Abstract
BACKGROUND:
The prevalence of metabolic syndrome (MetS) parallels the rise in childhood obesity. MetS is associated with neurocognitive impairments in adults, but this is thought to be a long-term effect of poor metabolism. It would be important to ascertain whether these brain complications are also present among adolescents with MetS, a group without clinically manifest vascular disease and relatively short duration of poor metabolism.
METHODS:
Forty-nine adolescents with and 62 without MetS, matched on age, socioeconomic status, school grade, gender, and ethnicity, received endocrine, MRI, and neuropsychological evaluations.
RESULTS:
Adolescents with MetS showed significantly lower arithmetic, spelling, attention, and mental flexibility and a trend for lower overall intelligence. They also had, in a MetS-dose–related fashion, smaller hippocampal volumes, increased brain cerebrospinal fluid, and reductions of microstructural integrity in major white matter tracts.
CONCLUSIONS:
We document lower cognitive performance and reductions in brain structural integrity among adolescents with MetS, thus suggesting that even relatively short-term impairments in metabolism, in the absence of clinically manifest vascular disease, may give rise to brain complications. In view of these alarming results, it is plausible that obesity-associated metabolic disease, short of type 2 diabetes mellitus, may be mechanistically linked to lower the academic and professional potential of adolescents. Although obesity may not be enough to stir clinicians or even parents into action, these results in adolescents strongly argue for an early and comprehensive intervention. We propose that brain function be introduced among the parameters that need to be evaluated when considering early treatment of childhood obesity.
KEY WORDS: metabolic syndrome, adolescence, obesity, diffusion tensor imaging, brain abnormalities, cognitive performance, hippocampal volumes, fractional anisotropy
What’s Known on This Subject:
Despite the dramatic rise in prevalence of metabolic syndrome (MetS) among children and adolescents, and that MetS is associated with cognitive and brain impairments among adults, no data on the impact of MetS on the brain exist in children.
What This Study Adds:
It provides the first data on the impact of MetS on brain in adolescence. We show reductions in cognitive function and brain structural integrity in nondiabetic adolescents with MetS, thus suggesting that even pre-clinical metabolic illness may give rise to brain complications.
As a result of the childhood obesity epidemic, in 2006, the prevalence of metabolic syndrome (MetS) was already 8.6% among all US children and adolescents.1 MetS in childhood predicts MetS and type 2 diabetes mellitus (T2DM) in adulthood.2 The MetS is composed of 5 obesity-associated components, namely elevations in fasting glucose levels or insulin resistance (IR) dependent on the definition used,3,4 lower high-density lipoprotein (HDL), hypertriglyceridemia, and hypertension in addition to abdominal obesity. MetS among middle-aged and older adults has been associated with cognitive dysfunction.5 However, to date, no brain data exist in youth.
We recently reported the presence of brain complications among obese adolescents with T2DM, including reduced hippocampal volumes, increased overall cerebrospinal fluid (CSF) volumes, and reduced white matter (WM) microstructural integrity.6,7 Studying nondiabetic youth with MetS presents a unique opportunity to evaluate whether brain structure and function are affected by metabolic dysregulation of relatively short duration and before the development of hyperglycemia or clinically manifest cardiovascular disease.
We aim to ascertain whether obesity and MetS, in the absence of T2DM, are associated with impairments in brain health. In addition to cognitive performance and measurements of hippocampal, dorsolateral prefrontal region (DLPFR), and overall CSF volumes, we also ascertained WM microstructural integrity by using sensitive diffusion tensor imaging methods.
Methods
MetS Classification
There is currently no general consensus to define pediatric MetS. The prevalence of impaired fasting glucose levels is very low in nondiabetic youth, and, therefore, measures of IR may offer higher sensitivity to detect metabolic abnormalities in this age group.8 The quantitative insulin sensitivity check index (QUICKI)9 has been validated as a measure of IR in nondiabetic children and adolescents.10 We chose a QUICKI value ≤0.3509 to indicate IR. We used the ATP III diagnostic criteria for abdominal obesity and hypertension in children,11 as well as the NHANES adolescent triglycerides cutoff.3 However, for HDL we used the more stringent adult criteria.12 In sum, the specific MetS component criteria we used were (1) abdominal obesity, waist circumference values ≥90th percentile for age and gender13; the adult cutoff value (waist circumference >88 cm [females] and >102 cm [males]) was used if it was lower than the children’s cutoff value; (2) reduced HDL, serum HDL levels <50 mg/dL (females) and <40 mg/dL (males); (3) hypertriglyceridemia, serum triglyceride levels >110 mg/dL; (4) hypertension, for those <18 years of age, blood pressure (BP) ≥90th percentile for age, gender, and height11; for those of ≥18 years of age, we used adult criteria, BP ≥130 mm Hg, diastolic BP ≥85 mm Hg; or use of antihypertensive medication; and (5) IR, a QUICKI value ≤0.350. An individual has MetS when he/she meets criteria for at least 3/5 of the MetS components.
