipids on the production of TGF-β by DCs was also tested. We found that there was a dose-dependent increase in the production of TGF-β by DCs in response to N. brasiliensis lipids at all of the time points analyzed (after 48 h, 154.3 ± 23.4 pg/ml with 10 μg of lipids versus 209.7 ± 43.5 pg/ml with 100 μg of N. brasiliensis lipids; P < 0.0001 [Fig. 4D]). These results suggest that the N. brasiliensis surface lipids are important inducers of the production of TGF-β in both macrophages and DCs.
Delipidated N. brasiliensis has a lower survival rate in BMDM than does wild-type N. brasiliensis.
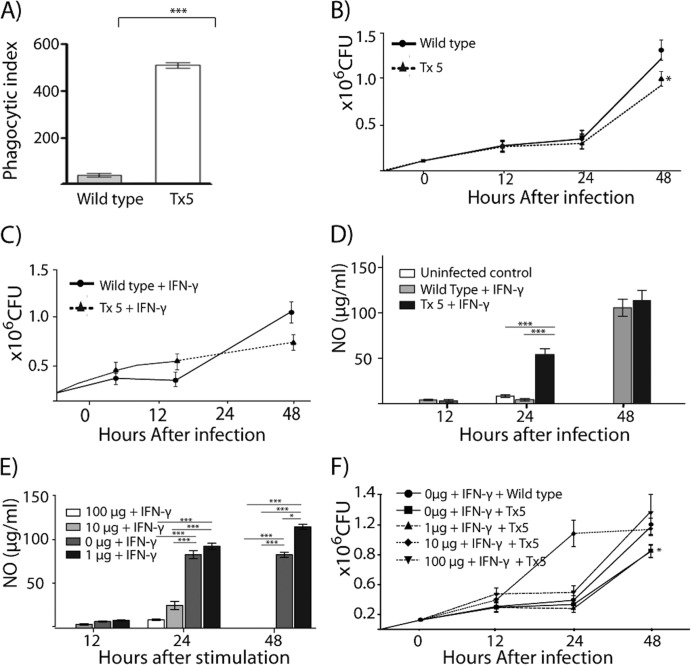
An important observation from our in vitro experiments was that after 48 h of infection a higher number of N. brasiliensis colonies were observed in the BMDM cultures infected with wild-type N. brasiliensis than in the macrophage cultures infected with Tx5 N. brasiliensis (Fig. 5B). In addition, when the BMDM cultures were fixed and Kinyoun stained, we observed that the wild-type N. brasiliensis organisms were found predominantly in the extracellular space whereas the Tx5 N. brasiliensis organisms were found mainly in the cytoplasm of the BMDM (Fig. 5A). These findings suggest that differences existed in the phagocytosis and the intracellular survival between wild-type and delipidated N. brasiliensis. Therefore, we investigated the differences in the phagocytic index for BMDM infected with wild-type and delipidated N. brasiliensis. We found that there was a higher phagocytic index in the BMDM infected with the Tx5 N. brasiliensis than in the macrophages infected with wild-type N. brasiliensis (Fig. 5A). The increase in the BMDM phagocytic index was directly proportional to the number of benzine extractions that the bacteria were subjected to.
Fig 5.
(A) Phagocytic index of BMDM infected with wild-type or Tx5 N. brasiliensis. (B) Number of CFU obtained in BMDM cultures infected with wild-type and Tx5 N. brasiliensis after several time intervals. (C) Number of CFU in IFN-γ-activated BMDM, infected with wild-type or Tx5 N. brasiliensis. (D) NO production in IFN-γ-activated BMDM, in response to infection with wild-type or Tx5 N. brasiliensis. (E) NO production by IFN-γ-activated BMDM stimulated with several concentrations of isolated N. brasiliensis wall-associated lipids. (F) Total number of CFU in cultures of BMDM stimulated with several concentrations of wall-associated lipids, activated with IFN-γ, and then infected with wild-type or Tx5 N. brasiliensis. Data are expressed as means ± standard deviations. *, P < 0.05; ***, P < 0.0001, as assessed using a Bonferroni posttest.
Next, we investigated the effects of the benzine treatment on the intracellular survival of N. brasiliensis in BMDM. BMDM were infected with wild-type or Tx5 N. brasiliensis, and after infection the numbers of CFU were assessed at several time points using an alamarBlue CFU determination assay. We observed fewer CFU in the BMDM cultures infected with Tx5 N. brasiliensis than in those infected with wild-type N. brasiliensis (after 48 h, 1.35 × 106 and 0.9 × 106 for wild type and Tx5, respectively; P < 0.01 [Fig. 5B]). This finding suggests that lipids interfere with the ability of macrophages to eliminate N. brasiliensis.
We analyzed the production of IFN-γ by BMDM infected with delipidated and with wild-type N. brasiliensis. It was determined that BMDM infected with Tx5 N. brasiliensis underwent an important peak in IFN-γ production after 48 h of infection, and this peak was absent in macrophages infected with wild-type N. brasiliensis (234.5 ± 37.6 pg/ml, not shown). This finding suggested that IFN-γ is an important mediator that regulates the response of macrophages against the delipidated N. brasiliensis. Next, we investigated the survival of the delipidated N. brasiliensis in IFN-γ-activated BMDM. We observed a lower number of N. brasiliensis CFU in the BMDM cultures infected with Tx5 N. brasiliensis than in the macrophages infected with wild-type N. brasiliensis. In this experiment, the reduction in the CFU was higher than the reduction that was observed in the non-IFN-γ-activated macrophages (after 48 h, the wild type had 1.1 × 106 and Tx5 had 0.6 × 106 CFU; P < 0.001 between wild type and Tx5 [Fig. 5C]).
