Abstract
OBJECTIVE:
To investigate whether a multidisciplinary, best-practice central line maintenance care bundle reduces central line-associated blood stream infection (CLABSI) rates in hospitalized pediatric oncology patients and to further delineate the epidemiology of CLABSIs in this population.
METHODS:
We performed a prospective, interrupted time series study of a best-practice bundle addressing all areas of central line care: reduction of entries, aseptic entries, and aseptic procedures when changing components. Based on a continuous quality improvement model, targeted interventions were instituted to improve compliance with each of the bundle elements. CLABSI rates and epidemiological data were collected for 10 months before and 24 months after implementation of the bundle and compared in a Poisson regression model.
RESULTS:
CLABSI rates decreased from 2.25 CLABSIs per 1000 central line days at baseline to 1.79 CLABSIs per 1000 central line days during the intervention period (incidence rate ratio [IRR]: 0.80, P = .58). Secondary analyses indicated CLABSI rates were reduced to 0.81 CLABSIs per 1000 central line days in the second 12 months of the intervention (IRR: 0.36, P = .091). Fifty-nine percent of infections resulted from Gram-positive pathogens, 37% of patients with a CLABSI required central line removal, and patients with Hickman catheters were more likely to have a CLABSI than patients with Infusaports (IRR: 4.62, P = .02).
CONCLUSIONS:
A best-practice central line maintenance care bundle can be implemented in hospitalized pediatric oncology patients, although long ramp-up times may be necessary to reap maximal benefits. Further research is needed to determine if this CLABSI rate reduction can be sustained and spread.
KEY WORDS: oncology, central line-associated blood stream infection, quality improvement, epidemiology, pediatric, central venous catheter/access device
Survival after a childhood cancer diagnosis has significantly increased over the past 25 years.1,2 One reason is intensive, data-driven chemotherapy regimens delivered through implanted and nonimplanted central lines.1,2 Unfortunately, these central lines put many immunocompromised children at risk for health care-associated infections, such as central line-associated blood stream infections (CLABSIs). In children, CLABSIs occur at a rate of 0.7 to 7.4 infections per 1000 catheter days and can cost an average of $45 000 per infection.3–7 Pediatric bone marrow transplant patients have the highest permanent central line pooled mean CLABSI rate (3.2 CLABSIs per 1000 central line days) in comparison with other adult oncology care areas,7 and these infections potentially carry a 1% mortality rate.8
Meticulous attention to central line maintenance strategies decreases CLABSI rates in hospitalized children.9–11 This includes handling central lines in an aseptic fashion each of the 30 to 50 times they may be used daily (Ascenzi J. Personal conversation regarding central line accesses in Johns Hopkins Children's Center Pediatric Intensive Care Unit. 2012). Centers for Disease Control and Prevention (CDC)–based best-practice central line care bundles have been successfully implemented in pediatric intensive care units, significantly reducing CLABSI rates.9,12 Similar best-practice bundles have decreased CLABSI rates in neonatal intensive care units, pediatric parenteral nutrition patients, and pediatric stem cell transplant recipients.10,13,14
Despite these efforts, no study has attempted to decrease CLABSI rates for an entire inpatient pediatric oncology cohort, including both stem cell transplant recipients and patients undergoing treatment of malignancies. These patients may be different from other pediatric populations because of their immunocompromised status, their disease-specific comorbidities, and the frequency with which they use their central lines. We hypothesized that rigorous attention to central line maintenance practices, combined with continuous quality improvement strategies, could significantly reduce CLABSI rates in an inpatient pediatric oncology population. Additionally, we hoped to further elucidate the epidemiology of CLABSIs in hospitalized pediatric oncology patients. Finally, we present interventions to improve compliance with best-practice care bundles in all populations to spread lessons learned to other institutions and patients.
Methods
Setting
This study was conducted in an urban, tertiary, university-affiliated hospital with a 186-bed Children’s Center. The pediatric oncology unit sees ∼200 new oncologic diagnoses and performs 35 stem cell transplants each year. The unit is an 18-bed, self-contained floor with consistent nursing and support staff devoted to patients with primary oncologic diagnoses.