Study Participants
A total of 129 nondiabetic adolescents (14–20 years of age) were screened for participation in a study to examine the brain consequences of MetS. This study was approved by the NYU School of Medicine institutional review board. All of the participants (and if <18 years of age, one of their parents) signed informed consent. Exclusion criteria were a diagnosis of T2DM or other significant medical conditions (other than IR, polycystic ovary disease, dyslipidemia, and hypertension), Tanner stage <4, use of psychoactive medications, a diagnosis of depression, a history of significant learning disability, or pregnancy. Of the 129 adolescents screened, 18 were excluded (9 did not meet entry criteria, 3 had clinical MRI abnormalities, and 6 did not complete the evaluation), resulting in 111 adolescents (49 with and 62 without MetS) included. One adolescent in the MetS group had a fasting glucose of 110 mg/dL (with a hemoglobin A1c level of 5.8%); all others had fasting glucose levels <100 mg/dL. Given our fasting glucose levels and normal hemoglobin A1c levels, it is highly unlikely we included any adolescents with undiagnosed T2DM.
Cognitive Evaluations
Cognitive testing was conducted blind to group membership in a standardized fashion ∼1 hour from the last meal over two 1.5-hour sessions. We used standard tests, described in detail elsewhere.14 For overall intellectual functioning we used the Wechsler Abbreviated Scale of Intelligence (WASI), and for academic achievement we used the Wide Range Achievement Test (WRAT). Memory skills were assessed with the Wide Range Assessment of Memory and Learning (WRAML), and executive function was assessed with the Wisconsin Card Sorting Test, Tower of London Test, Controlled Oral Word Association Test, and Trails B Test. Attention was measured with the Digit Vigilance Test (DVT), WRAML Attention-Concentration Index, and Trails A Test, and psychomotor efficiency was tested with the Digit Symbol Substitution Test. The Wechsler Abbreviated Scale of Intelligence, WRAT, and WRAML are adjusted for age; all others test scores are raw scores.
Obstructive sleep apnea is associated with obesity and can affect the brain15 and cognition.16 Sleep apnea was assessed with a 20-item questionnaire.17 A diagnosis of depression was exclusionary, but, to adjust for potential subclinical depressive symptoms on cognition, we administered the Beck Depression Inventory (BDI).18
MR Image Acquisition
Standardized MR scans were acquired by using identical parameters on the same 1.5 T Siemens Avanto System over 45 minutes. Please refer to Yau et al6 for details on the MR sequences.
Brain Volumetric Assessment
All brain volumes were determined blind to participants’ identity and diagnosis. We measured the intracranial vault (ICV) on the magnetization-prepared rapid acquisition gradient echo (MPRAGE) image by following the dural and tentorial margins. Overall, CSF volume was determined by using an intensity threshold to identify CSF voxels within the ICV. The volumes of right and left hippocampus were measured and then averaged by using a highly reliable19 and postmortem-validated method.20 The DLPFR region was outlined also by using a reliable method.21 To account for intersubject variability in brain size, measured brain volumes were adjusted (residualized) to the ICV volume by using linear regression.
Diffusion Tensor Imaging–based WM Microstructural Assessment
We used fractional anisotropy (FA) to assess WM microstructural integrity. To prepare the FA maps for voxelwise comparisons in Talaraich space, we used Automatic Registration Toolbox software,22 which is highly rated for image registration quality and accuracy.23 First, the skull-stripped structural native MPRAGE image was normalized to the standard Montreal Neurological Institute brain template by using a three-dimensional nonlinear warping algorithm. Second, a rigid-body linear transformation optimized the registration between T2 and MPRAGE by iteratively correcting for subject motion. Third, with a nonlinear two-dimensional warping algorithm, the non–diffusion-weighted b0 image was iteratively warped to correct for spatial distortions inherent in echo planar acquisitions by using the skull-stripped T2 image as a guide. Finally, to reduce interpolation errors, we combined transformation parameters from steps 1 to 3 and applied them to spatially correct and normalize the native FA maps to Talaraich space. Please refer to Yau et al24 for a more detailed description of these procedures.
Statistical Analyses
Before data analysis, we evaluated normality of continuous variables by using the Kolmogorov-Smirnov (for n ≥ 50) or Shapiro-Wilk (for n < 50) test. Normally distributed variables were evaluated by using 2-tailed independent samples t test (effect size expressed by Cohen d). For those that were nonnormally distributed, the Mann-Whitney U test (effect size expressed by r) was used. C-reactive protein (CRP) levels >10 mg/dL may indicate acute inflammation and were excluded casewise from analyses involving CRP. We used a WM mask created from the average of the MPRAGE images of all participants in Talaraich space to restrict group FA comparisons to WM. Two-tailed voxelwise analysis of covariance (VANCOVA) analysis examined the group differences in WM FA, with age as a covariate. Linear regression models were used to assess the differences in cognitive performance among adolescents who had 0, 1, 2, 3, 4+ (4 or 5) MetS components. Furthermore, stepwise regression analyses were used to understand whether any one or group of MetS components predicted the brain variables that were statistically different between MetS and non-MetS adolescents, after controlling for age and gender. For these analyses, we used the mean arterial BP (1/3 × systolic BP + 2/3 × diastolic BP) as a continuous measure of BP rather than the dichotomous classification used for MetS assignment. Extreme scores, >3 SDs from the respective group means, were excluded.
Descriptive statistics are presented as counts and percentages for categorical variables and as means and SDs (M ± SD) for continuous variables. For results of the regression analyses, the unstandardized β-values (β), proportion of variance explained by the independent variable (r2), and F-ratio are presented in parentheses in the text; the δ (∆) represents changes in the statistical values for the current step after accounting for covariates in the previous steps.