N. brasiliensis wall-associated lipids suppress the IFN-γ-mediated production of NO by BMDM.
Compared to wild-type N. brasiliensis, our in vitro experiments suggested that delipidated N. brasiliensis exhibited a reduced intracellular survival in IFN-γ-activated macrophages. This suggested that the wall-associated lipids affected the microbicidal response of macrophages in response to IFN-γ stimulation. Therefore, we investigated the production of NO by IFN-γ-activated macrophages in response to infection with wild-type or Tx5 N. brasiliensis. BMDM were activated with IFN-γ, infected with wild-type or Tx5 N. brasiliensis, and subsequently assessed for the production of NO at several time points after infection. No difference in the production of NO between the groups was observed after 12 h of infection. However, after 24 h of infection, we observed a statistically significant reduction in the IFN-γ-dependent NO production by macrophages infected with wild-type N. brasiliensis compared to macrophages infected with Tx5 N. brasiliensis (wild type, 12.6 ± 5.1 μg/ml; Tx5, 50.5 ± 13.1 μg/ml; P < 0.0001 between the wild type and Tx5 [Fig. 5E]). This difference was no longer evident after 48 h of infection (Fig. 5E). This suggests that the N. brasiliensis lipids partially inhibited the production of NO by macrophages in response to IFN-γ. Next, we assessed the effect of the isolated N. brasiliensis lipids on the production of NO by macrophages in response to IFN-γ. We observed a dose-dependent reduction in the production of NO by BMDM in response to IFN-γ stimulation after 24 and 48 h of lipid stimulation (Fig. 5F). At these time points, macrophages incubated with 100 μg and 10 μg of lipids and stimulated with IFN-γ exhibited a statistically significant reduction in the production of NO compared to macrophages stimulated with 1 μg or 0 μg of lipids. (In both cases, the P value was lower than 0.0001 [Fig. 5F]).
N. brasiliensis cell-associated lipids interfere with the IFN-γ-mediated microbicidal activity of macrophages.
Stimulation with the N. brasiliensis lipids affects the production of NO by macrophages in response to IFN-γ. Because NO is essential to mediate effective killing of intracellular microorganisms by macrophages, we hypothesized that stimulation with the N. brasiliensis lipids would interfere with the macrophages' capability to respond to IFN-γ and kill N. brasiliensis. To test this hypothesis, BMDM were stimulated with N. brasiliensis lipids in the presence or absence of IFN-γ. The BMDM were then infected with wild-type or Tx5 N. brasiliensis. After 48 h of infection, we observed 1.2 × 106 ± 0.18 × 106 CFU for the N. brasiliensis-infected cultures of macrophages that were not stimulated with lipids and were infected with wild-type N. brasiliensis (Fig. 5F). We observed significantly fewer CFU of N. brasiliensis in the macrophage cultures that were not stimulated with lipids and were infected with the Tx5 N. brasiliensis (0.85 × 106 ± 0.22 × 106; P < 0.001). Lipid stimulation decreased the ability of the macrophages to respond to IFN-γ stimulation and to kill the Tx5 N. brasiliensis in a dose-dependent fashion. We observed more bacterial CFU in the macrophage cultures stimulated with 100 μg of lipids and infected with Tx5 N. brasiliensis than in the macrophage cultures that were not stimulated with lipids prior to infection with wild-type N. brasiliensis (1.31 × 106 ± 0.28 × 106 in macrophages stimulated with lipids and infected with Tx5 N. brasiliensis compared to 1.2 × 106 ± 0.18 × 106 CFU in macrophages not stimulated with lipids and infected with wild-type N. brasiliensis). N. brasiliensis lipids are not toxic for macrophages even at the 100-μg concentration as determined by the trypan blue viability staining (data not shown). Altogether, these results suggest that the N. brasiliensis lipids impair the capacity of the macrophages to eliminate N. brasiliensis by inhibiting the macrophages' response to IFN-γ stimulation.
