Abstract
OBJECTIVES:
Bottle-feeding has been suggested to increase the risk of pyloric stenosis (PS). However, large population-based studies are needed. We examined the effect of bottle-feeding during the first 4 months after birth, by using detailed data about the timing of first exposure to bottle-feeding and extensive confounder information.
METHODS:
We performed a large population-based cohort study based on the Danish National Birth Cohort, which provided information on infants and feeding practice. Information about surgery for PS was obtained from the Danish National Patient Register. The association between bottle-feeding and the risk of PS was evaluated by hazard ratios (HRs) estimated in a Cox regression model, adjusting for possible confounders.
RESULTS:
Among 70 148 singleton infants, 65 infants had surgery for PS, of which 29 were bottle-fed before PS diagnosis. The overall HR of PS for bottle-fed infants compared with not bottle-fed infants was 4.62 (95% confidence interval [CI]: 2.78–7.65). Among bottle-fed infants, risk increases were similar for infants both breast and bottle-fed (HR: 3.36 [95% CI: 1.60–7.03]), formerly breastfed (HR: 5.38 [95% CI: 2.88–10.06]), and never breastfed (HR: 6.32 [95% CI: 2.45–16.26]) (P = .76). The increased risk of PS among bottle-fed infants was observed even after 30 days since first exposure to bottle-feeding and did not vary with age at first exposure to bottle-feeding.
CONCLUSIONS:
Bottle-fed infants experienced a 4.6-fold higher risk of PS compared with infants who were not bottle-fed. The result adds to the evidence supporting the advantage of exclusive breastfeeding in the first months after birth.
KEY WORDS: infantile hypertrophic pyloric stenosis, bottle-feeding, feeding practice, infants, risk factor
What’s Known on This Subject:
Pyloric stenosis is the most common condition requiring surgery in infants. It is typically not present at birth but develops within the first weeks after birth. The etiology is largely unknown, but bottle-feeding has been suggested as a risk factor.
What This Study Adds:
This study demonstrated that bottle-fed infants had a 4.6-fold increased risk of developing pyloric stenosis compared with infants who were not bottle-fed. The result adds to the evidence supporting the advantage of exclusive breastfeeding in the first months after birth.
Pyloric stenosis (PS), also known as infantile hypertrophic PS, is the most common condition requiring surgery in the first months after birth.1 The condition is caused by hypertrophy of the smooth circular muscle layer of the pyloric muscle, obstructing the gastric outlet to the small intestine and leading to severe postprandial vomiting. In Ramstedt pyloromyotomy, the circular muscle is incised longitudinally without closure, typically relieving the obstruction permanently.2 The incidence of PS in Denmark is 1 to 2 per 1000 live births.3,4
Although the clinical presentation, diagnosis, and treatment of PS are well-established, the etiology remains unclear. Male gender3,5–7 and a family history of PS3,5,6,8 are consistently reported risk factors and suggest a genetic component to the etiology.3,6 However, the change in PS incidence reported in several countries9 indicates that environmental factors are also important. Symptoms usually do not arise until the second or third week after birth10 and only exceptionally before,11 suggesting that early exposures such as feeding practices could be important risk factors. In Denmark, the frequency of breastfeeding increased during the 1990s (http://www.sst.dk/publ/Publ2009/CFF/Boernesundhed/Amning09.pdf). In the same period, the incidence of PS in Denmark decreased.4,12
Parallels between breastfeeding and PS risk have been documented in other countries raising discussion about whether breastfeeding protects or is a risk factor for PS.13–18 Two case-control studies revealed a two- to threefold higher risk for infants being bottle-fed.19,20 The authors of these studies examined the association between feeding practice at the time of postdelivery discharge or the first week after birth and the subsequent risk of PS. However, PS onset is typically between 3 and 8 weeks of age,7 several weeks after this feeding information was obtained. Thus, mothers classified as breastfeeding soon after discharge may have changed to bottle-feeding well before the onset of PS symptoms, thereby likely underestimating the risk of PS with respect to bottle-feeding. Using data from a nationwide birth cohort, we were able to examine in detail the association between the timing of first exposure to bottle-feeding and the subsequent risk of PS.