Results
Demographic and Endocrine Data
Groups did not differ significantly on age, socioeconomic status, school grade, gender, or ethnicity (Table 1). As expected, adolescents with MetS had significantly larger waist circumference and BMI, higher degree of IR, worse lipid profile, and poorer BP control (only 1 participant was receiving an antihypertensive medication). Adolescents with MetS also had significant elevations in plasma acute-phase reactant markers of inflammation (CRP and fibrinogen). The groups did not differ significantly on self-reported ratings of obstructive sleep apnea, or subclinical scores of depressive symptoms.
TABLE 1.
Demographic and Endocrine Data
| Measures | MetS (n = 49), Mean ± SD | Non-MetS (n = 62), Mean ± SD | Effect Size | P |
|---|---|---|---|---|
| Age | 17.77 ± 1.42 | 17.48 ± 1.65 | 0.19 | .33 |
| (14.29–20.50) | (14.28–20.83) | |||
| Ages 14/15–19/20 | 1 / 44 / 4 | 4 / 53 / 5 | ||
| Gendera | 31 F/18 M | 34 F/28 M | .37 | |
| Socioeconomic statusb | 2.07 ± 1.03 | 2.40 ± 1.38 | 0.09 | .36 |
| School grade | 11.79 ± 1.76 | 11.81 ± 1.91 | 0.01 | .95 |
| Ethnicity, %a | .93 | |||
| White | 20 | 23 | ||
| Hispanic | 41 | 39 | ||
| African American | 29 | 25 | ||
| Asian | 10 | 13 | ||
| Meets IR criterion, %a | 100 | 37 | <.001 | |
| Meets waist criterion, %a | 96 | 27 | <.001 | |
| Meets HDL criterion, %a | 77 | 26 | <.001 | |
| Meets triglyceride criterion, %a | 42 | 6 | <.001 | |
| Meets hypertension criterion, %a | 20 | 6 | .02 | |
| BMIb | 38.43 ± 7.17 | 27.09 ± 9.59 | 0.64 | <.001 |
| Waist measurement, cmb | 115.97 ± 17.70 | 88.52 ± 21.28 | 0.64 | <.001 |
| QUICKI scorec | 0.31 ± 0.02 | 0.36 ± 0.04 | 1.65 | <.001 |
| Fasting glucose (mg/dL)b | 78.96 ± 8.85 | 75.53 ± 7.38 | 0.20 | .04 |
| Fasting insulin (μIU/mL)b | 23.91 ± 12.73 | 10.22 ± 9.05 | 0.67 | <.001 |
| HbA1C (%)b | 5.44 ± 0.33 | 5.25 ± 0.32 | 0.28 | .003 |
| HDL (mg/dL)c | 41.25 ± 6.47 | 51.37 ± 11.47 | 1.05 | <.001 |
| Triglycerides (mg/dL)b | 105.29 ± 42.08 | 69.76 ± 25.40 | 0.44 | <.001 |
| Systolic BP (mm Hg)b | 115.86 ± 12.72 | 104.94 ± 10.70 | 0.43 | <.001 |
| Diastolic BP (mm Hg)b | 71.51 ± 10.13 | 63.42 ± 7.09 | 0.42 | <.001 |
| CRP (mg/L)b | 3.11 ± 1.91 | 1.72 ± 2.59 | 0.46 | <.001 |
| Fibrinogen (mg/dL)b | 379.64 ± 101.79 | 298.77 ± 61.55 | 0.45 | <.001 |
| BDI scoreb | 10.66 ± 8.97 | 7.98 ± 6.82 | 0.15 | .14 |
| Self-rating of sleep apneab | 0.23 ± 0.16 | 0.18 ± 0.13 | 0.12 | .22 |
Normally distributed continuous variables were evaluated with the t test (effect size Cohen d) unless indicated otherwise. BDI, Beck Depression Inventory; F, female; HbA1c, hemoglobin A1c; M, male.
The χ2 test was used for categorical variables.
Mann-Whitney U test was used (effect size r: 0.1, small; 0.3, medium; 0.5, large). Effect sizes are expressed as absolute values.
Adjusted for unequal variances.
Cognitive Performance Results
Adolescents with MetS had lower academic achievement (spelling and arithmetic) and tended to have a lower IQ (Table 2). They also scored lower on measures of attention and mental flexibility (completion time on the Trails B Test), but no other test of executive function was affected. The groups did not differ on memory performance or psychomotor efficiency.
TABLE 2.