Intervention
In November 2009, our institution joined a 27-institution, Hematology/Oncology quality transformation effort, organized by the Children’s Hospital Association (CHA), focused on eliminating CLABSIs by implementing best-practice central line care bundles. Both our local efforts and our unit’s participation in a national collaborative were supported by oncology leadership and hospital-wide physician and nursing leadership. In contrast to previously published CLABSI reduction initiatives that addressed bothcatheter insertion and maintenance procedures,9,11 this effort focused solely on optimizing catheter maintenance procedures. This maintenance-only focus was chosen given previous evidence suggesting that improved maintenance practices were significantly associated with decreased infection rates,9 a previous hospital focus on improving temporary central line insertion practices through a dedicated bedside central line insertion team15 and the knowledge that the majority of oncology patient lines are placed in the operating room, an arena that necessitates significantly different quality improvement strategies than our oncology unit. The Strategy for Translating Evidence into Practice was used as a conceptual model: (1) summarize the evidence, (2) identify local barriers, (3) measure performance, and (4) ensure that all patients receive the intervention through Engage, Educate, Execute, and Evaluate.16
The CHA central line care bundle9 (Table 1), based on CDC recommendations,17 emphasizes best practices in all areas of central line care: reduction of line entries, aseptic entries into the line, and aseptic procedures when changing line components. Education on the bundle began in November 2009. Anonymous nursing self-practice audits were performed on a randomly chosen nursing shift, 1 day every week, as a sampling strategy for all unit patients with central lines (Supplemental Appendix A). Through these audits, compliance of bedside nurses with the 3 main bundle elements was tracked and reinforced: (1) daily discussion of line entry reduction with medical team, (2) aseptic entries into the line, and (3) aseptic procedures when changing line components. An all-or-none measurement strategy was used for audits: nursing practice was only recorded as compliant with one of the bundle elements if every part of that bundle element was done appropriately.18 For example, if a nurse wore sterile gloves, a mask, shielded the patient, and scrubbed the needle site appropriately, but did not record the date and time, this patient interaction was scored as not compliant. Based on a continuous quality improvement model,16,19,20 targeted interventions were instituted to improve compliance with each of the bundle elements (Table 2). Results from the intervention, including CLABSI rates and audit compliance rates, were displayed graphically in the nursing break room.
TABLE 1.
Best-Practice Central Line Maintenance Care Bundle
| Central Line Maintenance Care Bundlea |
|---|
| 1. Daily assessment of line necessity and consolidation and/or elimination of catheter entries (CDC recommended) |
| 2. Daily dressing/site assessment performed (CDC recommended)17 |
| 3. Catheter entries |
| a. Hand hygiene performed before all catheter entries (CDC recommended) |
| b. Nonsterile gloves worn for all catheter entries |
| c. Cap scrubbed with alcohol (15 s scrub and 15 s dry) or Chlorhexidine Gluconate (CHG) (30 s scrub and 30–60 s dry) for each entry (CDC recommended) |
| 4. Cap/tubing/dressing/needle changes: |
| a. Sterile gloves and mask worn by provider/assistantb |
| b. Cap connection site scrubbed with alcohol or CHG before removal of old cap (CDC recommended) |
| c. Dressing/needle site scrubbed with CHG (CDC recommended) |
| d. For dressing/port needle changes, shield patient face or tracheotomy from dressing change site |
| e. Old and new cap/tubing/dressing/needle date and time clear |
| 5. Catheter site care |
| a. No iodine ointment (CDC recommended) |
| b. Change needle every 7 d; unless soiled, loosened, dislodged, or infiltrated |
| c. Change gauze dressings every 2 d; unless soiled, dampened, loosened (CDC recommended) |
| d. Change clear dressing every 7 d; unless soiled, dampened, loosened (CDC recommended) |
| e. Prepackaged dressing change kit |
| 6. Catheter hub/cap/tubing care |
| a. Replace administration sets, including add on devices at 96 h, unless soiled or suspected to be infected (CDC recommended) |
| b. Replace tubing used to administer blood, blood products, or lipids at 24 h (CDC recommended) |
| c. Change caps at 72 h but should be replaced when administration set is changed (CDC recommended) |
| d. Prepackaged cap change kit/cart/central location |
If not noted, bundle components were chosen based on Children’s Hospital Association expert consensus.
This piece of the best-practice central line maintenance care bundle was not implemented in our institution for cap and tubing changes owing to the resource-intensive nature of these interventions. Clean gloves were worn instead. CDC recommendations suggest either sterile or clean gloves can be used for dressing and needle changes.
TABLE 2.