Methods
Study Cohort
The study was based on the Danish National Birth Cohort,21 which enrolled 101 042 pregnancies in 91 827 Danish women during the period from 1996 to 2002. Twice during pregnancy (weeks 12 and 30) and twice after delivery (6 months and 18 months), women were interviewed by telephone to obtain detailed exposure data.
For the current study, inclusion criteria required being a live-born singleton infant included in the Danish National Birth Cohort and having a mother who answered the questions about feeding practice in the 6-month interview. In total, 70 148 infants fulfilled these criteria. The reduction in numbers occurred largely because participation in the study decreased at each interview (92% participated in the first interview, 87% in the second interview, and 70% in the third interview) but also because multiple-birth infants were excluded. Multiple births were excluded because they are special pregnancies with a higher risk of PS.3
Exposure
Information on feeding practice was obtained from the 6-month telephone interview. In this interview, the mothers were asked (1) whether they were currently breastfeeding, (2) for how long they had fully breastfed their children, and (3) how old their children were when they stopped breastfeeding. The mothers indicated the length of breastfeeding in days, weeks (7 days), or months (30.5 days) plus weeks, but all answers were translated into days from birth. We assumed that all infants <4 months of age who were not fully breastfed were bottle-fed. Thus all infants who were not fully breastfed are labeled as bottle-fed infants. Only 48 women (no PS cases) in our study gave their own (45 women) or another mother’s milk (3 women) by bottle. We therefore assumed that the bottle-fed infants were fed artificial formula milk. Information on the brand of formulas was not available from the interview.
Outcome
Information on PS diagnosis was obtained from the Danish National Patient Register.22 PS cases were defined as infants who had a pyloromyotomy determined by the following surgery codes: KJDH60 and KJDH61 (Nordic Classification of Surgical Procedures). Of the 101 042 pregnancies enrolled in the Danish National Birth Cohort, 75 infants had a surgery code for PS. Thirty-two infants had a diagnosis code for PS according to the Danish National Patient Register (Q40.0, DK31.1, K31.1A, DK31.3, and DK31.8B [International Classifications of Diseases, 10th Revision]), but no codes documented surgery for PS. Their medical files were individually reviewed, and when a definite record of surgery for PS was found, the child was included as a case (14 cases). Of the 89 cases, 3 cases were not singleton, 20 cases were missing information about feeding practice from the 6-month interview, and 1 case was diagnosed at age 5 months, resulting in 65 PS cases included in the study. Date of PS diagnosis was defined as the date of first admission for PS diagnosis.
Covariates
From the telephone interviews, we gathered information on mothers’ socioeconomic status (occupation and years of schooling) and smoking habits. Information on the infant’s gender, date and place of birth, maternal age, birth order, emigration, and updated information on vital status was obtained from the Danish Civil Registration System, data being available since April 1968. The Danish Civil Registration System includes a unique personal identification number assigned to each Danish resident permitting accurate linkage of individual-level information between the nationwide registers in Denmark.23 Data on gestational age, birth weight, and type of birth were based on information from the Danish Medical Birth Registry.24
Statistical Analysis
The association between bottle-feeding and the risk of PS was evaluated by hazard ratios (HRs) estimated in a Cox regression model by using PROC TPHREG in SAS software, version 9.1 (SAS Institute, Inc, Cary, NC). Age was used as the underlying time scale and, unless otherwise stated, all HRs were adjusted for gender, gestational age (<37, 37–<42, and ≥42 weeks), birth weight (<2500, 2500–<3000, 3000–<3500, 3500–<4000, 4000–<4500, and ≥4500 g), birth order (1, 2, and 3+), maternal age (<25, 25–<30, 30–<35, and ≥35 years), and maternal smoking during pregnancy. Infants were considered at risk from birth to 4 months of age, death, emigration, or PS diagnosis, whichever occurred first.