Cognitive Data
| Measures | MetS (n = 49), Mean ± SD | Non-MetS (n = 62), Mean ± SD | Effect Size | P |
|---|---|---|---|---|
| Intellectual functioning and academic achievement | ||||
| Estimated full-scale IQa | 102.00 ± 11.63 | 105.95 ± 12.35 | 0.17 | .09 |
| WRAT reading standard scorea | 106.44 ± 11.57 | 107.64 ± 10.74 | 0.02 | .83 |
| WRAT spelling standard scorea | 101.22 ± 13.04 | 105.39 ± 10.94 | 0.21 | .04 |
| WRAT arithmetic standard score | 93.02 ± 13.25 | 102.09 ± 13.04 | 0.69 | .001 |
| Memory function | ||||
| WRAML general memory index | 103.05 ± 14.14 | 106.37 ± 13.59 | 0.24 | .24 |
| WRAML verbal memory indexa | 105.60 ± 13.20 | 104.76 ± 12.45 | 0.001 | .99 |
| WRAML visual memory indexa | 99.05 ± 13.13 | 101.35 ± 13.03 | 0.06 | .55 |
| WRAML working memory index | 101.02 ± 16.19 | 105.00 ± 15.76 | 0.25 | .22 |
| Executive function | ||||
| WCST – perseverative errorsa | 10.57 ± 8.17 | 9.81 ± 6.21 | 0.05 | .63 |
| TOL – excess movesa | 15.66 ± 14.78 | 12.62 ± 8.92 | 0.02 | .87 |
| Stroop interference score | −1.24 ± 8.92 | −1.57 ± 6.24 | 0.04 | .82 |
| COWAT total scorea | 37.20 ± 12.03 | 37.67 ± 12.58 | 0.03 | .79 |
| Trails B time (s)a | 69.39 ± 31.41 | 59.18 ± 30.80 | 0.2 | .04 |
| Attention and psychomotor efficiency | ||||
| DVT total timea | 403.91 ± 118.96 | 354.96 ± 83.27 | 0.23 | .02 |
| WRAML attention-concentration index | 102.40 ± 14.48 | 108.13 ± 16.06 | 0.37 | .07 |
| Trails A time (s)a | 28.68 ± 8.71 | 25.84 ± 8.02 | 0.19 | .06 |
| DSST total score | 60.37 ± 10.54 | 63.61 ± 11.28 | 0.3 | .14 |
Normally distributed continuous variables were evaluated with the t test (effect size Cohen d), unless indicated otherwise. COWAT, Controlled Oral Word Association Test; DVT, Digit Vigilance Test; DSST, Digit Symbol Substitution Test; TOL, Tower of London Test; WCST, Wisconsin Card Sorting Test; WRAML, Wide Range Assessment of Memory and Learning; WRAT, Wide Range Achievement Test.
Mann-Whitney U test was used (effect size r: 0.1, small; 0.3, medium; 0.5, large). Effect sizes are expressed as absolute values.
Although the groups did not differ in self-ratings of obstructive sleep apnea or depressive symptoms, to err on the side of caution, we confirmed that the significant cognitive differences were largely unchanged after controlling for those ratings.
Imaging Results
Of the 111 participants included, 18 did not have MRI data (most of them could not be accommodated by the scanner because of their large body size), which resulted in 93 adolescents with MR scans (34 MetS and 59 non-MetS adolescents). We found no significant differences in ICV volume (MetS, 1178.65 ± 102.76 mL; non-MetS, 1202.74 ± 116.17 mL, t[91] = −1.00, P = .32, d = 0.22). Adolescents with MetS had significantly smaller ICV-adjusted hippocampal volumes (MetS, 2.68 ± 0.31 versus non-MetS, 2.91 ± 0.35 mL, t[90] = −2.93, P < .01, d = 0.63 [medium to large effect size]) and larger ICV-adjusted overall CSF volume (MetS, 42.08 ± 20.51 versus non-MetS, 31.35 ± 13.75 mL, t[91] = 3.08, P < .01, d = 0.73 [medium to large effect size]). The groups did not differ in the ICV-adjusted DLPFR volume (MetS, 235.62 ± 29.18 versus non-MetS, 239.61 ± 35.00 mL, t[85] = 0.04, P = .97, d = 0.01).
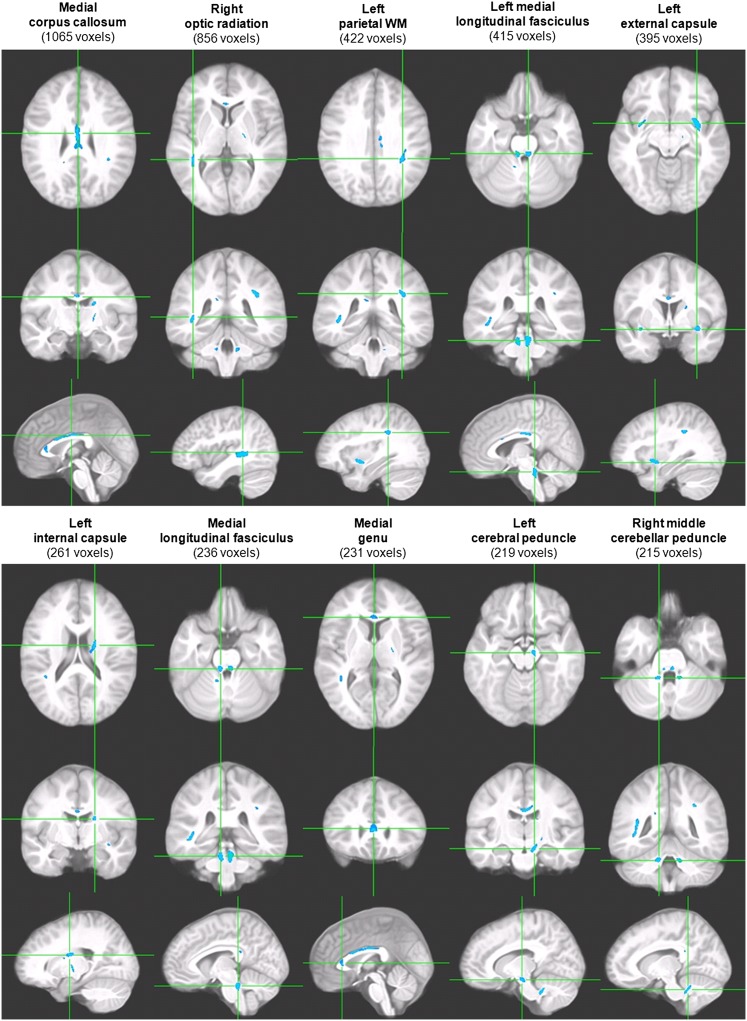
No participant had clinically relevant WM hyperintensities. VANCOVA analyses assessing WM microstructural integrity and utilizing conservative statistics with a cluster size of 100 contiguous voxels and a false discovery rate of 1%25 identified a total of 14 clusters (overall, 4.90 mL in volume), all showing reduction in FA, an indication of diminished fiber organization, among adolescents with MetS (P < .001). The significant clusters were located in major fiber tracts such as the corpus callosum, optic radiations, and medial longitudinal fasciculi. See Fig 1 where we present the 10 largest clusters. Given that 20% of participants with and 6% without MetS were hypertensive and that hypertension is associated with WM disease,26 we confirmed that the group differences remained when also controlling for mean arterial BP (data not shown).