Interventions to Improve Compliance With Bundle Elements
| Intervention | Date | Leadership Impression of Efficacy | Improvements After Implementation |
|---|---|---|---|
| Nurse education: "Scrub the hub," "Mask for the task," "Prime the line," "Disconnect and you infect." "Be a line saver campaign" | Fall 2009 | Very effective, used visual cues, and slogans. Rolled out each task individually. | |
| Developed and implemented nursing audit tool | Fall/winter 2009/2010 | Very effective and still in use. | |
| Central line on-line data entry system to track central line days and types of lines | Fall 2009 | Eased paper data recording requirement for central line days. Allowed multiple stakeholders to access the data. | Website logic implemented and 1 nurse responsible for data entry to decrease data entry errors |
| Central line entry cards attached to IV poles to track number of times nurses entering central lines | Fall 2010 | The cards were difficult to maintain on IV poles, frequently lost, felt like double charting to RN staff. | Created daily line entry sheet and changed to tracking 2 days per week. |
| Discussing central line entries on daily rounds with all disciplines present. Laminated cards placed on conference table to remind clinicians to decrease line entries. | Fall 2010 | Initially effective with some attending physicians, but not embraced by all. | Placed "Are there problems with the patient’s central line?" on the resident sign-out sheet and nursing charge report sheets |
| Developed "workgroup" of pediatric oncology nurses to keep the momentum of the study going, assist with disseminating information to the nursing staff, and developing projects. | Fall 2010 | Improved unitwide acceptance of intervention and still in use. | |
| Presenting "Root Cause Analysis" results on CLABSI bulletin board in nursing break room. | Winter 2010 | Helpful for the staff to see the patient information and any changes we made to our practice based on our findings of the infection. | Added board where nurses can anonymously write "Who is at risk for the next CLABSI" and why. This helps get them engaged and likely focused on vulnerable patients. |
| Parent wallet cards were created to identify patients’ central line type and proper maintenance techniques. The cards are kept by parents and presented to outside hospitals for education on how to handle the central line. | Spring 2011 | Cards are very much appreciated by the families. Compliance with making them for each patient when they receive a new CL has been an issue. Still in use. | Planning on printing out the 4 different versions and having them located on the nursing unit to be individualized and laminated by the nurses when needed. We are hoping this will improve compliance. |
| CLABSI newsletter –“CABSI in the know” includes information on recent CLABSIs and changes made as a result of them. | Spring 2011 | Helpful in disseminating information in a creative way. | Working to produce newsletters at least quarterly. |
A team of front-line nurses, the unit nurse manager, physicians, pharmacists, quality improvement specialists, and infection preventionists (IPs) met monthly to complete mini-root cause analyses of all CLABSIs (Supplemental Appendix B) and discuss systems changes to improve compliance with the bundle. These meetings were led by front-line nurses, who had protected time to engage in this quality improvement initiative and were supported by the unit nurse manager. The mini-root cause analysis process began as soon as a patient had a positive blood culture and involved discussions with all care providers working with the patient. The analyses looked for 13 patient and systems factors that could have led to the CLABSI and allowed us to learn from every infection.21 The results of the mini-root cause analyses led to systems changes such as tracking central line entries and parent wallet cards, and often included tests of change by the next monthly meeting (Table 2). Additionally, nonpunitive, individual, and group education and reinforcement of policies often resulted after mini-root causes analyses. Semiannually, key members of the CLABSI prevention team attended national collaborative learning sessions aimed at improving compliance with the best-practice bundle and decreasing CLABSI rates. Additionally, team members participated in monthly webinars organized by CHA on various topics relevant to CLABSI prevention in hematology/oncology units.
Although families were not expressly included in planning this intervention, many observed nursing central line care and quickly learned the bundle procedures. Given the high level of family-centered care present on the unit, families acted as an additional check to ensure provider compliance in our unit as well as in settings throughout the hospital such as interventional radiology and the post-operative recovery unit. Some families felt uncomfortable broaching the subject in real time themselves, but pointed out differences in care to their pediatric oncology team, who both served as their advocates and provided them with scripts to advocate for their own children directly. Oral scripts were developed to explain the project to families, solicit family feedback, and respond to criticisms without defensiveness.
Definitions and Data Sources
In January 2009, we began prospectively tracking CLABSI rates in our pediatric oncology unit. Potential cases were identified by passive surveillance, as floor nurses reported all laboratory-confirmed, positive blood cultures to the IP, and active surveillance, as IPs independently searched laboratory blood culture databases. A trained IP independently adjudicated all positive blood cultures following the National Healthcare Safety Network guidelines for CLABSIs and utilizing both chart and laboratory review.22 Patient demographic characteristics, infection characteristics, and patient outcomes were collected on all patients with CLABSIs. Baseline data included 10 months from January 2009 through the start of the intervention in November 2009. CLABSI rates were defined as CLABSIs per 1000 central line days. In accordance with CDC recommendations,23 each patient with a central line contributed only 1 central line day per hospital day, even if the patient had >1 central line. The census of pediatric oncology patients with central lines was tracked manually each day by floor nurses. Starting with bundle implementation in November 2009, we began tracking central line days by central line type as well as total central line days.
Statistical Analyses
Descriptive statistics were calculated for demographic characteristics of the cohort. The Wilcoxon rank-sum test was used to compare differences between skewed distributions. Monthly CLABSI rates and compliance with the bundle elements were displayed graphically as a function of calendar time. The primary analysis estimated the difference between the baseline average monthly CLABSI rate and the post-bundle implementation average monthly CLABSI rate by using a Poisson regression model with a single covariate; an indicator for bundle implementation. A secondary analysis allowed the post-bundle implementation period to be partitioned into years and estimated the CLABSI rate separately for the baseline and each year after bundle implementation. This analysis was done because evidence from several studies demonstrated that infection rates change differently during initial adoption of the bundle, and the stable effect period after adoption of the bundle.9,24 Additionally, we tested for associations between compliance with bundle elements and CLABSI rates by using 3 separate Poisson regression models with a single covariate for each of the 3 bundle elements; the bundle elements were not included in the same model because compliance was highly correlated across the 3 bundle elements. Stata 11.1 (Stata Corp, College Station, TX) was used for all analyses.