Based on the answers from the questions about feeding practice in the 6-month interview, we defined the child’s age at change from full breastfeeding to both breast and bottle-feeding and the age at change from both breast and bottle-feeding to exclusive bottle-feeding. These ages were used to define the time-dependent feeding practice variables (ie, combination of breast and bottle-feeding, time since first exposure to bottle-feeding, and age at first exposure to bottle-feeding) for every day during follow-up. Analyses of effect modification by gender and attained age of child were conducted by including an interaction term in the Cox regression model. The assumption of proportional hazards in the Cox regression model was evaluated by the effect modification with attained age. All tests were homogeneity Wald test. Cumulative risk of PS by age in months was estimated by the Kaplan–Meier estimates.
The association between case status (case versus noncase) and whether the mother reported time of first exposure to bottle-feeding in days, weeks, or months plus weeks was evaluated by logistic regression with adjustment for age at exposure to first bottle-feeding, using the procedure GENMOD in SAS.
Ethics
Women in the study gave consent to participation, and permission to perform the analyses was granted by the Danish National Birth Cohort steering committee and the Danish Data Protection Board.
Results
The cohort of 70 148 singleton infants was followed for 4 months during which 65 infants had surgery for PS, which amounts to an overall risk of 0.1%. Of the 65 PS cases, 59 (91%) were boys and 6 (9%) were girls. The median age at diagnosis was 35 days (minimum: 9; maximum: 96), and 95% of the cases were diagnosed between 13 and 69 days of age. Of the 65 PS cases, 29 infants were bottle-fed before PS diagnosis. Among ever breastfed infants, the median age at first exposure to bottle-feeding was 91 days for PS cases and 122 days for noncases. Follow-up included 4781 person-years for bottle-fed infants (with or without breastfeeding in addition) and 18 635 person-years for infants while not bottle-fed.
Table 1 shows the HRs of PS according to feeding practice characteristics. The overall HR of PS for infants who were bottle-fed compared with infants who were not bottle-fed was 4.62 (95% confidence interval [CI]: 2.78–7.65). Among the bottle-fed infants, there were no significant differences in risk between the both breast and bottle-fed (HR: 3.36 [95% CI: 1.60–7.03]), the formerly breastfed (HR: 5.38 [95% CI: 2.88–10.06]), and the never breastfed infants (HR: 6.32 [95% CI: 2.45–16.26]) (P = .76). Furthermore, the increased risk of PS among infants who were bottle-fed was observed even after 14 days, as well as 30 days since first exposure to bottle-feeding. Among both breast and bottle-fed infants, we observed an HR of 4.35 (95% CI: 1.70–11.20) with <14 days of exposure to bottle-feeding and 2.60 (95% CI: 0.91–7.41) with >14 days of exposure to bottle-feeding. The risk of PS among bottle-fed infants did not vary with age at first exposure to bottle-feeding.
TABLE 1.
HRs of PS According to Feeding Practice Characteristics
| Feeding Practice Characteristics | Number of PS Cases | HR (95% CI)a | P |
|---|---|---|---|
| Exposure to bottle-feeding | |||
| Not bottle-fed | 36 | 1 (reference) | <.0001 |
| Bottle-fed | 29 | 4.62b (2.78–7.65) | — |
| Combination of breast and bottle-feeding | |||
| Not bottle-fed | 36 | 1 (reference) | — |
| Bottle-fedc | |||
| Both breast and bottle-fed | 9 | 3.36d (1.60–7.03) | |
| Formerly breastfed | 15 | 5.38 (2.88–10.06) | .76 |
| Never breastfed | 5 | 6.32d (2.45–16.26) | |
| Time since first exposure to bottle-feedinge | |||
| Not bottle-fed | 36 | 1 (reference) | — |
| Bottle-fed | |||
| <30 d | 18 | 4.50 (2.53–8.01) | .98 |
| ≥30 d | 11 | 4.85 (2.33–10.07) | |
| Age at first exposure to bottle-feeding | |||
| Not bottle-fed | 36 | 1 (reference) | — |
| Bottle-fed | |||
| <30 d | 25 | 5.00 (2.97–8.41) | .26 |
| ≥30 d | 4 | 2.95 (0.99–8.72) |
HR adjusted for age, gender, gestational age, birth weight, birth order, maternal age, and maternal smoking during pregnancy. Adjusting only for age increases HR to 5.05 (3.07–8.30), primarily due to not adjusting for maternal smoking during pregnancy.