FIGURE 1.
Representative clusters of lower WM FA value among adolescents with MetS. Each column displays 1 cluster of FA reduction in axial, coronal, and sagittal orientations with the axes going through the cluster centroid. Cluster size (minimum 100 voxels, equivalent to 0.1 mL in volume) is shown in parentheses.
Our non-MetS (control) group also had varying degrees of metabolic dysregulation (see Table 1); thus, we also contrasted our adolescents with MetS (n = 49) with those without any positive MetS components or “completely healthy” (n = 21); this more restricted control group also had similar demographic characteristics to the MetS group. We found the hippocampal volume reductions and increased CSF volumes remained significant and that the cognitive group differences were more dramatic, with 10 of the 17 (up from 7/17) cognitive measures now showing at least a statistical trend, all with larger effect sizes (data not shown). In addition, the VANCOVA analysis (P < .005, also at false discovery rate = 1%), revealed a total of 16 clusters (overall, 4.47 mL in volume) all demonstrating FA reductions in the adolescents with MetS.
Impact of Higher MetS Burden on the Cognitive and Brain Findings
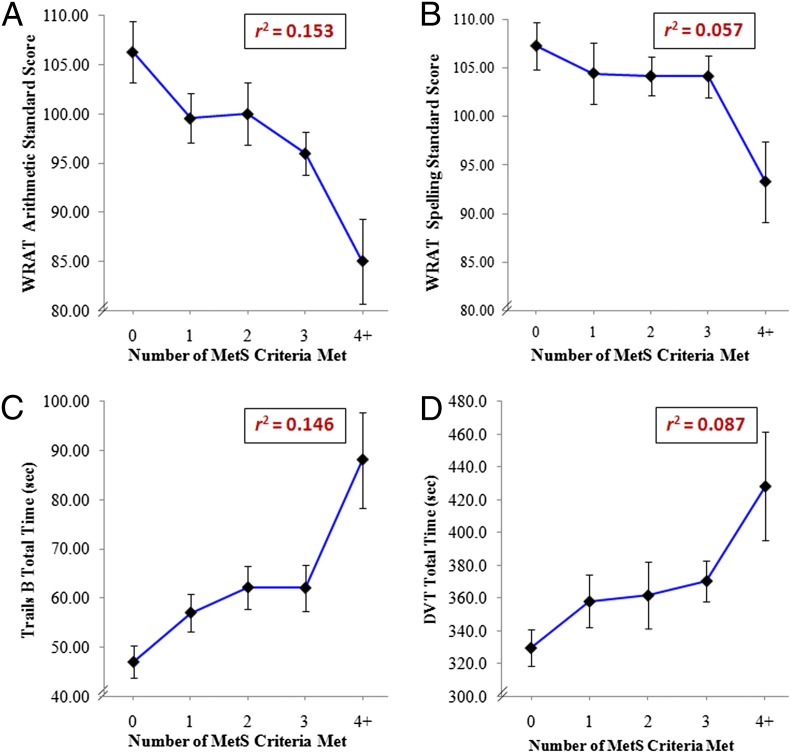
Only 2 adolescents met criteria for all 5 MetS components; thus, we combined those with 4 and 5 components. As seen on Fig 2 A–D, linear regression analyses revealed that, for an increasing number of MetS component criteria met, adolescents had progressive reductions in performance for WRAT Arithmetic (β = −4.11, r2 = 0.153, F[1,96] = 17.31, P < .001) and Spelling Standard Scores (β = −2.18, r2 = 0.057, F[1,97] = 5.82, P = .02), Trails B total time (β = 7.52, r2 = 0.146, F[1,98] = 16.80, P < .001), and DVT total time (β = 18.50, r2=0.087, F[1100] = 9.58, P < .01).
FIGURE 2.
Lower cognitive performance with increasing number of MetS components present. A, WRAT arithmetic standard score. B, WRAT spelling standard score. C, Trails B total time. D, DVT total time for individuals who met 0 criterion (n = 21), 1 criterion (n = 18), 2 criteria (n = 23), 3 criteria (n = 36), or 4+ criteria (n = 13). Data presented are mean ± SEM.
The same pattern existed for smaller ICV-adjusted hippocampal volumes (β = −0.07, r2 = 0.081, F[1,90] = 7.91, P = .01) and increased overall CSF volume (β = 4.38, r2 = 0.123, F[1,90] = 12.58, P = .001) (no figure shown). Inflammation as indicated by fibrinogen (β = 33.23, r2 = 0.261, F[1,78] = 27.51, P < .001) and CRP levels (β = 0.81, r2 = 0.192, F[1,90] = 21.32, P < .001) also increased with increasing number of MetS components present (no figure shown).