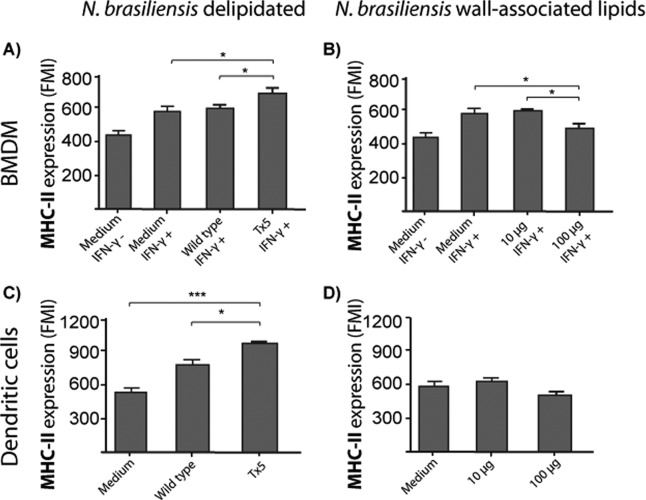
N. brasiliensis wall-associated lipids inhibit the expression of the MHC-II molecule in BMDM and dendritic cells.
In addition to induction of NO production, IFN-γ activates antigen presentation and induces MHC-II molecule expression on macrophages. We analyzed, by flow cytometry, MHC-II molecule expression in IFN-γ-activated BMDM infected with wild-type or Tx5 N. brasiliensis. We observed higher MHC-II expression in IFN-γ-activated macrophages infected with Tx5 N. brasiliensis than in IFN-γ-stimulated macrophages infected with wild-type N. brasiliensis (621.3 ± 46.7 versus 576.6 ± 34.5 fluorescence mean intensity [FMI]; P < 0.05 [Fig. 6A]).Compared with macrophages stimulated with IFN-γ alone, we also observed a reduced expression of MHC-II on IFN-γ-activated macrophages stimulated with 100 μg of purified N. brasiliensis wall-associated lipids (no lipids, 571.3 ± 48.3 FMI; 10 μg lipids, 581.8 ± 15.6 FMI; 100 μg, 487.7 FMI; P < 0.01 [Fig. 6B]), suggesting that at a high concentration, N. brasiliensis wall-associated lipids can inhibit macrophage expression of MHC-II molecules in response to IFN-γ.
Fig 6.
(A) Bar graphs showing the expression of MHC-II molecules in IFN-γ-activated bone marrow-derived macrophages (BMDM) infected with wild-type or Tx5 N. brasiliensis. As controls, BMDM were incubated in medium with (medium IFN-γ+) or without (medium IFN-γ−) IFN-γ, and expression of MHC-II molecules was assessed by flow cytometry. (B) Expression of MHC-II molecules in IFN-γ-activated BMDM stimulated with several concentrations of N. brasiliensis wall-associated lipids. As controls, BMDM were incubated in medium with (medium IFN-γ+) or without (medium IFN-γ−) IFN-γ, and expression of MHC-II molecules was assessed by flow cytometry. (C) MHC-II molecules in dendritic cells infected with wild-type or Tx5 N. brasiliensis. As controls, DCs were incubated in medium alone, and expression of MHC-II molecules was assessed by flow cytometry. (D) Expression of MHC-II molecules in DCs stimulated with several concentrations of N. brasiliensis wall-associated lipids. As controls, DCs were incubated in medium alone, and expression of MHC-II molecules was assessed by flow cytometry. Data expressed as means ± standard deviations of the fluorescence mean intensity (FMI) of MHC-II expression. *, P < 0.05; ***, P < 0.0001 as assessed using a Bonferroni posttest.
We also investigated MHC-II expression on DCs infected with wild-type or Tx5 N. brasiliensis, finding a higher MHC-II expression on DCs infected with Tx5 N. brasiliensis than on DCs infected with wild-type N. brasiliensis (P < 0.01 [Fig. 6C]). When the effect of isolated lipids on MHC-II expression was investigated, we also observed lower MHC-II expression on DCs stimulated with higher concentrations (100 μg) of N. brasiliensis lipids, although this effect was not statistically significant compared to stimulation with lower lipid concentration (10 μg) or basal MHC-II expression in DCs (Fig. 6D).
