Abstract
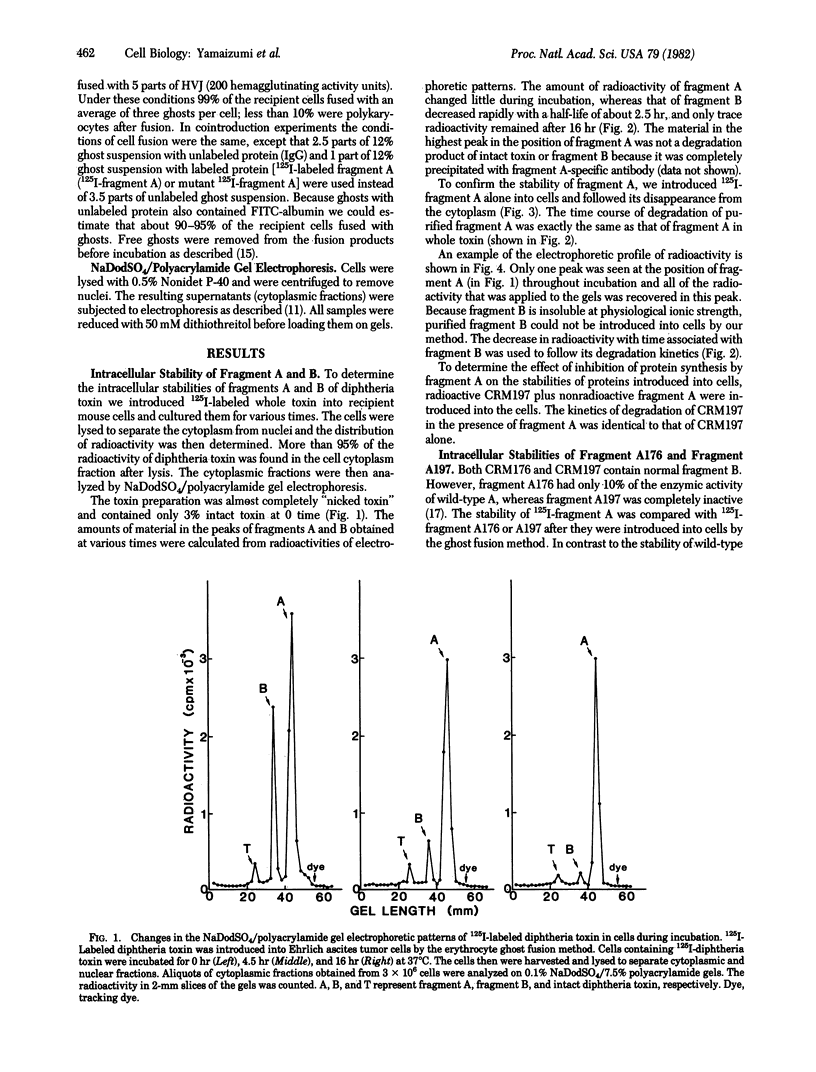
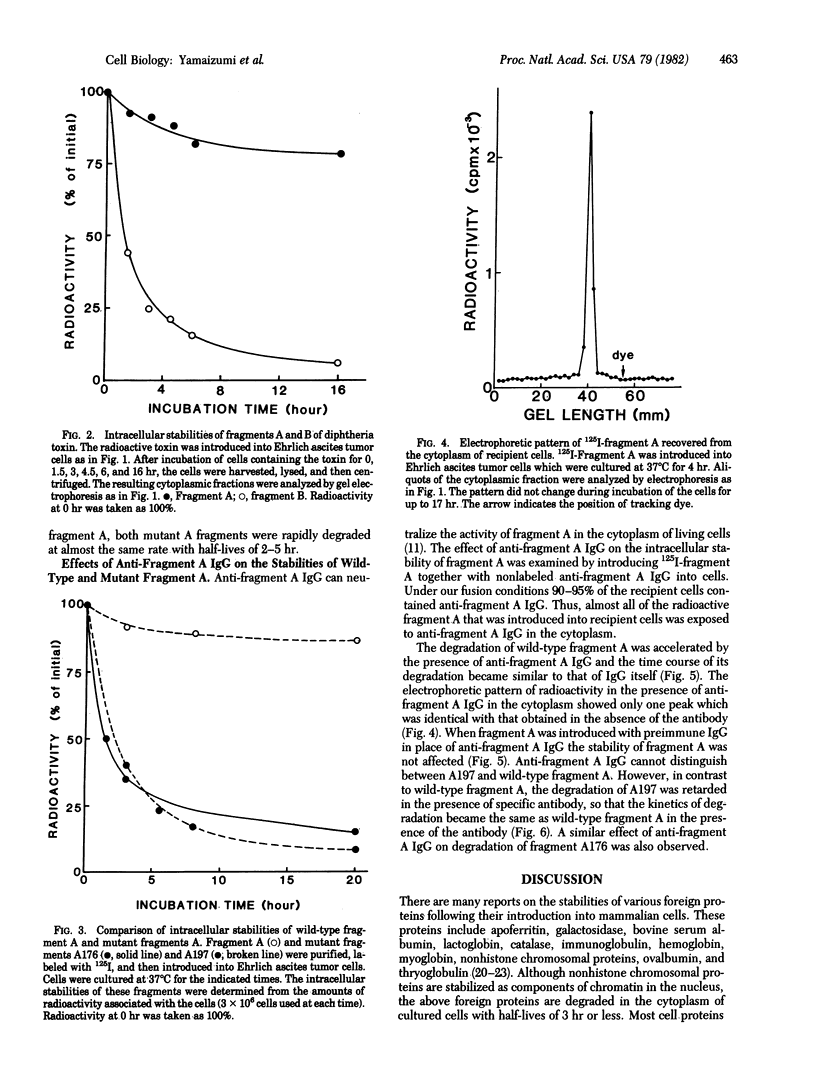
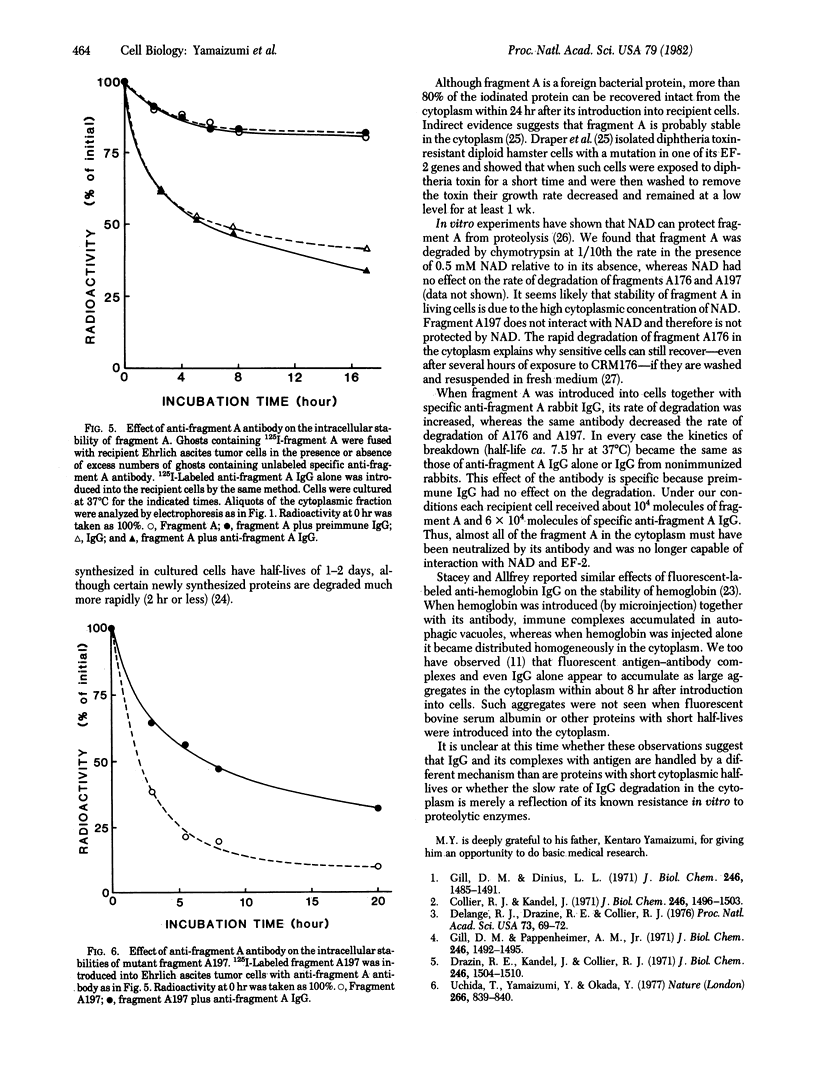
The erythrocyte ghost function method was used to introduce 125I-labeled diphtheria toxin, its fragments A and B, and two labeled crossreacting material (CRM), mutant proteins CRM176 and CRM197, into cultured mouse cells. Fragment A was relatively stable in mouse cytoplasm at 37 degrees C and at least 80% was recovered from cells after 24 hr of incubation. In contrast, wild-type fragment B and A fragments from CRM176 and CRM197 were unstable and were degraded with half-lives of about 2.5 hr under similar conditions. When a rabbit anti-fragment A IgG fraction was introduced with wild-type A, the rate of degradation of A was accelerated, whereas the rates of degradation of A176 and A197 were retarded by the same antibody. In every instance the degradation rate appeared to be that of the IgG fraction itself with a half-life of about 7.5 hr.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Collier R. J., Kandel J. Structure and activity of diphtheria toxin. I. Thiol-dependent dissociation of a fraction of toxin into enzymically active and inactive fragments. J Biol Chem. 1971 Mar 10;246(5):1496–1503. [PubMed] [Google Scholar]
- DeLange R. J., Drazin R. E., Collier R. J. Amino-acid sequence of fragment A, an enzymically active fragment from diphtheria toxin. Proc Natl Acad Sci U S A. 1976 Jan;73(1):69–72. doi: 10.1073/pnas.73.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper R. K., Chin D., Eurey-Owens D., Scheffler I. E., Simon M. I. Biochemical and genetic characterization of three hamster cell mutants resistant to diphtheria toxin. J Cell Biol. 1979 Oct;83(1):116–125. doi: 10.1083/jcb.83.1.