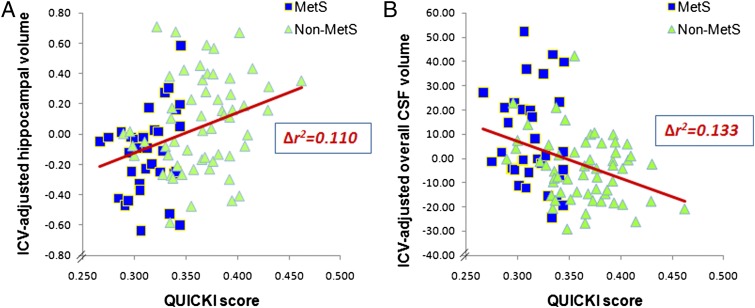
In stepwise regression analyses to ascertain which of the 5 MetS components best predicted the brain volumes, we found that after controlling for age and gender (βage = −0.02, βgender = 0.006, ∆r2 = 0.005, ∆F[2,88] = 0.22, ∆P = .80), IR as estimated by the QUICKI score was the only significant MetS component associated with ICV-adjusted hippocampal volumes (β = 2.60, ∆r2 = 0.110, ∆F[1,87] = 10.84, ∆P = .001; see Fig 3A). Similarly, after adjusting for age and gender (βage = 1.96, βgender = −2.98, ∆r2 = 0.043, ∆F[2,89] = 2.01, ∆P = .14), IR was the only significant MetS component associated with ICV-adjusted CSF volume (β = −157.96, ∆r2 = 0.133, ∆F[1,88] = 14.18, ∆P < .001; see Fig 3B).
FIGURE 3.
Lower QUICKI scores (more IR) were associated with smaller ICV-adjusted hippocampal volumes (n = 91) (A) and larger ICV-adjusted overall CSF volumes (n = 92) (B).
To ensure that the associations above (Fig 3 A and B) between IR and brain volumes were independent of overall obesity, as reflected by BMI (or waist circumference), we conducted hierarchical regression analyses confirming that the QUICKI score, after accounting for age, gender, and BMI (or waist circumference), still predicted a significant proportion of the variance for both hippocampal volume (β = 2.70, ∆r2 = 0.073, ∆F[1,87] = 7.29, ∆P = .01) and overall CSF volume (β = −157.91, ∆r2 = 0.084, ∆F[1,88] = 8.92, ∆P < .01) (no figure shown).
Discussion
To the best of our knowledge, this is the first report demonstrating brain abnormalities among obese nondiabetic adolescents with MetS. In addition to lower scores on cognitive measures, we also demonstrated that adolescents with MetS have reduced hippocampal volumes, increased overall CSF volume, and compromised WM microstructural integrity. These findings are conservative, because many of our control adolescents met criteria for some of the MetS components, just not 3/5 (see Table 1); when we used a subset of controls not meeting criteria for any MetS component, the group differences became more pronounced.
Overall, nondiabetic adolescents with MetS, although still performing in the normal range, scored lower across all the cognitive domains assessed than those without MetS; they had significantly lower academic achievement (ie, spelling and arithmetic), attention, and mental flexibility and trended to have lower estimated intellectual functioning. This suggests that these obesity-associated medical abnormalities, short of T2DM, may have a dampening effect on academic performance, which may impact professional potential and perhaps lifelong learning. We have recently reported that obese adolescents with T2DM also have cognitive dysfunction,6 but more prominent than those reported here and also included memory difficulties.
Hippocampal volume reductions have been described in adults27 and obese adolescents with T2DM.7 The current finding of smaller hippocampal volumes among nondiabetic adolescents with MetS was unexpected. These data suggest that among obese adolescents the hippocampus may already be affected in the prediabetic stages of metabolic disease. In addition, we observed an increase in overall CSF volume among adolescents with MetS. Given that our study groups did not differ in ICV volume, whose volume is likely determined by the early growth of the brain, this suggests that the increased overall CSF volume among obese adolescents with MetS is likely caused by brain parenchyma volume loss rather than differences in early brain development.
We found lower WM microstructural integrity among adolescents with MetS than what we had found among obese adolescents with T2DM.6 However, in the current study, we had nearly 4 times the sample size, and the more extensive current findings are likely a function of more robust statistics and not disease. Consistent with findings in adults with MetS,28 we found compromised WM microstructural integrity in major fiber tracts involved in interhemispheric or corticosubcortical communications. In the future, prospective studies should clarify whether these WM microstructural changes represent a delay in the WM maturation, which is still ongoing during adolescence,29 or actual damage.
We found that IR was the most significant predictor of brain volume changes, and the only one that was a significant predictor in a multivariate stepwise analysis. IR is thought to be central to MetS,30 and this was confirmed by the fact that it remained significantly associated to smaller hippocampal volumes and increased CSF volumes even after accounting for BMI (or waist circumference). Nevertheless, the other MetS components also contributed to the brain abnormalities, in that the more MetS components reached threshold, the smaller the hippocampal volumes and greater overall CSF volumes.
We found worsening cognitive performance with increasing number of MetS components present, with variance explained ranging from 5.7% (small to medium effect size) to 15.3% (medium to large effect size), which is consistent with adult data.31 However, unlike the associations of brain volume changes with IR, cognition was not significantly associated with IR. These findings suggest that, to impair cognitive performance, obesity or hypertension may be sufficient, but that to affect structural brain changes such as hippocampal volume reductions or increased overall CSF volume, further metabolic dysregulation, such as marked fasting hyperinsulinemia, may be required. These somewhat speculative conclusions will need to be confirmed in a larger prospective longitudinal study.