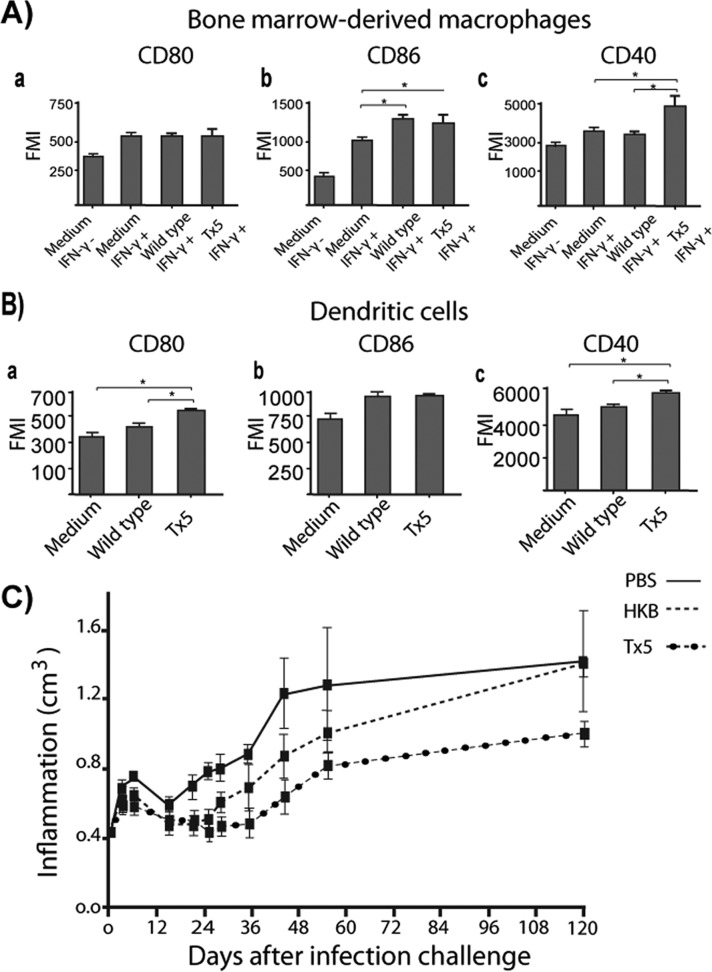
N. brasiliensis lipids regulate the expression of T cell costimulatory molecules in macrophages and dendritic cells.
In addition to the presentation of peptide antigens on MHC-II molecules, another requirement for T cell activation is the expression of T cell costimulatory molecules by antigen-presenting cells. Therefore, we investigated the expression of the costimulatory molecules CD80, CD86, and CD40 and the negative costimulatory molecule PDL-2 in macrophages stimulated with IFN-γ and infected with wild-type or Tx5 N. brasiliensis using flow cytometry. We observed no difference in the expression of CD80 or PDL-2 between the groups (Fig. 7A, subpanels a and b). CD86 expression was found to be significantly increased in the wild-type and the Tx5 groups compared to the positive and negative controls. However, these differences were not statistically significant. The expression of CD40 was higher in the macrophages infected with the Tx5 N. brasiliensis than in those infected with wild-type N. brasiliensis and controls (478.6 ± 86.5 FMI in Tx5 versus 324.7 ± 27.2 FMI in the wild type and 349.8 ± 35.9 FMI in the positive control; P < 0.01 [Fig. 7A, subpanel c]). These data suggest that N. brasiliensis lipids decrease the expression of CD40 by IFN-γ-activated macrophages.
Fig 7.
(A) Bar graphs showing the expression of several T cell costimulatory molecules in IFN-γ-activated BMDM infected with wild-type or Tx5 N. brasiliensis. As controls, BMDM were incubated in medium with (medium IFN-γ+) or without (medium IFN-γ−) IFN-γ, and expression of MHC-II molecules was assessed by flow cytometry. (B) Expression of several T cell costimulatory molecules in dendritic cells infected with wild-type or Tx5 N. brasiliensis. As controls, DCs were incubated in medium alone, and expression of MHC-II molecules was assessed by flow cytometry. Data expressed as means ± standard deviations of the fluorescence mean intensity (FMI) of MHC-II expression. *, P < 0.05; ***, P < 0.0001 as assessed using a Bonferroni posttest. (C) Dot plot showing the level of footpad inflammation (in cm3) in mice immunized with heat-killed N. brasiliensis (HKB), PBS, or Tx5 N. brasiliensis and challenged with wild-type N. brasiliensis 15 days after immunization.
The expression of the costimulatory molecules CD80, CD86, and CD40 and the inhibitory costimulatory molecule PDL-2 in uninfected DCs (exposed to medium alone) and in DCs infected with wild-type or with Tx5 N. brasiliensis was also determined. We did not observe a difference in the expression of CD86 or PDL-2 between the groups (Fig. 7B, subpanels a and b). However, we observed a statistically significant increase in the expression of CD80 (524.4 ± 19.5 FMI in the Tx5 group versus 410.1 ± 34.5 FMI in the wild-type group and 358.9 ± 39.7 FMI in the medium-alone group; P < 0.01 [Fig. 7B, subpanel a]) and CD40 (5,878.5 ± 195 FMI in the Tx5 group versus 5,010.1 ± 345.7 FMI in the wild-type group and 4,589 ± 739.7 FMI in the medium-alone group; P < 0.01 [Fig. 7B, subpanel c]) in DCs infected with the Tx5 N. brasiliensis compared to the uninfected DCs and the DCs infected with the wild-type N. brasiliensis.
Delipidated N. brasiliensis induces a partial protective immunity against N. brasiliensis.
Compared to wild-type N. brasiliensis, macrophages and DCs infected with delipidated N. brasiliensis expressed higher levels of MHC-II and the T cell costimulatory molecules CD80, CD86, and CD40. This suggests that the delipidation of N. brasiliensis may result in a more efficient presentation of bacterial antigens and could lead to the induction of protective immunity against N. brasiliensis. To test this hypothesis, we immunized mice with live Tx5 N. brasiliensis, heat-killed N. brasiliensis (HKB), or only vehicle (PBS) as a control. Fifteen days after immunization, mice where challenged with 10 mg of wild-type N. brasiliensis (wet weight) and analyzed for the development of mycetoma for 120 days. As previously presented in Fig. 1, mice immunized with the Tx5 N. brasiliensis did not develop mycetoma. When challenged with the wild-type N. brasiliensis after 15 days, mice immunized with Tx5 N. brasiliensis did develop mycetoma. Importantly, differences were observed in the patterns of the mycetoma development of the mice immunized with the Tx5 N. brasiliensis and the controls. First, mice immunized with the Tx5 N. brasiliensis developed significantly less inflammation than did the mice immunized with HKB or PBS (Fig. 7C). Second, there was a delay in the development of the mycetoma in the mice immunized with the Tx5 N. brasiliensis. Mycetoma was observed around day 30 or 40 postinfection in mice that were immunized with vehicle or HKB, respectively. In mice immunized with Tx5 N. brasiliensis, mycetoma was observed after 60 days of infection (Fig. 7C). Clinically, the mycetoma that developed in mice immunized with Tx5 N. brasiliensis was significantly smaller, caused a reduced deformation of the footpad, and presented less severe clinical features of actinomycetoma, such as the fistulas and nodules that were present in the mice immunized with vehicle or HKB. These results indicate that immunization with the delipidated Tx5 N. brasiliensis conferred partial protection against actinomycetoma development in mice experimentally infected with wild-type N. brasiliensis.
DISCUSSION
The cellular and molecular mechanisms involved in the N. brasiliensis-induced actinomycetoma remain largely unknown. Several protein molecules, including proteases (28), catalases (3), and superoxide dismutases (3, 36), have been implicated as determinants of the virulence of Nocardia spp. However, these factors seem to play a role in the context of macrophage infection and do not account for the extensive inflammatory response and the tissue destruction that characterize the disease induced by the whole microorganism. Here, we report that the N. brasiliensis wall-associated lipids are implicated in the development of actinomycetoma primarily by inducing a strong inflammatory response, by inhibiting important microbicidal effects by macrophages, and by suppressing the expression of MHC-II and T cell costimulatory molecules by macrophages and DCs.