This study was approved by the Johns Hopkins University School of Medicine Institutional Review Board.
Results
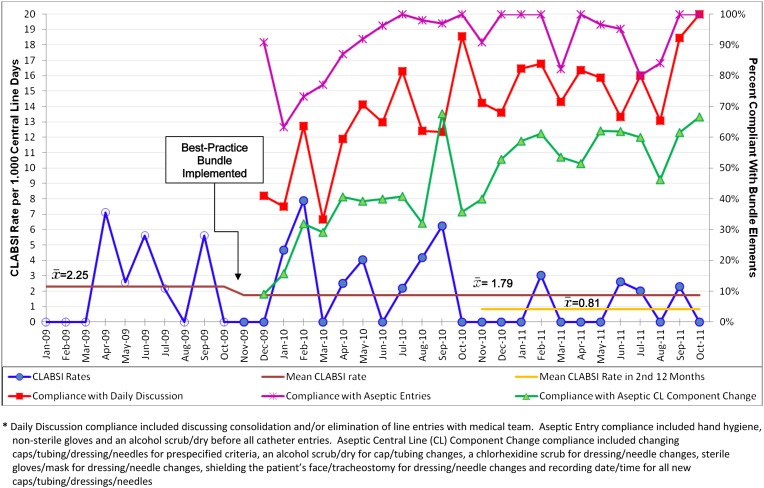
There were 14 987 patient days and 14 059 central line days on our pediatric oncology unit during the baseline and intervention periods (94% of patients with one or more central lines daily). During the baseline period from January through October 2009, our inpatient pediatric oncology unit experienced 9 CLABSIs and 4007 central line days, for a CLABSI rate of 2.25 CLABSIs per 1000 central line days (95% confidence interval [CI]: 1.02–4.26). During the 24 months of the intervention, our inpatient unit experienced 18 CLABSIs and 10 052 central line days, for a CLABSI rate of 1.79 CLABSIs per 1000 central line days (95% CI: 1.06–2.83). The incidence rate ratio (IRR) comparing the intervention period CLABSI rate with the baseline CLABSI rate was 0.80 (P = .58). (Fig 1 and Table 3) Rates of non-CLABSI bacteremias did not increase after implementation of the bundle: 5.2 non-CLABSI bacteremias per 1000 central line days preintervention versus 5.1 non-CLABSI bacteremias per 1000 central line days postintervention.
FIGURE 1.
CLABSI Rates pre and post best-practice bundle implementation and nursing compliance with bundle elements.
TABLE 3.
CLABSI Rates Before and After Bundle Implementation and by Central Line Type
| CLABSIs | Total Central Line Days | CLABSIs per 1000 Central Line Days | IRR (95% CI) | |
|---|---|---|---|---|
| Pre- vs postintervention comparison | ||||
| Baseline (1/09–10/09) | 9 | 4007 | 2.25 | ref |
| Postintervention (11/09–10/11) | 18 | 10 052 | 1.79 | 0.80 (0.36–1.77) |
| 2nd year of intervention (11/10–10/11) | 4 | 4193 | 0.81 | 0.36 (0.11–1.17) |
| Central line type comparison (postintervention only, 11/09–10/11) | ||||
| Infusaport central linesa | 2 | 3550 | 0.56 | ref |
| Hickman central lines | 14 | 5376 | 2.6 | 4.62 (1.06–41.9) |
| Peripherally inserted central catheters | 1 | 1411 | 0.71 | 1.26 (0.02–24.2) |
| Other central lines | 1 | 778 | 1.29 | 2.28 (0.04–43.8) |
Total central line days for Infusaports, Hickmans, peripherally inserted central catheters, and other central lines does not equal total central line days for postintervention because, in accordance with CDC recommendations, each patient in the pre- vs postintervention comparison contributed a maximum of 1 central line day per day, even if the patient had >1 central line.23
Given the apparent decline in CLABSI rates after the first 12 months of the intervention, a secondary analysis was performed under the assumption that the intervention’s stable effect period did not occur until the intervention’s second year. During the second year of the intervention, our unit experienced 4 CLABSIs and 4913 central line days, for an average CLABSI rate of 0.81 CLABSIs per 1000 central line days (95% CI: 0.22–2.08). The IRR comparing the second year’s CLABSI rate with the baseline CLABSI rate was 0.36 (P = .091).
Although nursing compliance with each bundle element increased during the intervention (Fig 1), 35% of patients were not receiving all bundle elements at the end of 24 months of continuous quality improvement efforts. The association between CLABSI rates and a hypothetical 10% increase in compliance on each bundle element was (1) daily discussion of line entry reduction with medical team: IRR 0.84 (P = .21), (2) aseptic entries into the line: IRR 0.73 (P = .12), and (3) aseptic procedures when changing line components: IRR 0.93 (P = .64).