Additionally adjusting for mothers’ socioeconomic status (occupation and school skills), type of birth (vaginal/caesarean section) and maternal smoking during breastfeeding, and postnatal yield similar results.
Among the bottle-fed infants, the median age at diagnosis was 40 d for both breast and bottle-feeding infants, and 35 d for formerly breastfed infants now receiving only bottle-feeding. Five infants with PS were never breast-fed, and among them, the age at diagnosis ranged from 13 to 35 d, with a median age of 26 d. Furthermore, among the infants who were bottle-fed, the time of follow-up was 2027 person-years for those both breast and bottle-fed, 2332 person-years for those formerly breastfed, and 422 person-years for those never breastfed (exclusively bottle-fed).
The ratio between the HR for both breast and bottle-fed infants and the HR for never breastfed was 1.88 (0.63–5.66).
Among the bottle-fed infants, the HR of PS was 4.91 (95% CI: 2.34–10.31) <14 d since first exposure to bottle-feeding and 4.48 (95% CI: 2.53–7.94) >14 d since first exposure to bottle-feeding. Less than 14 d since first exposure to bottle-feeding, there were 9 cases: 3 cases within the first 48 h and 6 cases in the second week.
We found no significant modification of the overall association with bottle-feeding and PS risk according to attained age of the infant (<30 days: HR: 5.82 [95% CI: 2.47–13.73]; ≥30 days: HR: 4.12 [95% CI: 2.24–7.60]; P = .52) or gender of the infant (boy infants: HR: 3.91 [95% CI: 2.29–6.68]; girl infants: HR: 30.09 [95% CI: 3.50–258.63]; P = .07).
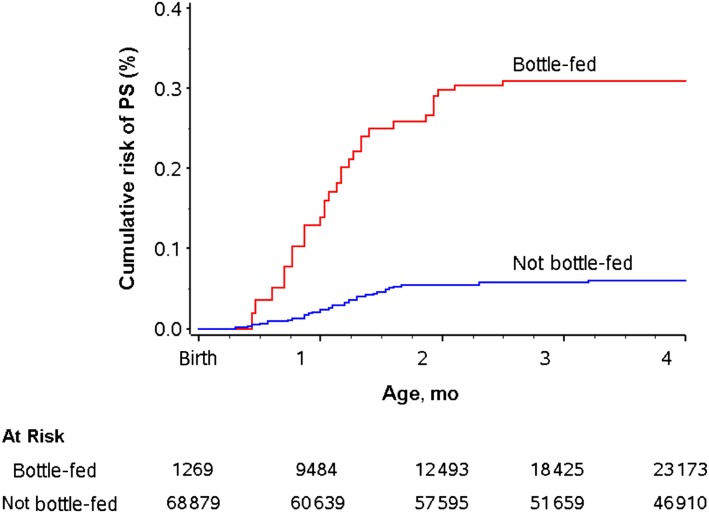
Figure 1 shows the estimated cumulative risk of PS by age in bottle-fed and in not bottle-fed infants. It was estimated that among bottle-fed infants, 0.31% developed PS during the first 4 months after birth compared with 0.05% among infants who were not bottle-fed during the first 4 months.
FIGURE 1.
Cumulative risk of PS by age in bottle-fed and not bottle-fed infants.
Whether the mother reported time at first exposure to bottle-feeding in days, weeks, or months plus weeks was not associated with case status after adjustment for age at first exposure to bottle-feeding (P = .29).
Adjusting covariates for bottle-feeding did not affect the estimates of the covariates.
Discussion
It is surprising that feeding practice has not been more intensively investigated in relation to PS. It is an obvious candidate as an early postnatal risk factor for this disease. In this large cohort study, we found that infants who were bottle-fed had a 4.6-fold increased risk of developing PS compared with infants who were not bottle-fed, even after adjustment for other known risk factors and possible confounders. The increased risk was seen in all bottle-fed groups; in infants who were both breast and bottle-fed, in infants who were formerly breastfed, and in infants who were never breastfed (exclusively bottle-fed).