The effect of the N. brasiliensis wall-associated lipids in the development of actinomycetoma was investigated using chemical delipidation with benzine as originally described by Bloch (9, 10). Delipidation with benzine has been extensively used to investigate the role of the wall-associated lipids in the pathogenesis of microorganisms of the Mycobacterium genus because it removes the wall lipids and glycolipids important for bacterial pathogenesis without affecting the viability of the microorganism (9, 10, 21, 44). In agreement with previous reports on the Mycobacterium spp., we found that treatment with benzine removed lipids from the outermost, electron-dense layer of N. brasiliensis (Fig. 1D) without affecting the viability of the bacteria (Fig. 1A). In addition, as observed in the Mycobacterium spp., our chemical analyses revealed the presence of TDM as one of the predominant lipids extracted from N. brasiliensis by benzine, but in Mycobacterium spp., removing TDM does not affect bacterial acid-fastness (9, 10, 21, 41) in contrast to our finding showing its modification. An explanation for this discrepancy could be related to our observation that, in addition to the extraction of TDM, benzine extraction resulted in the extraction of a highly apolar lipid as the second most abundant compound with an isoprenoid structure. These types of apolar compounds, which also include free mycolates, have been reported to be important constituents of the Corynebacterineae cell envelope. Particularly in Mycobacterium tuberculosis, the loss of mycolates by genetic mutations, such as those that occur in the phoP and ΔkasB mutant strains, resulted in the loss of acid-fastness. These findings are similar to what we observed after the benzine treatment of N. brasiliensis (5, 6).
An important finding of our study was that delipidated N. brasiliensis did not induce actinomycetoma, in contrast to the severe lesion induced by wild-type N. brasiliensis. Delipidated N. brasiliensis induced a weak, transitory inflammatory process that was resolved after 30 days of infection (Fig. 1B). Considering the inflammatory nature of actinomycetoma, our finding strongly suggests that one of the most important mechanisms by which N. brasiliensis mediates the development of actinomycetoma is through the induction of inflammation.
Our laboratory has previously demonstrated that during the course of infection with N. brasiliensis there is a high production of proinflammatory cytokines, particularly IL-1β and IL-6 (43). Based on our in vivo data showing that delipidated N. brasiliensis does not induce actinomycetoma and considering the inflammatory nature of the disease, we hypothesized that the wall-associated lipids were implicated in the induction of the inflammatory response and found that macrophages infected with delipidated N. brasiliensis induced lower levels of IL-1β, IL-6, and CCL-2 than did wild-type N. brasiliensis. These findings mirror what has been previously reported with benzine-delipidated M. tuberculosis. Similarly, a reduction in the production of IL-1β and IL-6 in macrophages infected with the benzine-delipidated M. tuberculosis compared to macrophages infected with wild-type bacteria was observed (21). We also showed that macrophages stimulated with N. brasiliensis wall-associated lipids induced the production of IL-1β, IL-6, and CCL-2 to levels similar to those observed when the cells were infected with the complete microorganisms. This confirms the role of the N. brasiliensis wall-associated lipids in the stimulation of the production of the proinflammatory cytokines.
We previously reported that N. brasiliensis does not induce the production of TNF-α in experimental actinomycetoma (43). TNF-α mediates important aspects of the immune response against intracellular microorganisms as suggested by the increased prevalence of tuberculosis in patients receiving anti-TNF-α treatment and higher mortality rate in TNF-α knockout (KO) mice infected with M. tuberculosis (4, 18, 31). It has been reported that hypervirulent M. tuberculosis strains suppress the induction of TNF-α, and interestingly, a wall-associated phenolic glycolipid has been implicated in this process (17). Here, we report that the N. brasiliensis wall-associated lipids suppress the production of TNF-α by BMDM (Fig. 3D and H). Although this was not investigated further in our study, we think that the wall-associated lipids might be implicated in the suppression of the production of TNF-α that we observed in vivo during the course of infection with N. brasiliensis (43).
In addition to modulation of cytokines, wall-associated lipids affected macrophage phagocytosis of N. brasiliensis, suggesting their role as inhibitors of phagocytosis. The most intriguing difference found in our in vitro infection experiments was that the delipidated N. brasiliensis cells exhibited a reduced survival in macrophages compared to the wild-type bacteria (Fig. 5B). This is similar to what has been observed in macrophages that are infected with delipidated M. tuberculosis (21). In M. tuberculosis, it has been found that some of the wall-associated lipids that are removed after the benzine treatment arrest the maturation of the phagosome within macrophages by inhibiting the fusion of the phagosome with the lysosome (22). Consequently, delipidated M. tuberculosis has a lower survival within macrophages. Similar observations have been made for N. asteroides (7, 8). Interestingly, the N. asteroides TDM, which is readily removed from the cell envelope by benzine extraction, has been implicated in this process (45). Therefore, it seems likely that some of the lipids removed from the N. brasiliensis cell envelope after treatment with benzine inhibit phagosome-lysosome fusion. Thus, it is possible that TDM present in our preparations from N. brasiliensis benzine extracts might block phagosome-lysosome fusion in macrophages. However, due to contamination with other lipids, we cannot exclude the participation of the apolar lipid or the MMT in this process. Further research with purified lipids must be conducted to determine which compound is responsible for the observed results with macrophages in vitro.