Table 4 presents the patient demographic characteristics, infection characteristics, and patient outcomes for all patients with CLABSIs before and after the intervention. Thirty-seven percent of patients had their central lines removed because of their CLABSI, and 7% of patients died during the hospitalization. Both before and after the intervention, a slight majority of infections were due to Gram-positive organisms. In patients with a bone marrow transplant within 100 days of their CLABSI, 75% of pathogenic CLABSI organisms were Gram-positive as opposed to only 45% in patients without a recent bone marrow transplant. In patients with a concurrent diagnosis of mucositis, 78% of pathogenic CLABSI organisms were Gram-positive as opposed to only 52% in patients without mucositis. Median days between central line insertion and patient CLABSI were lower in the second year of the intervention (median 9, interquartile range [IQR] 6.5–33.5) in comparison with the baseline period (median 65, IQR 17, 146, Wilcoxon rank-sum P = .09). There were 3 patients who each had 2 CLABSIs across the entire study period.
TABLE 4.
Demographics of Patients with CLABSIs Before and After Bundle Implementation
| Baseline (1/09–10/09) n = 9 | Postintervention (11/09–10/11) n = 18 | 2nd Year of Intervention (11/10–10/11)n = 4 | |
|---|---|---|---|
| Patient characteristics | |||
| No. of unique patients | 8 | 18 | 4 |
| Age at infection, y (SD) | 10.4 (8.2) | 11.2 (7.0) | 9.8 (3.9) |
| Race, n (%) | |||
| White | 5 (56) | 7 (38) | 3 (75) |
| African American | 3 (33) | 5 (28) | — |
| Asian/Pacific Islander | 1(11) | 1 (6) | — |
| Other | — | 5 (28) | 1 (25) |
| Ethnicity: not Hispanic, n (%) | 9 (100) | 15 (83) | 3 (75) |
| Gender (% male) | 6 (67) | 9 (50) | 3 (75) |
| Tumor, n (%) | |||
| Hematologic | 7 (78) | 10 (56) | 3 (75) |
| Solid | 2 (22) | 8 (44) | 1 (25) |
| Active malignancy, n (%) | 3 (33) | 5 (28) | 1 (25) |
| Bone marrow transplant, n (%), | |||
| Ever bone marrow transplant | 3 (33) | 9 (50) | 1 (25) |
| Bone marrow transplant within 100 d of infection | 3 (33) | 6 (33) | 0 |
| Bone marrow transplant within 7 d of infection | 2 (22) | 4 (22) | 0 |
| Mucositis, n (%) | 3 (33) | 5 (28) | 1 (25) |
| Neutropenia, n (%) | 7 (78) | 10 (56) | 2 (50) |
| Active graft versus host disease, n (%) | 3 (33) | 5 (28) | 1 (25) |
| Type of central line, n (%) | |||
| Double lumen Hickman | 4 (44) | 14 (78) | 3 (75) |
| Single lumen Hickman | 2 (22) | — | — |
| Infusaport | 2 (22) | 2 (11) | 1 (25) |
| PICC line | 1 (12) | 1 (6) | — |
| Othera | — | 1 (6) | — |
| Median days from central line insertion to CLABSI, n (IQR) | 65(17–146) | 37.5(13–187) | 9(6.5–33.5) |
| Infection characteristics | |||
| Polymicrobial, n (%) | 1 (11) | 3 (17) | 0 |
| Median length of stay Before CLABSI, d (IQR) | 10(1–19) | 13.5(6–19) | 7(5.5–11.5) |
| Infectious organisms: total | 11 | 23 | 4 |
| Gram positive, n (%) | 6 (55) | 14 (61) | 3 (75) |
| Coagulase-negative Staphylococcus | 1 | 4 | 1 |
| Enterobacter cloacae | 3 | 1 | — |
| Enterococcus faecalis | 1 | 3 | — |
| Streptococcus viridians | — | 2 | 1 |
| Enterococcus gallinarum | — | 1 | — |
| Staphylococcus aureus | — | 1 | 1 |
| Citrobacter | 1 | — | — |
| Corynebacterium spp. | — | 1 | — |
| Clostridium tertium | — | 1 | — |
| Gram negative, n (%) | 5 (45) | 8 (35) | 1 (25) |
| Klebsiella spp. | 3 | 2 | — |
| Escherichia coli | 1 | 2 | — |
| Acinetobacter spp. | — | 2 | — |
| Moraxella osloensis | — | 1 | 1 |
| Pseudomonas aeruginosa | 1 | — | — |
| Stenotrophomonas maltophilia | — | 1 | — |
| Fungal (%) | — | 1 (4) | — |
| Candida glabrata | — | 1 | — |
| Patient outcomes | |||
| Mean length of stay after CLABSI, d (SD) | 26 (12) | 28 (28) | 53 (46) |
| PICU stay after CLABSI, n (%) | 1 (11) | 2 (11) | 1 (25) |
| Death during hospitalization, n (%) | 1 (11) | 1 (6) | 0 |
| Central line removed owing to CLABSI, n (%) | 4 (44) | 6 (33) | 1 (25) |
–, indicate zero values.