The current study has significant strengths. The groups were moderate in size and had distributions that minimized socioeconomic and education bias. In addition, we used reliable and validated brain volume measurements as well as sensitive WM microstructural assessment methods with rigorous thresholds for statistical significance. Moreover, our results are likely conservative, because the control group was not totally free of metabolic dysregulation. These cognitive and brain findings in adolescents with MetS are in line with our previous reports on adolescents with T2DM, just showing smaller effect sizes. Taken together, these data suggest that there may be dose effects in brain complications as we move along the spectrum linking obesity to T2DM.
Study participants were recruited from the community and evaluated at the medical center. Although not a clinical population, our participants are not representative of the general population. In addition, given that we used IR, rather than hyperglycemia, as one of our MetS components, the results presented here may not be directly comparable to those of other studies. Although other studies of adolescents have used fasting glucose level ≥110 mg/dL,32 all of those studies included adolescents with diabetes. Had we used a fasting glucose of ≥110 mg/dL, only one of our obese adolescents would have met that criterion. Nevertheless, had we had sufficient participants with impaired fasting glucose level, the brain abnormalities would have likely been worse, because that group is closer to T2DM than our group with IR but normal glucose levels. Insulin sensitivity has been shown to worsen during pubertal progression,33 and, thus, the use of the QUICKI score cutoff of ≤0.350 to indicate IR could potentially bias against younger children being included in the MetS group. However, in these data we did not have such potential bias, because QUICKI scores rose, nonsignificantly, with age.
Another possible limitation of the current study is that we did not control for multiple comparisons when testing for group differences in cognitive performance. However, given that all the cognitive measures were lower in the MetS group, with 7 of 17 cognitive measures showing at least a statistical trend, we felt justified for this first report of brain and cognitive impairments in adolescents with MetS in not controlling for multiple comparisons.
Conclusions
Although obesity may not be enough to stir clinicians or even parents into action, these results among youth with MetS strongly argue for an early and comprehensive intervention. We propose that brain function be introduced among the parameters that need to be evaluated when considering early treatment of childhood obesity. Future work should also ascertain whether the reductions in cognitive performance and structural brain abnormalities are reversible with significant weight loss and reversal of the obesity-associated MetS components.
Glossary
- BP
blood pressure
- CRP
C-reactive protein
- CSF
cerebrospinal fluid
- DLPFR
dorsolateral prefrontal region
- DVT
Digit Vigilance Test
- FA
fractional anisotropy
- HDL
high-density lipoprotein
- ICV
intracranial vault
- IR
insulin resistance
- MetS
metabolic syndrome
- MPRAGE
magnetization-prepared rapid acquisition gradient echo
- QUICKI
quantitative insulin sensitivity check index
- T2DM
type 2 diabetes mellitus
- VANCOVA
voxelwise analysis of covariance
- WM
white matter
- WRAML
Wide Range Assessment of Memory and Learning
- WRAT
Wide Range Achievement Test
Footnotes
Each author made substantial contributions to this article. Dr Convit designed, performed, and supervised the study; Drs Yau and Convit, W. H. Tsui, M. G. Castro, and A. Tagani acquired and analyzed the data; Drs Yau and Convit wrote the article; all authors have seen and approved the final version of the manuscript.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Funded by the National Institutes of Health DK 083537 and, in part, by grant 1UL1RR029893 from the National Center for Research Resources. Funded by the National Institutes of Health (NIH).
References
- 1.Johnson WD, Kroon JJ, Greenway FL, Bouchard C, Ryan D, Katzmarzyk PT. Prevalence of risk factors for metabolic syndrome in adolescents: National Health and Nutrition Examination Survey (NHANES), 2001-2006. Arch Pediatr Adolesc Med. 2009;163(4):371–377 [DOI] [PubMed] [Google Scholar]
- 2.Morrison JA, Friedman LA, Wang P, Glueck CJ. Metabolic syndrome in childhood predicts adult metabolic syndrome and type 2 diabetes mellitus 25 to 30 years later. J Pediatr. 2008;152(2):201–206 [DOI] [PubMed] [Google Scholar]
- 3.Cook S, Auinger P, Li C, Ford ES. Metabolic syndrome rates in United States adolescents, from the National Health and Nutrition Examination Survey, 1999-2002. J Pediatr. 2008;152(2):165–170 [DOI] [PubMed] [Google Scholar]
- 4.Cruz ML, Weigensberg MJ, Huang TT, Ball G, Shaibi GQ, Goran MI. The metabolic syndrome in overweight Hispanic youth and the role of insulin sensitivity. J Clin Endocrinol Metab. 2004;89(1):108–113 [DOI] [PubMed] [Google Scholar]