The role of feeding practice has been examined primarily by ecological studies in which changes in risk of PS over time has been correlated with changes in rates of breastfeeding/bottle-feeding.14–17 Conflicting results have been reported. In 2 older studies in which the authors used survey information, bottle-feeding had no impact on PS risk.13,18 However, a few recent analytical studies have revealed a distinct risk association between early bottle-feeding and PS.19,20,25 In a 1:1 case-control study, Habbick et al19 examined feeding practice at the time of postdelivery discharge and found that bottle-feeding was 2.9 times more common among infants who developed PS than among the control subjects. A recent 30-year retrospective study of infants diagnosed with PS in Nigeria revealed that all 57 infants developing PS in the survey area were infants who used artificial feeds.25 Pisacane et al20 observed that infants developing PS were less likely to have been exclusively breastfed during the first week after birth compared with control infants. Consistent with our findings, they estimated a similar risk of PS for the exclusively formula-fed infants and the both breast and formula-fed infants.
To our knowledge, no previous studies have evaluated the importance of the timing of first exposure to bottle-feeding and the risk of PS or presented the modification of the PS risk according to attained age and gender. The prospective design and information on perinatal and feeding practices in the Danish National Birth Cohort allowed us such an opportunity. We found no significant difference in the overall effect according to these characteristics. Despite small numbers, the 30-fold elevation in HR for girls and the nearly eightfold difference in the girl:boy ratio of HRs raises the possibility of an increased female susceptibility to bottle-feeding. However, as the baseline rate is much lower in girls, it could simply reflect that the rates in boys and girls are similar among bottle-fed infants. This observation needs further examination. We found no significant variation of the overall result according to attained age, and we found time since first exposure to bottle-feeding similarly increased less and above 30 days.
We documented a higher risk of PS for bottle-fed infants compared with infants who were not bottle-fed. Two possible explanations are that either breastfeeding confers protection or bottle-feeding is itself the risk factor for PS responsible for the association.
Under the hypothesis that breast milk confers protection against PS, one suggestion has been that the presence of high levels of hormones such as vasoactive intestinal peptide in human milk favor pyloric relaxation.20 Breast milk also has lower osmolarity, which could provide better gastric emptying.26 It is also theoretically possible that breastfeeding protects against an infectious trigger by unknown agents because previous studies have revealed that breastfeeding confers protection against gastrointestinal infections.27–29 The protective effect of prebiotics might be contributing. Bifidobacteria and lactobacilli make up 90% of the bacteria in the stomach of breastfed infants but only 40% to 60% in bottle-fed infants.30–32 However, no infectious cause is known to explain PS.33
We find it more likely that bottle-feeding is the important feeding risk factor. In support, we demonstrated a significant increased risk among both never (sixfold) and formerly breastfed infants (fivefold) as compared with infants who were not bottle-fed (fully breastfed). In both bottle-exposed groups, infants were exclusively being bottle-fed at PS onset. Among the bottle-fed infants, infants both breast and bottle-fed were a mixed group, and the proportion of milk coming from the breast and from bottle-feeding, respectively, was not reported. By experience, in the beginning of the both breast and bottle-fed period, bottle use may be minimal. However, even within 14 days of bottle-exposure, among the infants both breast and bottle-fed, the risk was increased. This observation suggests that limited exposure to bottle-feeding quickly results in a higher risk of PS. Although the point estimate for both breast and bottle-fed infants was lower than for the exclusively bottle-fed infants (never breastfed), there was no statistical difference. This suggests that breast milk provided little if any protective effect.
On the basis of our study, it is not possible to determine whether it is the formula or the mechanism of bottle-feeding or both that make up the high risk. Formula has a higher level of whey and particularly casein proteins than breast milk and is likely more difficult for the infants to digest.34 Furthermore, the gastric emptying is affected by the composition of the feed and is slower in formula-fed infants.26,35 The gastric and intestinal hormones may contribute to this difference.36 Bottle-fed infants consume a larger volume in less time. We therefore speculate that infants given formula by bottle ingest a larger volume of milk and retain it for a longer period of time in the stomach. This burden of overfeeding may challenge the pylorus muscle and lead to hypertrophy. Boy infants likewise may consume a larger volume or take food faster because they gain weight faster than girl infants.37,38 If so, overfeeding might contribute to the higher risk of PS in boy infants. Formulas have been improved over time and now approach the composition of breast milk. This could be a contributing reason for the decrease in PS incidence.