Reduced survival of the delipidated N. brasiliensis compared to the wild-type bacteria was more evident when the macrophages were stimulated with IFN-γ. This suggests that, in addition to the possible arrest in the maturation of the phagosome, the N. brasiliensis wall-associated lipids interfere with the IFN-γ-dependent activation of macrophages. Our findings support the notion that the N. brasiliensis lipids mediate an early suppression of the IFN-γ-mediated production of NO by macrophages in response to N. brasiliensis infection. Although the production of NO was restored later during the infection, it is possible that the early NO suppression allows bacterial growth to the extent that the macrophages are no longer able to control the infection when NO levels are restored (Fig. 5E).
Suppression of NO production in IFN-γ-activated macrophages by the wall-associated lipids was also demonstrated in experiments with purified N. brasiliensis lipids in which the ability of the IFN-γ-activated macrophages to eliminate delipidated N. brasiliensis was abolished in a dose-dependent manner after stimulation with lipids. This strongly suggests that the N. brasiliensis wall-associated lipids impair the ability of the macrophages to respond to IFN-γ.
The wall-associated lipids suppressed the expression of MHC-II molecules on macrophages activated with IFN-γ. Moreover, stimulation with the wall-associated lipids inhibited the expression of the MHC-II molecules on macrophages in a dose-dependent manner (Fig. 6A and B). Furthermore, the N. brasiliensis wall-associated lipids inhibited the expression of the T cell costimulatory molecules CD86 and CD40 in macrophages activated with IFN-γ. A similar observation has been reported for macrophages infected with benzine-delipidated M. tuberculosis, demonstrating higher expression of MHC-II, CD40, and CD86 than macrophages infected with wild-type bacteria (24), suggesting TDM as the main compound involved in the inhibition of the expression of MHC-II and T cell costimulatory molecules by macrophages. Because of the presence of TDM in our lipid extract preparation, it is also possible that the effects observed in vitro in the macrophages stimulated with the N. brasiliensis wall-associated lipids were caused by TDM.
It is clear from the infection experiments that the N. brasiliensis wall-associated lipids regulate the expression of the MHC-II and T cell costimulatory molecules in DCs and other functions, such as the induction of the expression of TGF-β. Importantly, the stimulation of DCs with wall-associated lipids induced the production of TGF-β in a dose-dependent fashion. This finding is important because the production of TGF-β by the DCs at the time of antigen presentation may skew the response toward one that is Treg mediated, instead of a T-CD4 Th1 and a T-CD8 cytotoxic response (47), which is necessary to control infection by intracellular microorganisms such as N. brasiliensis. Evidence of this can be seen in the increased incidence of nocardiosis in immunocompromised individuals (29). Together with the reduced expression of antigen-presenting molecules, the N. brasiliensis wall-associated lipids may impair the development of an effective, long-lasting, protective immune response, which is required to eliminate the microorganism from the host. We were able to induce partial protection against the development of actinomycetoma after immunization with delipidated N. brasiliensis. The reason why only partial protection was induced by delipidated N. brasiliensis is not completely understood. A possible explanation is that, because of the chemical and not the genetic delipidation of the bacteria used in our study, N. brasiliensis could relipidize in vivo and restore the inhibition of the antigen presentation and the inhibition of T cell activation. For this reason, we suggest that a genetically delipidated strain of N. brasiliensis could be a promising candidate as a vaccine for actinomycetoma. Similar approaches to prevent M. tuberculosis infections have led to production of attenuated strains as candidates for vaccines against pulmonary tuberculosis (5, 14, 25).
In conclusion, we demonstrate the implications of the cell wall-associated lipids in the pathogenesis of another member of the Corynebacterineae suborder, N. brasiliensis. Our results suggest that either decreasing or eliminating the production of certain apolar lipids of the N. brasiliensis cell wall will help to understand pathogenesis and to design a vaccine.
ACKNOWLEDGMENTS
This work fulfills in part the requirements for a Doctorate degree for J. Humberto Trevino-Villarreal.
We thank Julio Sepulveda for his assistance with the electron microscopy and Adrian Rosas for his technical assistance. We also thank Reynaldo Pecina Rodriguez for his skilled assistance in the animal facility.
This work was partially supported by grants from CONACYT 2009.2010 Mexico and PAICYT UANL.