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drazin R., Kandel J., Collier R. J. Structure and activity of diphtheria toxin. II. Attack by trypsin at a specific site within the intact toxin molecule. J Biol Chem. 1971 Mar 10;246(5):1504–1510. [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill D. M., Dinius L. L. Observations on the structure of diphtheria toxin. J Biol Chem. 1971 Mar 10;246(5):1485–1491. [PubMed] [Google Scholar]
- Gill D. M., Pappenheimer A. M., Jr Structure-activity relationships in diphtheria toxin. J Biol Chem. 1971 Mar 10;246(5):1492–1495. [PubMed] [Google Scholar]
- Gill D. M., Pappenheimer A. M., Jr, Uchida T. Diphtheria toxin, protein synthesis, and the cell. Fed Proc. 1973 Apr;32(4):1508–1515. [PubMed] [Google Scholar]
- Goldberg A. L., St John A. C. Intracellular protein degradation in mammalian and bacterial cells: Part 2. Annu Rev Biochem. 1976;45:747–803. doi: 10.1146/annurev.bi.45.070176.003531. [DOI] [PubMed] [Google Scholar]
- Kandel J., Collier R. J., Chung D. W. Interaction of fragment A from diphtheria toxin with nicotinamide adenine dinucleotide. J Biol Chem. 1974 Apr 10;249(7):2088–2097. [PubMed] [Google Scholar]
- Moynihan M. R., Pappenheimer A. M., Jr Kinetics of adenosinediphosphoribosylation of elongation factor 2 in cells exposed to diphtheria toxin. Infect Immun. 1981 May;32(2):575–582. doi: 10.1128/iai.32.2.575-582.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y., Murayama F. Requirement of calcium ions for the cell fusion reaction of animal cells by HVJ. Exp Cell Res. 1966 Nov-Dec;44(2):527–551. doi: 10.1016/0014-4827(66)90458-7. [DOI] [PubMed] [Google Scholar]
