Abstract
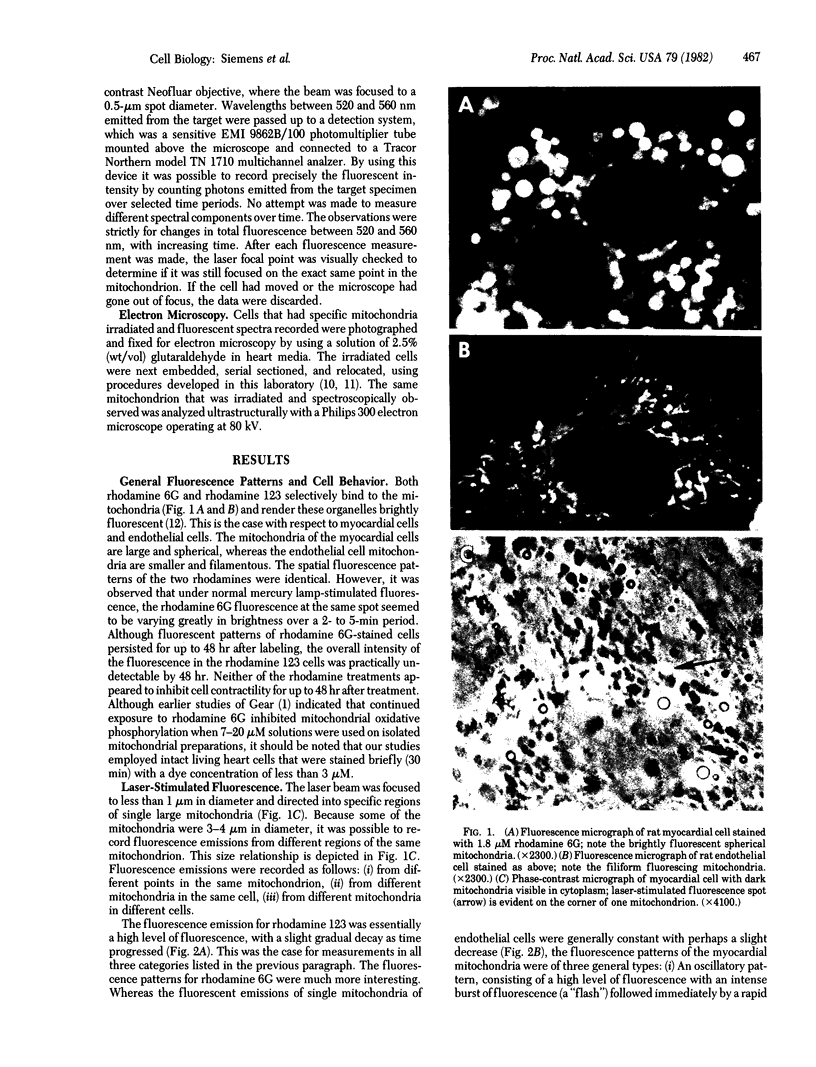
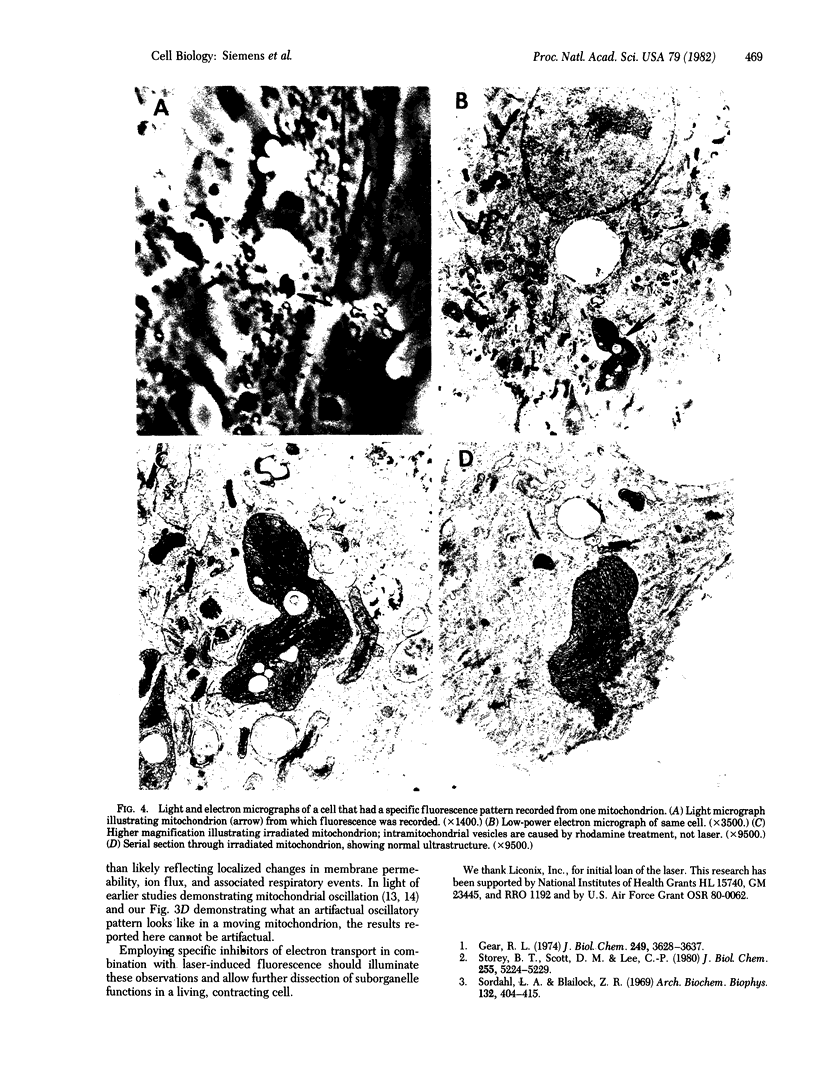
A focused laser beam of 442 nm was used to stimulate fluorescence in 0.5-micrometer spots in single mitochondria of myocardial and endothelial cells in culture. Cells were treated with rhodamine 6G or 123 in order to render the mitochondria fluorescent. Rhodamine 123-treated cells exhibited a gradual decrease in fluorescence over several minutes, whereas the rhodamine 6G-treated myocardial cells exhibited three distinct patterns of variable fluorescence intensity. These patterns were detected at different points within the same mitochondrion or in different mitochondria. Mitochondria from nonmyocardial endothelial cells did not exhibit any variable intensity patterns of fluorescence. Electron microscopy revealed no ultrastructural damage attributable to laser exposure of the mitochondria. The variable fluorescence patterns are hypothesized to be indicative of localized alterations in molecules or ions at the suborganelle level.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiuchi T., Tanabe H., Kurihara K., Kobatake Y. Fluorescence changes of rhodamine 6G associated with chemotactic responses in Tetrahymena pyriformis. Biochim Biophys Acta. 1980 Mar 20;628(3):355–364. doi: 10.1016/0304-4165(80)90385-2. [DOI] [PubMed] [Google Scholar]
- Berns M. W., Gamaleja N., Olson R., Duffy C., Rounds D. E. Argon laser micro-irradiation of mitochondria in rat myocardial cells in tissue culture. J Cell Physiol. 1970 Oct;76(2):207–213. doi: 10.1002/jcp.1040760211. [DOI] [PubMed] [Google Scholar]
- Gear A. R. Rhodamine 6G. A potent inhibitor of mitochondrial oxidative phosphorylation. J Biol Chem. 1974 Jun 10;249(11):3628–3637. [PubMed] [Google Scholar]
- Johnson L. V., Walsh M. L., Chen L. B. Localization of mitochondria in living cells with rhodamine 123. Proc Natl Acad Sci U S A. 1980 Feb;77(2):990–994. doi: 10.1073/pnas.77.2.