Footnotes
Published ahead of print 30 July 2012
REFERENCES
- 1. Beaman BL. 1975. Structural and biochemical alterations of Nocardia asteroides cell walls during its growth cycle. J. Bacteriol. 123:1235–1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Beaman BL, Beaman L. 1994. Nocardia species: host-parasite relationships. Clin. Microbiol. Rev. 7:213–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Beaman BL, Black CM, Doughty F, Beaman L. 1985. Role of superoxide dismutase and catalase as determinants of pathogenicity of Nocardia asteroides: importance in resistance to microbicidal activities of human polymorphonuclear neutrophils. Infect. Immun. 47:135–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bean AG, et al. 1999. Structural deficiencies in granuloma formation in TNF gene-targeted mice underlie the heightened susceptibility to aerosol Mycobacterium tuberculosis infection, which is not compensated for by lymphotoxin. J. Immunol. 162:3504–3511 [PubMed] [Google Scholar]
- 5. Bhatt A, et al. 2007. Deletion of kasB in Mycobacterium tuberculosis causes loss of acid-fastness and subclinical latent tuberculosis in immunocompetent mice. Proc. Natl. Acad. Sci. U. S. A. 104:5157–5162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bhatt A, Molle V, Besra GS, Jacobs WR, Kremer L. 2007. The Mycobacterium tuberculosis FAS-II condensing enzymes: their role in mycolic acid biosynthesis, acid-fastness, pathogenesis and in future drug development. Mol. Microbiol. 64:1442–1454 [DOI] [PubMed] [Google Scholar]
- 7. Black CM, Paliescheskey M, Beaman BL, Donovan RM, Goldstein E. 1986. Acidification of phagosomes in murine macrophages: blockage by Nocardia asteroides. J. Infect. Dis. 154:952–958 [DOI] [PubMed] [Google Scholar]
- 8. Black CM, Paliescheskey M, Beaman BL, Donovan RM, Goldstein E. 1986. Modulation of lysosomal protease-esterase and lysozyme in Kupffer cells and peritoneal macrophages infected with Nocardia asteroides. Infect. Immun. 54:917–919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bloch H. 1950. Studies on the virulence of tubercle bacilli; isolation and biological properties of a constituent of virulent organisms. J. Exp. Med. 91:197–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bloch H. 1950. Studies on the virulence of tubercle bacilli: the relationship of the physiological state of the organisms to their pathogenicity. J. Exp. Med. 92:507–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brennan PJ, Nikaido H. 1995. The envelope of mycobacteria. Annu. Rev. Biochem. 64:29–63 [DOI] [PubMed] [Google Scholar]
- 12. Brennan PJ. 2003. Structure, function, and biogenesis of the cell wall of Mycobacterium tuberculosis. Tuberculosis (Edinb.) 83:91–97 [DOI] [PubMed] [Google Scholar]
- 13. Chacon-Moreno BE, et al. 2009. Efficacy of ciprofloxacin and moxifloxacin against Nocardia brasiliensis in vitro and in an experimental model of actinomycetoma in BALB/c mice. Antimicrob. Agents Chemother. 53:295–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Copenhaver RH, et al. 2004. A mutant of Mycobacterium tuberculosis H37Rv that lacks expression of antigen 85A is attenuated in mice but retains vaccinogenic potential. Infect. Immun. 72:7084–7095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Daffe M, Laneelle MA, Puzo G. 1983. Structural elucidation by field desorption and electron-impact mass spectrometry of the c-mycosides isolated from Mycobacterium smegmatis. Biochim. Biophys. Acta 751:439–443 [DOI] [PubMed] [Google Scholar]
- 16. Daffe M, Draper P. 1998. The envelope layers of mycobacteria with reference to their pathogenicity. Adv. Microb. Physiol. 39:131–203 [DOI] [PubMed] [Google Scholar]
- 17. De Lorimier R, Hellinga HW, Spicer LD. 1996. NMR studies of structure, hydrogen exchange, and main-chain dynamics in a disrupted-core mutant of thioredoxin. Protein Sci. 5:2552–2565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Flynn JL, et al. 1995. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity 2:561–572 [DOI] [PubMed] [Google Scholar]
- 19. Han Y, Kawada N, Yano I. 1998. Granuloma formation and in vitro macrophage activation in mice by mycoloyl glycolipids from Nocardia asteroides and related taxa. Osaka City Med. J. 44:201–217 [PubMed] [Google Scholar]
- 20. Hoffmann C, Leis A, Niederweis M, Plitzko JM, Engelhardt H. 2008. Disclosure of the mycobacterial outer membrane: cryo-electron tomography and vitreous sections reveal the lipid bilayer structure. Proc. Natl. Acad. Sci. U. S. A. 105:3963–3967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Indrigo J, Hunter RLJ, Actor JK. 2002. Influence of trehalose 6,6′-dimycolate (TDM) during mycobacterial infection of bone marrow macrophages. Microbiology 148:1991–1998 [DOI] [PubMed] [Google Scholar]
- 22. Indrigo J, Hunter RLJ, Actor JK. 2003. Cord factor trehalose 6,6′-dimycolate (TDM) mediates trafficking events during mycobacterial infection of murine macrophages. Microbiology 149:2049–2059 [DOI] [PubMed] [Google Scholar]
- 23. Ioneda T, Beaman BL, Viscaya L, Almeida ET. 1993. Composition and toxicity of diethyl ether soluble lipids from Nocardia asteroides GUH-2 and Nocardia asteroides 10905. Chem. Phys. Lipids 65:171–178 [DOI] [PubMed] [Google Scholar]