- 5.Hassenstab JJ, Sweat V, Bruehl H, Convit A. Metabolic syndrome is associated with learning and recall impairment in middle age. Dement Geriatr Cogn Disord. 2010;29(4):356–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yau PL, Javier DC, Ryan CM, et al. Preliminary evidence for brain complications in obese adolescents with type 2 diabetes mellitus. Diabetologia. 2010;53(11):2298–2306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bruehl H, Sweat V, Tirsi A, Shah B, Convit A. Obese adolescents with type 2 diabetes mellitus have hippocampal and frontal lobe volume reductions. Neurosci Med. 2011;2(1):34–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharma S, Lustig RH, Fleming SE. Identifying metabolic syndrome in African American children using fasting HOMA-IR in place of glucose. Prev Chronic Dis. 2011;8(3):A64. [PMC free article] [PubMed] [Google Scholar]
- 9.Katz A, Nambi SS, Mather K, et al. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85(7):2402–2410 [DOI] [PubMed] [Google Scholar]
- 10.Gungor N, Saad R, Janosky J, Arslanian S. Validation of surrogate estimates of insulin sensitivity and insulin secretion in children and adolescents. J Pediatr. 2004;144(1):47–55 [DOI] [PubMed] [Google Scholar]
- 11.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents . The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114(2 suppl 4th report):555–576 [PubMed] [Google Scholar]
- 12.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) . Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation and treatment of high cholesterol in adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–3421 [PubMed] [Google Scholar]
- 13.Fernández JR, Redden DT, Pietrobelli A, Allison DB. Waist circumference percentiles in nationally representative samples of African-American, European-American, and Mexican-American children and adolescents. J Pediatr. 2004;145(4):439–444 [DOI] [PubMed] [Google Scholar]
- 14.Lezak MD, Howieson DB, Loring DW. Neuropsychological Assessment. New York, NY: Oxford University Press; 2004 [Google Scholar]
- 15.Macey PM, Kumar R, Woo MA, Valladares EM, Yan-Go FL, Harper RM. Brain structural changes in obstructive sleep apnea. Sleep. 2008;31(7):967–977 [PMC free article] [PubMed] [Google Scholar]
- 16.Gozal D, Kheirandish-Gozal L. Neurocognitive and behavioral morbidity in children with sleep disorders. Curr Opin Pulm Med. 2007;13(6):505–509 [DOI] [PubMed] [Google Scholar]
- 17.Mindell JA, Owens JA. A Clinical Guide to Pediatric Sleep: Diagnosis and Management of Sleep Problems. Philadelphia, PA: Lippincott Williams & Wilkins; 2003 [Google Scholar]
- 18.Beck A, Steer RA, Brown GK. Manual for Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996 [Google Scholar]
- 19.Convit A, de Leon MJ, Tarshish C, et al. Hippocampal volume losses in minimally impaired elderly. Lancet. 1995;345(8944):266. [DOI] [PubMed] [Google Scholar]
- 20.Bobinski M, de Leon MJ, Wegiel J, et al. The histological validation of post mortem magnetic resonance imaging-determined hippocampal volume in Alzheimer’s disease. Neuroscience. 2000;95(3):721–725 [DOI] [PubMed] [Google Scholar]
- 21.Convit A, Wolf OT, de Leon MJ, et al. Volumetric analysis of the pre-frontal regions: findings in aging and schizophrenia. Psychiatry Res. 2001;107(2):61–73 [DOI] [PubMed] [Google Scholar]
- 22.Ardekani BA, Guckemus S, Bachman A, Hoptman MJ, Wojtaszek M, Nierenberg J. Quantitative comparison of algorithms for inter-subject registration of 3D volumetric brain MRI scans. J Neurosci Methods. 2005;142(1):67–76 [DOI] [PubMed] [Google Scholar]
- 23.Klein A, Andersson J, Ardekani BA, et al. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. Neuroimage. 2009;46(3):786–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yau PL, Javier D, Tsui W, et al. Emotional and neutral declarative memory impairments and associated white matter microstructural abnormalities in adults with type 2 diabetes. Psychiatry Res. 2009;174(3):223–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995;57(1):289–300 [Google Scholar]
- 26.Sierra C, Lopez-Soto A, Coca A. Connecting cerebral white matter lesions and hypertensive target organ damage. J Aging Res. 2011;2011:438978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gold SM, Dziobek I, Sweat V, et al. Hippocampal damage and memory impairments as possible early brain complications of type 2 diabetes. Diabetologia. 2007;50(4):711–719 [DOI] [PubMed] [Google Scholar]
- 28.Segura B, Jurado MA, Freixenet N, Falcón C, Junqué C, Arboix A. Microstructural white matter changes in metabolic syndrome: a diffusion tensor imaging study. Neurology. 2009;73(6):438–444 [DOI] [PubMed] [Google Scholar]
- 29.Barnea-Goraly N, Menon V, Eckert M, et al. White matter development during childhood and adolescence: a cross-sectional diffusion tensor imaging study. Cereb Cortex. 2005;15(12):1848–1854 [DOI] [PubMed] [Google Scholar]
- 30.Ferrannini E, Haffner SM, Mitchell BD, Stern MP. Hyperinsulinaemia: the key feature of a cardiovascular and metabolic syndrome. Diabetologia. 1991;34(6):416–422 [DOI] [PubMed] [Google Scholar]
- 31.Gatto NM, Henderson VW, St John JA, McCleary C, Hodis HN, Mack WJ. Metabolic syndrome and cognitive function in healthy middle-aged and older adults without diabetes. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2008;15(5):627–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McNeal C, Wilson DP. Metabolic syndrome and dyslipidemia in youth. J Clin Lipidol. 2008;2(3):147–155 [DOI] [PubMed] [Google Scholar]
- 33.Lindgren F, Dahlquist G, Efendić S, Persson B, Skottner A. Insulin sensitivity and glucose-induced insulin response changes during adolescence. Acta Paediatr Scand. 1990;79(4):431–436 [DOI] [PubMed] [Google Scholar]