Strengths and Limitations
Major strengths of this study included its design, size, and the utilization of unique Danish national registers. The detailed information in the Danish National Birth Cohort provided a unique opportunity to perform a large study with extensive information on breastfeeding status and adjustment for potential confounders by using information obtained before possible PS. PS diagnoses were obtained from the Danish National Patient Register in which hospital discharge diagnoses are mandatorily recorded for the entire country. In particular, surgical diagnoses must be considered both accurate and well-recorded.39 In addition, we furthermore evaluated medical records to ensure the correctness of the diagnosis. We therefore consider misclassification of the diagnosis unlikely.
However, some limitations should be considered in the interpretation of the observed association. Information about feeding practice was obtained 6 months after birth. Thus, the observed association could at least partly be explained by differential recall (ie, that mothers of infants who develop PS reported feeding practice differently compared with other mothers). However, it is difficult to imagine that these mothers on average should report the introduction of bottle-feeding 31 days (=122 days − 91 days) earlier because of the disease of the infant. Furthermore, even though these mothers potentially could use the time of PS diagnosis to date the introduction of bottle-feeding more precisely, they did not more than expected report time of introduction of bottle-feeding by using the more precise form of weeks and days rather than months and weeks. We therefore do not find it likely that the higher risk of PS in bottle-fed infants can be ascribed to recall bias in the mothers.
Another concern could be whether the observed higher risk of PS in bottle-fed infants occurred because mothers ended full breastfeeding due to disease symptoms in the infant (ie, inverse causality). However, we observed a similar increase in PS risk among infants who were first bottle-exposed close to PS surgery and those first exposed >30 days before PS surgery, well before symptoms were likely to have occurred. Furthermore, the infants who were never breastfed had the same high risk as infants who had formerly been breastfed. Therefore, inverse causality is not a likely explanation for our findings.
Finally, the observed difference in risk could in theory be due to a different risk factor profile in mothers who never breastfed their infants compared with those who breastfed (ie, confounding). However, with respect to gestational age, maternal age, the mother’s smoking habits, and other known variables, the group that never breastfed was similar in distribution to other cases. Furthermore, the difference in risk was also seen among breastfed children (eg, fully breastfed versus formerly breastfed). In addition, the minor effect of adjusting for the potentially most likely confounders did not support that the higher risk in bottle-fed infants can be ascribed to differences in risk profile.
Thus, we find it unlikely that the risk association observed in this study was caused by either recall bias, inverse causality, or confounding.
Conclusions
We found that infants who were bottle-fed had a 4.6-fold increased risk of developing PS compared with infants who were not bottle-fed. The result adds to the evidence supporting the advantage of exclusive breastfeeding in the first months after birth.
Acknowledgment
We thank the Danish National Birth Cohort for permission to use the data.
Glossary
- CI
confidence interval
- HR
hazard ratio
- PS
pyloric stenosis
Footnotes
Drs Krogh, Biggar, Fischer, Mr Lindholm, Mr Wohlfahrt, and Dr Melbye made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; Drs Krogh, Biggar, Fischer, Mr Wohlfahrt, and Dr Melbye contributed to drafting the article or revising it critically for important intellectual content; and Drs Krogh, Biggar, Fischer, Mr Lindholm, Mr Wohlfahrt, and Dr Melbye contributed to the final approval of the version to be published.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: This study was supported by grants from the Faculty of Health Sciences (University of Copenhagen) and the Danish Agency for Science, Technology, and Innovation. None of the Foundations had a role in the design and conduct of the study; in the collection, management, analysis, and interpretation of the data; or in the preparation, review, or approval of the article.