- Stacey D. W., Allfrey V. G. Evidence for the autophagy of microinjected proteins in HeLA cells. J Cell Biol. 1977 Dec;75(3):807–817. doi: 10.1083/jcb.75.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida T., Kim J., Yamaizumi M., Miyake Y., Okada Y. Reconstitution of lipid vesicles associated with HVJ (Sendai virus) sikes. Purification and some properties of vesicles containing nontoxic fragment A of diphtheria toxin. J Cell Biol. 1979 Jan;80(1):10–20. doi: 10.1083/jcb.80.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida T., Miyake Y., Yamaizumi M., Mekada E., Okada Y. Improved methods using HVJ(Sendai virus) for introducing substances into cells. Biochem Biophys Res Commun. 1979 Mar 30;87(2):371–379. doi: 10.1016/0006-291x(79)91806-0. [DOI] [PubMed] [Google Scholar]
- Uchida T., Pappenheimer A. M., Jr, Greany R. Diphtheria toxin and related proteins. I. Isolation and properties of mutant proteins serologically related to diphtheria toxin. J Biol Chem. 1973 Jun 10;248(11):3838–3844. [PubMed] [Google Scholar]
- Uchida T., Pappenheimer A. M., Jr, Harper A. A. Diphtheria toxin and related proteins. II. Kinetic studies on intoxication of HeLa cells by diphtheria toxin and related proteins. J Biol Chem. 1973 Jun 10;248(11):3845–3850. [PubMed] [Google Scholar]
- Uchida T., Pappenheimer A. M., Jr, Harper A. A. Reconstitution of diphtheria toxin from two nontoxic cross-reacting mutant proteins. Science. 1972 Feb 25;175(4024):901–903. doi: 10.1126/science.175.4024.901. [DOI] [PubMed] [Google Scholar]
- Uchida T., Yamaizumi M., Okada Y. Reassembled HVJ (Sendai virus) envelopes containing non-toxic mutant proteins of diphtheria toxin show toxicity to mouse L cell. Nature. 1977 Apr 28;266(5605):839–840. doi: 10.1038/266839a0. [DOI] [PubMed] [Google Scholar]