- 24. Kan-Sutton C, Jagannath C, Hunter R., Jr 2009. Trehalose 6,6′-dimycolate on the surface of Mycobacterium tuberculosis modulates surface marker expression for antigen presentation and costimulation in murine macrophages. Microbes Infect. 11:40–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Katti MK, et al. 2008. The ΔfbpA mutant derived from Mycobacterium tuberculosis H37Rv has an enhanced susceptibility to intracellular antimicrobial oxidative mechanisms, undergoes limited phagosome maturation and activates macrophages and dendritic cells. Cell. Microbiol. 10:1286–1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Klein CE, Steinmayer T, Kaufmann D, Weber L, Brocker E-B. 1991. Identification of a melanoma progression antigen as integrin VLA-2. J. Invest. Dermatol. 96:281–284 [DOI] [PubMed] [Google Scholar]
- 27. Lichon V, Khachemoune A. 2006. Mycetoma: a review. Am. J. Clin. Dermatol. 7:315–321 [DOI] [PubMed] [Google Scholar]
- 28. Licón-Trillo Á, Castro-Corona MÁ, Salinas-Carmona MC. 2003. Immunogenicity and biophysical properties of a protease involved in pathogenesis of mycetoma. FEMS Immunol. Med. Microbiol. 37:37–44 [DOI] [PubMed] [Google Scholar]
- 29. Martínez R, Reyes S, Menéndez R. 2008. Pulmonary nocardiosis: risk factors, clinical features, diagnosis and prognosis. Curr. Opin. Pulm. Med. 14:219–227 [DOI] [PubMed] [Google Scholar]
- 30. Minnikin DE. 1982. Lipids: complex lipids, their chemistry, biosynthesis and roles, p 95–184 In Ratledge C. (ed), The biology of the mycobacteria: physiology, identification and classification. Academic Press, San Diego, CA [Google Scholar]
- 31. Nacci F, Matucci-Cerinic M. 2011. Tuberculosis and other infections in the anti-tumour necrosis factor-alpha (anti-TNF-α) era. Best Pract. Res. Clin. Rheumatol. 25:375–388 [DOI] [PubMed] [Google Scholar]
- 32. Niederweis M, Danilchanka O, Huff J, Hoffmann C, Engelhardt H. 2010. Mycobacterial outer membranes: in search of proteins. Trends Microbiol. 18:109–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Niescher S, Wray V, Lang S, Kaschabek SR, Schlömann M. 2006. Identification and structural characterisation of novel trehalose dinocardiomycolates from n-alkane-grown Rhodococcus opacus 1CP. Appl. Microbiol. Biotechnol. 70:605–611 [DOI] [PubMed] [Google Scholar]
- 34. Puech V, et al. 2001. Structure of the cell envelope of corynebacteria: importance of the non-covalently bound lipids in the formation of the cell wall permeability barrier and fracture plane. Microbiology 147:1365–1382 [DOI] [PubMed] [Google Scholar]
- 35. Ratledge C, Patel PV. 1976. Isolation, properties and taxonomic relevance of lipid-soluble, iron-binding compounds (the nocobactins) from Nocardia. J. Gen. Microbiol. 93:141–152 [DOI] [PubMed] [Google Scholar]
- 36. Revol A, Espinoza-Ruiz M, Medina-Villanueva I, Salinas-Carmona MC. 2006. Expression of superoxide dismutase during the early infection of murine peritoneal macrophages. Can. J. Microbiol. 52:1255–1260 [DOI] [PubMed] [Google Scholar]
- 37. Salinas-Carmona MC. 2000. Nocardia brasiliensis: from microbe to human and experimental infections. Microbes Infect. 2:1373–1381 [DOI] [PubMed] [Google Scholar]
- 38. Salinas-Carmona MC, Torres-Lopez E, Ramos AI, Licon-Trillo A, Gonzalez-Spencer D. 1999. Immune response to Nocardia brasiliensis antigens in an experimental model of actinomycetoma in BALB/c mice. Infect. Immun. 67:2428–2432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Salinas-Carmona MC, Welsh O, Casillas SM. 1993. Enzyme-linked immunosorbent assay for serological diagnosis of Nocardia brasiliensis and clinical correlation with mycetoma infections. J. Clin. Microbiol. 31:2901–2906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shiloh MU, Ruan J, Nathan C. 1997. Evaluation of bacterial survival and phagocyte function with a fluorescence-based microplate assay. Infect. Immun. 65:3193–3198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Silva CL, Ekizlerian SM, Fazioli RA. 1985. Role of cord factor in the modulation of infection caused by mycobacteria. Am. J. Pathol. 118:238–247 [PMC free article] [PubMed] [Google Scholar]
- 42. Silva CL, Tincani I, Brandao Filho SL, Faccioli LH. 1988. Mouse cachexia induced by trehalose dimycolate from Nocardia asteroides. J. Gen. Microbiol. 134:1629–1633 [DOI] [PubMed] [Google Scholar]
- 43. Solis-Soto JM, et al. 2008. In situ detection and distribution of inflammatory cytokines during the course of infection with Nocardia brasiliensis. Histol. Histopathol. 23:573–581 [DOI] [PubMed] [Google Scholar]
- 44. Sorkin E, Erlenmeyer H, Bloch H. 1952. Purification of a lipid material (‘cord factor’) obtained from young cultures of tubercle bacilli. Nature 170:124. [DOI] [PubMed] [Google Scholar]
- 45. Spargo BJ, Crowe LM, Ioneda T, Beaman BL, Crowe JH. 1991. Cord factor (alpha,alpha-trehalose 6,6′-dimycolate) inhibits fusion between phospholipid vesicles. Proc. Natl. Acad. Sci. U. S. A. 88:737–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Welsh O, Vera-Cabrera L, Salinas-Carmona MC. 2007. Mycetoma. Clin. Dermatol. 25:195–202 [DOI] [PubMed] [Google Scholar]
- 47. Yamazaki S, Steinman RM. 2009. Dendritic cells as controllers of antigen-specific Foxp3+ regulatory T cells. J. Dermatol. Sci. 54:69–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yano I, et al. 1987. Isolation of mycolic acid-containing glycolipids in Nocardia rubra and their granuloma forming activity in mice. J. Pharmacobiodyn. 10:113–123 [DOI] [PubMed] [Google Scholar]
- 49. Zuber B, et al. 2008. Direct visualization of the outer membrane of mycobacteria and corynebacteria in their native state. J. Bacteriol. 190:5672–5680 [DOI] [PMC free article] [PubMed] [Google Scholar]