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppel D. E., Axelrod D., Schlessinger J., Elson E. L., Webb W. W. Dynamics of fluorescence marker concentration as a probe of mobility. Biophys J. 1976 Nov;16(11):1315–1329. doi: 10.1016/S0006-3495(76)85776-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liaw L. H., Berns M. W. Electron microscope autoradiography on serial sections of preselected single living cells. J Ultrastruct Res. 1981 May;75(2):187–194. doi: 10.1016/s0022-5320(81)80134-7. [DOI] [PubMed] [Google Scholar]
- Mustafa M. G., Utsumi K., Packer L. Damped oscillatory control of mitochondrial respiration and volume. Biochem Biophys Res Commun. 1966 Aug 12;24(3):381–385. doi: 10.1016/0006-291x(66)90168-9. [DOI] [PubMed] [Google Scholar]
- Packer L., Utsumi R., Mustafa M. G. Oscillatory states of mitochondria. 1. Electron and energy transfer pathways. Arch Biochem Biophys. 1966 Nov;117(2):381–393. doi: 10.1016/0003-9861(66)90426-7. [DOI] [PubMed] [Google Scholar]
- Rattner J. B., Berns M. W. Light and electron microscopy of laser microirradiated chromosomes. J Cell Biol. 1974 Aug;62(2):526–533. doi: 10.1083/jcb.62.2.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sordahl L. A., Blailock Z. R., Kraft G. H., Schwartz A. The possible relationship between ultrastructure and biochemical state of heart mitochondria. Arch Biochem Biophys. 1969 Jul;132(2):404–415. doi: 10.1016/0003-9861(69)90382-8. [DOI] [PubMed] [Google Scholar]
- Sordahl L. A., Blailock Z. R., Liebelt A. G., Kraft G. H., Schwartz A. Some ultrastructural and biochemical characteristics of tumor mitochondria isolated in albumin-containing media. Cancer Res. 1969 Nov;29(11):2002–2009. [PubMed] [Google Scholar]
- Storey B. T., Scott D. M., Lee C. Energy-linked quinacrine fluorescence changes in submitochondrial particles from skeletal muscle mitochondria. Evidence for intramembrane H+ transfer as a primary reaction of energy coupling. J Biol Chem. 1980 Jun 10;255(11):5224–5229. [PubMed] [Google Scholar]
- Yaginuma N., Hirose S., Inada Y. Spectral change of rhodamine 6G caused by the energization of mitochondria, in relation to charge separation. J Biochem. 1973 Oct;74(4):811–815. doi: 10.1093/oxfordjournals.jbchem.a130307. [DOI] [PubMed] [Google Scholar]