This infection was present in a Cook catheter.
During the intervention period, the CLABSI rate for patients with Hickman catheters was 2.6 CLABSIs per 1000 central line days, and the CLABSI rate for patients with Infusaports was 0.56 CLABSIs per 1000 central line days (IRR 4.62, P = .02). The CLABSI rate for patients with peripherally inserted central catheters was 0.71 CLABSIs per 1000 central line days (IRR compared with Infusaports 1.26, P = .83) (Table 3).
Discussion
Our tertiary care oncology unit experienced a 20% decline in CLABSI rates after the implementation of a best-practice central line care bundle (P = .58). Secondary analyses indicated the second year of the intervention realized a 64% decline in CLABSI rates below baseline (P = .091), suggesting that a long ramp-up period may be necessary to achieve effective change. Qualitative keys to successful implementation of this best-practice bundle included front-line staff buyin and leadership, physician support, continuous quality improvement efforts, data dissemination and transparency, and a persistent focus on expected behaviors though audits and mini-root cause analyses of all CLABSIs.
Quality improvement work and behavioral change can be slow processes that take time and energy to mature and succeed.25 Despite extensive continuous quality improvement efforts, our unit was only able to reach 65% compliance with one of the bundle elements. This speaks to the pace of behavioral change and the importance of persistence in any quality improvement effort. The apparent decline of CLABSI rates during the second year of our study suggests that commitment to quality improvement efforts must be maintained during a potentially prolonged ramp-up period to fully realize the benefit of maintenance care bundles. Front line staff attributed the apparent reduction in CLABSI rates to the consistent and sustained focus on CLABSIs on our unit and the repeated efforts to involve and listen to nurses who are dealing with central line maintenance every day. Our investigation suggests the importance of committed quality improvement efforts that look past short-term time horizons.
Although previous studies have demonstrated dramatic CLABSI reductions in 18 months or less,9,15,24 these interventions started with higher baseline CLABSI rates and therefore had larger room for improvement. We observed a baseline CLABSI rate in inpatient pediatric oncology patients of 2.25 CLABSIs per 1000 central line days, which is appreciably lower than reported in previous pediatric CLABSI reduction studies.9,11,13,14 This may have contributed to the lack of statistical significance found in our results. Despite this, there was a large effect size appreciated within our study (64% decline in the second year), and the lack of statistical significance could have been related to a limited sample size. Again, this study argues for a long-term vision when pursing quality improvement efforts.
With regard to the epidemiology of CLABSIs in hospitalized pediatric oncology patients, our study parallels previous work that found slightly more Gram-positive than Gram-negative pathogens in pediatric oncology CLABSIs.14,26,27 Although a recent study found a preponderance of Enterococcus faecalis isolates in inpatient pediatric oncology CLABSIs,27 our study identified coagulase-negative Staphylococcus as the most frequent isolate. The differences among studies in the distribution of pathogens suggest that regional and institutional factors probably influence the epidemiology of CLABSIs in pediatric oncology patients. Further study is needed to help clinicians determine appropriate empirical antibiotics for patients with suspected CLABSIs, and these antibiotics may need to be institution specific. Our study found significant morbidity from CLABSIs, with affected patients having their central line removed in 37% of cases. It is unclear if antibiotic-impregnated catheters and ethanol lock strategies could reduce pediatric oncology CLABSIs or reduce the frequency of central line removal after a CLABSI.28 Finally, patients with Hickman catheters were >4 times as likely to experience a CLABSI (P = .02), a finding that may be confounded because bone marrow transplant patients often receive Hickman catheters and are likely at greater risk for infection.27 Clinicians should carefully weigh the benefits of utilizing a catheter type that has been repeatedly shown to carry a higher risk of infection in multiple pediatric oncology cohorts.3,27
There are a number of limitations to the current study. CLABSI rates have decreased nationally over the past decade.29 Given this study’s interrupted time series design, it is impossible to know if confounding factors, such as an increased national focus on CLABSIs and public CLABSI reporting efforts, contributed to the reduction of CLABSI rates.30 We have no data on whether hospital-wide awareness campaigns regarding CLABSIs affected our staff and patient behaviors. It is unclear if the results from our single-institution study can be generalized to nontertiary care inpatient pediatric oncology units that do not care for a large number of bone marrow transplant patients. Although our unit experienced an apparent decline in CLABSI rates during the second 12 months of our intervention, we must continue to observe CLABSI rates to ensure that this reduction is sustained. Given that 1 of 3 patients did not receive care completely in compliance with the bundle, additional CLABSI rate reduction may occur with improved compliance.25 Finally, our study is at risk for misclassification bias, because adjudicators may have mistakenly labeled infections as CLABSIs.22 This concern is increased in patients with mucositis and severe neutropenia, comorbidities that provide a non–central line-related reason for bacteremia but are not accounted for in the National Healthcare Safety Network CLABSI definitions.22 The risk for misclassification bias is potentially increased because the IP was not blinded to the time period of the intervention. We believe this risk is low, because rates of non-CLABSI bacteremias did not increase after implementation of the bundle.