References
- 1.Chung E. Infantile hypertrophic pyloric stenosis: genes and environment. Arch Dis Child. 2008;93(12):1003–1004 [DOI] [PubMed] [Google Scholar]
- 2.Ramstedt C. Zur opration der angeborenen pylorus-stenose. Med Klin. 1912;8:1702–1705 [Google Scholar]
- 3.Krogh C, Fischer TK, Skotte L, et al. Familial aggregation and heritability of pyloric stenosis. JAMA. 2010;303(23):2393–2399 [DOI] [PubMed] [Google Scholar]
- 4.Nielsen JP, Haahr P, Haahr J. Infantile hypertrophic pyloric stenosis. Decreasing incidence. Dan Med Bull. 2000;47(3):223–225 [PubMed] [Google Scholar]
- 5.MacMahon B. The continuing enigma of pyloric stenosis of infancy: a review. Epidemiology. 2006;17(2):195–201 [DOI] [PubMed] [Google Scholar]
- 6.Mitchell LE, Risch N. The genetics of infantile hypertrophic pyloric stenosis. A reanalysis. Am J Dis Child. 1993;147(11):1203–1211 [DOI] [PubMed] [Google Scholar]
- 7.Schechter R, Torfs CP, Bateson TF. The epidemiology of infantile hypertrophic pyloric stenosis. Paediatr Perinat Epidemiol. 1997;11(4):407–427 [DOI] [PubMed] [Google Scholar]
- 8.Carter CO, Evans KA. Inheritance of congenital pyloric stenosis. J Med Genet. 1969;6(3):233–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pedersen RN, Garne E, Loane M, Korsholm L, Husby S, EUROCAT Working Group . Infantile hypertrophic pyloric stenosis: a comparative study of incidence and other epidemiological characteristics in seven European regions. J Matern Fetal Neonatal Med. 2008;21(9):599–604 [DOI] [PubMed] [Google Scholar]
- 10.Rollins MD, Shields MD, Quinn RJ, Wooldridge MA. Pyloric stenosis: congenital or acquired? Arch Dis Child. 1989;64(1):138–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tashjian DB, Konefal SH. Hypertrophic pyloric stenosis in utero. Pediatr Surg Int. 2002;18(5-6):539–540 [DOI] [PubMed] [Google Scholar]
- 12.Sørensen HT, Nørgård B, Pedersen L, Larsen H, Johnsen SP. Maternal smoking and risk of hypertrophic infantile pyloric stenosis: 10 year population based cohort study. BMJ. 2002;325(7371):1011–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dodge JA. Infantile hypertrophic pyloric stenosis in Belfast, 1957-1969. Arch Dis Child. 1975;50(3):171–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jedd MB, Melton LJ, III, Griffin MR, et al. Factors associated with infantile hypertrophic pyloric stenosis. Am J Dis Child. 1988;142(3):334–337 [DOI] [PubMed] [Google Scholar]
- 15.Knox EG, Armstrong E, Haynes R. Changing incidence of infantile hypertrophic pyloric stenosis. Arch Dis Child. 1983;58(8):582–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lammer EJ, Edmonds LD. Trends in pyloric stenosis incidence, Atlanta, 1968 to 1982. J Med Genet. 1987;24(8):482–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Donoghue JM, Connolly KD, Gallagher MM, O’Hanlon D, Doyle J, Flynn JR. The increasing incidence of infantile hypertrophic pyloric stenosis. Ir J Med Sci. 1993;162(5):175–176 [DOI] [PubMed] [Google Scholar]
- 18.Webb AR, Lari J, Dodge JA. Infantile hypertrophic pyloric stenosis in South Glamorgan 1970-9. Effects of changes in feeding practice. Arch Dis Child. 1983;58(8):586–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Habbick BF, Khanna C, To T. Infantile hypertrophic pyloric stenosis: a study of feeding practices and other possible causes. CMAJ. 1989;140(4):401–404 [PMC free article] [PubMed] [Google Scholar]