- Wasserman M., Kulka R. G., Loyter A. Degradation and localization of IgG injected into Friend erythroleukemic cells by fusion with erythrocyte ghosts. FEBS Lett. 1977 Nov 1;83(1):48–52. doi: 10.1016/0014-5793(77)80639-x. [DOI] [PubMed] [Google Scholar]
- Yamaizumi M., Mekada E., Uchida T., Okada Y. One molecule of diphtheria toxin fragment A introduced into a cell can kill the cell. Cell. 1978 Sep;15(1):245–250. doi: 10.1016/0092-8674(78)90099-5. [DOI] [PubMed] [Google Scholar]
- Yamaizumi M., Uchida T., Mekada E., Okada Y. Antibodies introduced into living cells by red cell ghosts are functionally stable in the cytoplasm of the cells. Cell. 1979 Dec;18(4):1009–1014. doi: 10.1016/0092-8674(79)90213-7. [DOI] [PubMed] [Google Scholar]
- Yamaizumi M., Uchida T., Okada Y., Furusawa M., Mitsui H. Rapid transfer of non-histone chromosomal proteins to the nucleus of living cells. Nature. 1978 Jun 29;273(5665):782–784. doi: 10.1038/273782a0. [DOI] [PubMed] [Google Scholar]
- Yamaizumi M., Uchida T., Okada Y., Furusawa M. Neutralization of diphtheria toxin in living cells by microinjection of antifragment A contained within resealed erythrocyte ghosts. Cell. 1978 Feb;13(2):227–232. doi: 10.1016/0092-8674(78)90191-5. [DOI] [PubMed] [Google Scholar]
- Yamaizumi M., Uchida T., Okada Y. Macromolecules can penetrate the host cell membrane during the early period of incubation with HVJ (Sendai virus). Virology. 1979 May;95(1):218–221. doi: 10.1016/0042-6822(79)90418-5. [DOI] [PubMed] [Google Scholar]
- Zavortink M., Thacher T., Rechsteiner M. Degradation of proteins microinjected into cultured mammalian cells. J Cell Physiol. 1979 Jul;100(1):175–185. doi: 10.1002/jcp.1041000118. [DOI] [PubMed] [Google Scholar]