In conclusion, this study suggests best-practice central line bundles can be implemented in inpatient pediatric oncology patients and presents practical interventions that can be applied in other institutions aiming to reduce CLABSIs. CLABSI prevention efforts focusing on central line maintenance are arduous, rely heavily on front-line staff, require patience for culture change, and likely need to use nonstatistically significant trends to motivate staff given small numbers of infections. Despite these difficulties, CLABSI prevention efforts can ultimately be successful and reduce harmful infections in vulnerable populations. Further research is needed to determine if the observed reduction in harmful health care-associated infections can be sustained and spread to other non-ICU arenas, such as to ambulatory oncology patients.
Supplementary Material
Glossary
- CDC
Centers for Disease Control and Prevention
- CHA
Children’s Hospital Association
- CI
confidence interval
- CLABSI
central line-associated blood stream infection
- IP
infection preventionist
- IQR
interquartile range
- IRR
incidence rate ratio
Footnotes
Each of the authors contributed significantly to the conception and design, acquisition of data or analysis and interpretation of data; participated in drafting the article or revising it critically for important intellectual content; and gave final approval of the version to be published.
FINANCIAL DISCLOSURE: Dr Milstone received grant support from Sage Products, Inc; the other authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Dr Rinke was supported by grant 5KL2RR025006 from the National Center for Research Resources, a component of the National Institutes of Health and the Roadmap for Medical Research. Dr Miller is supported by the Children’s Hospital Association for her work. Funded by the National Institutes of Health (NIH).
References
- 1.Pulte D, Gondos A, Brenner H. Trends in 5- and 10-year survival after diagnosis with childhood hematologic malignancies in the United States, 1990–2004. J Natl Cancer Inst. 2008;100(18):1301–1309 [DOI] [PubMed] [Google Scholar]
- 2.Pulte D, Gondos A, Brenner H. Trends in survival after diagnosis with hematologic malignancy in adolescence or young adulthood in the United States, 1981–2005. Cancer. 2009;115(21):4973–4979 [DOI] [PubMed] [Google Scholar]
- 3.Adler A, Yaniv I, Solter E, et al. Catheter-associated bloodstream infections in pediatric hematology-oncology patients: factors associated with catheter removal and recurrence. J Pediatr Hematol Oncol 2006;28(1):23–28 [PubMed]
- 4.Ingram J, Weitzman S, Greenberg ML, Parkin P, Filler R. Complications of indwelling venous access lines in the pediatric hematology patient: a prospective comparison of external venous catheters and subcutaneous ports. Am J Pediatr Hematol Oncol. 1991;13(2):130–136 [DOI] [PubMed] [Google Scholar]
- 5.O'Grady NP, Alexander M, Dellinger EP, Gerberding JL, Heard SO, Maki DG. Guidelines for the prevention of intravascular catheter-related infections. Infect Control Hosp Epidemiol. 2002;23(12):759–769 [DOI] [PubMed]
- 6.Pittet D, Tarara D, Wenzel RP. Nosocomial bloodstream infection in critically ill patients. Excess length of stay, extra costs, and attributable mortality. JAMA. 1994;271(20):1598–1601 [DOI] [PubMed] [Google Scholar]
- 7.Dudeck MA, Horan TC, Peterson KD, et al. National Healthcare Safety Network (NHSN) Report, data summary for 2010, device-associated module. Am J Infect Control. 2011;39(10):798–816 [DOI] [PubMed] [Google Scholar]
- 8.Downes KJ, Metlay JP, Bell LM, McGowan KL, Elliott MR, Shah SS. Polymicrobial bloodstream infections among children and adolescents with central venous catheters evaluated in ambulatory care. Clin Infect Dis. 2008;46(3):387–394 [DOI] [PubMed]
- 9.Miller MR, Griswold M, Harris JM II, et al. Decreasing PICU catheter-associated bloodstream infections: NACHRI’s quality transformation efforts. Pediatrics. 2010;125(2):206–213 [DOI] [PubMed] [Google Scholar]
- 10.Schulman J, Stricof R, Stevens TP, et al; New York State Regional Perinatal Care Centers. Statewide NICU central-line-associated bloodstream infection rates decline after bundles and checklists. Pediatrics. 2011;127(3):436–444 [DOI] [PubMed] [Google Scholar]
- 11.Wheeler DS, Giaccone MJ, Hutchinson N, et al. A hospital-wide quality-improvement collaborative to reduce catheter-associated bloodstream infections. Pediatrics. 2011;128(4). Available at: www.pediatrics.org/cgi/content/full/128/4/e995 [DOI] [PubMed] [Google Scholar]