- 20.Pisacane A, de Luca U, Criscuolo L, et al. Breast feeding and hypertrophic pyloric stenosis: population based case-control study. BMJ. 1996;312(7033):745–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olsen J, Melbye M, Olsen SF, et al. The Danish National Birth Cohort—its background, structure and aim. Scand J Public Health. 2001;29(4):300–307 [DOI] [PubMed] [Google Scholar]
- 22.Andersen TF, Madsen M, Jørgensen J, Mellemkjoer L, Olsen JH. The Danish National Hospital Register. A valuable source of data for modern health sciences. Dan Med Bull. 1999;46(3):263–268 [PubMed] [Google Scholar]
- 23.Pedersen CB, Gøtzsche H, Møller JO, Mortensen PB. The Danish Civil Registration System. A cohort of eight million persons. Dan Med Bull. 2006;53(4):441–449 [PubMed] [Google Scholar]
- 24.Knudsen LB, Olsen J. The Danish Medical Birth Registry. Dan Med Bull. 1998;45(3):320–323 [PubMed] [Google Scholar]
- 25.Osifo DO, Evbuomwan I. Does exclusive breastfeeding confer protection against infantile hypertrophic pyloric stenosis? A 30-year experience in Benin City, Nigeria. J Trop Pediatr. 2009;55(2):132–134 [DOI] [PubMed] [Google Scholar]
- 26.Cavell B. Gastric emptying in infants fed human milk or infant formula. Acta Paediatr Scand. 1981;70(5):639–641 [PubMed] [Google Scholar]
- 27.Howie PW, Forsyth JS, Ogston SA, Clark A, Florey CD. Protective effect of breast feeding against infection. BMJ. 1990;300(6716):11–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oddy WH. Breastfeeding protects against illness and infection in infants and children: a review of the evidence. Breastfeed Rev. 2001;9(2):11–18 [PubMed] [Google Scholar]
- 29.Beaudry M, Dufour R, Marcoux S. Relation between infant feeding and infections during the first six months of life. J Pediatr. 1995;126(2):191–197 [DOI] [PubMed] [Google Scholar]
- 30.Dai D, Walker WA. Protective nutrients and bacterial colonization in the immature human gut. Adv Pediatr. 1999;46:353–382 [PubMed] [Google Scholar]
- 31.Coppa GV, Zampini L, Galeazzi T, Gabrielli O. Prebiotics in human milk: a review. Dig Liver Dis. 2006;38(suppl 2):S291–S294 [DOI] [PubMed] [Google Scholar]
- 32.Penders J, Thijs C, Vink C, et al. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics. 2006;118(2):511–521 [DOI] [PubMed] [Google Scholar]
- 33.Sherwood W, Choudhry M, Lakhoo K. Infantile hypertrophic pyloric stenosis: an infectious cause? Pediatr Surg Int. 2007;23(1):61–63 [DOI] [PubMed] [Google Scholar]
- 34.Agbasi GG. Your decision to breast feed is best. GRMA News. 1992;4(14):4–, 7. [PubMed] [Google Scholar]
- 35.Van Den Driessche M, Peeters K, Marien P, Ghoos Y, Devlieger H, Veereman-Wauters G. Gastric emptying in formula-fed and breast-fed infants measured with the 13C-octanoic acid breath test. J Pediatr Gastroenterol Nutr. 1999;29(1):46–51 [DOI] [PubMed] [Google Scholar]
- 36.Lucas A, Sarson DL, Blackburn AM, Adrian TE, Aynsley-Green A, Bloom SR. Breast vs bottle: endocrine responses are different with formula feeding. Lancet. 1980;1(8181):1267–1269 [DOI] [PubMed] [Google Scholar]
- 37.van’t Hof MA, Haschke F, Darvay S, Euro-Growth Study Group . Euro-Growth references on increments in length, weight, and head and arm circumferences during the first 3 years of life. J Pediatr Gastroenterol Nutr. 2000;31(suppl 1):S39–S47 [DOI] [PubMed] [Google Scholar]
- 38.Agostoni C, Grandi F, Giannì ML, et al. Growth patterns of breast fed and formula fed infants in the first 12 months of life: an Italian study. Arch Dis Child. 1999;81(5):395–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mosbech J, Jørgensen J, Madsen M, Rostgaard K, Thornberg K, Poulsen TD. [The national patient registry. Evaluation of data quality]. Ugeskr Laeger. 1995;157(26):3741–3745 [PubMed] [Google Scholar]