- 12.Miller MR, Niedner MF, Huskins WC, et al; National Association of Children’s Hospitals and Related Institutions Pediatric Intensive Care Unit Central Line-Associated Bloodstream Infection Quality Transformation Teams. Reducing PICU central line-associated bloodstream infections: 3-year results. Pediatrics. 2011;128(5). Available at: www.pediatrics.org/cgi/content/full/128/4/e1077 [DOI] [PubMed] [Google Scholar]
- 13.Castello FV, Maher A, Cable G. Reducing bloodstream infections in pediatric rehabilitation patients receiving parenteral nutrition. Pediatrics. 2011;128(5). Available at: www.pediatrics.org/cgi/content/full/128/5/e1273 [DOI] [PubMed] [Google Scholar]
- 14.Barrell C, Covington L, Bhatia M, et al. Preventive strategies for central line-associated bloodstream infections in pediatric hematopoietic stem cell transplant recipients. Am J Infect Control. 2012;40(5):434–439 [DOI] [PubMed] [Google Scholar]
- 15.Taylor T, Massaro A, Williams L, et al. Effect of a dedicated percutaneously inserted central catheter team on neonatal catheter-related bloodstream infection. Adv Neonatal Care. 2011;11(2):122–128 [DOI] [PubMed] [Google Scholar]
- 16.Pronovost PJ, Berenholtz SM, Needham DM. Translating evidence into practice: a model for large scale knowledge translation. BMJ. 2008;337:a1714 [DOI] [PubMed]
- 17.O’Grady NP, Alexander M, Burns LA, et al; Healthcare Infection Control Practices Advisory Committee. Guidelines for the prevention of intravascular catheter-related infections. Am J Infect Control. 2011;39(4 suppl 1):S1–S34 [DOI] [PubMed] [Google Scholar]
- 18.Nolan T, Berwick DM. All-or-none measurement raises the bar on performance. JAMA. 2006;295(10):1168–1170 [DOI] [PubMed] [Google Scholar]
- 19.Kritchevsky SB, Simmons BP. Continuous quality improvement. Concepts and applications for physician care. JAMA. 1991;266(13):1817–1823 [DOI] [PubMed] [Google Scholar]
- 20.Langley GJ, Moen R, Nolan KM, Nolan TW, Norman CL, Provost LP. The Improvement Guide: A Practical Approach to Enhancing Organizational Performance. 2nd ed. San Francisco, CA: Jossey-Bass; 2009 [Google Scholar]
- 21.Agency for Healthcare Research and Quality. Becoming a High Reliability Organization: Operational Advice for Hospital Leaders. 2008. Available at: www.ahrq.gov/qual/hroadvice/hroadvice.pdf. Accessed March 31, 2012
- 22.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36(5):309–332 [DOI] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention. Central Line-Associated Bloodstream Infection (CLABSI) Event. Available at: www.cdc.gov/nhsn/pdfs/pscmanual/4psc_clabscurrent.pdf. Published 2011. Accessed January 18, 2012
- 24.Pronovost P, Needham D, Berenholtz S, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med. 2006;355(26):2725–2732 [DOI] [PubMed] [Google Scholar]
- 25.Miller MR, Bundy DG. Quality transformation efforts: no quick fixes. Pediatrics. 2011;127(3):571–572 [DOI] [PubMed] [Google Scholar]
- 26.Adler A, Yaniv I, Steinberg R, et al. Infectious complications of implantable ports and Hickman catheters in paediatric haematology-oncology patients. J Hosp Infect. 2006;62(3):358–365 [DOI] [PubMed] [Google Scholar]
- 27.Kelly M, Conway M, Wirth K, Potter-Bynoe G, Billett AL, Sandora TJ. Moving CLABSI prevention beyond the intensive care unit: risk factors in pediatric oncology patients. Infect Control Hosp Epidemiol. 2011;32(11):1079–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang EY, Chen C, Abdullah F, et al; 2011 American Pediatric Surgical Association Outcomes and Clinical Trials Committee. Strategies for the prevention of central venous catheter infections: an American Pediatric Surgical Association Outcomes and Clinical Trials Committee systematic review. J Pediatr Surg. 2011;46(10):2000–2011 [DOI] [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention (CDC). Vital signs: central line-associated blood stream infections—United States, 2001, 2008, and 2009. MMWR Morb Mortal Wkly Rep. 2011;60(8):243–248 [PubMed] [Google Scholar]
- 30.Ketelaar NA, Faber MJ, Flottorp S, Rygh LH, Deane KH, Eccles MP. Public release of performance data in changing the behaviour of healthcare consumers, professionals or organisations. Cochrane Database Syst Rev. 2011; (11):CD004538 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.