Abstract
BACKGROUND
Flexible bronchoscopy with bronchoalveolar lavage (BAL) is performed widely for the diagnosis of pulmonary infections in patients with cancer, but there is no consensus regarding the technical parameters of the lavage procedure in this setting.
METHODS
The authors evaluated the mechanics (instilled and recovered volumes), diagnostic yield, and safety of a standardized BAL protocol in 284 patients with cancer who underwent bronchoscopy for the evaluation of new radiologic infiltrates.
RESULTS
Physician adherence to the BAL protocol was > 90%. The most common protocol deviations were reductions in the saline volume instilled because of actual or anticipated oxyhemoglobin desaturation during the procedure. The mean volume instilled was 121.5 ± 13.9 mL, the mean volume recovered was 68.7 ± 18.1 mL, and the mean ratio of volume instilled to that recovered was 56.7% ± 14.5%. The overall diagnostic yield of BAL was 33.8% and was higher in the nonhematologic malignancy group (42.3% vs 29.4%; P = .021). The diagnostic yield in neutropenic patients was significantly higher than in nonneutropenic patients (41.5% vs 24.6%; P = .019). No major complications were encountered.
CONCLUSIONS
In summary, the diagnostic performance of a standardized BAL protocol was comparable to that of nonprotocolized BAL reported in the literature with few complications. Adherence to a standardized BAL protocol may improve clinical and laboratory comparisons between studies, potentially facilitating research into the diagnosis and management of pneumonia in patients with cancer.
Keywords: pneumonia, bronchoalveolar lavage, immunocompromise, cancer, diagnosis
Pneumonia remains a major diagnostic and therapeutic challenge, with attributed mortality reaching 55% in immunocompromised patients who have cancer.1–4 Up to 30% of infections in these patients are classified as pneumonias, but they rarely have microbiologic confirmation.2 Moreover, the spectrum of the involved pathogens is broad and is constantly changing.1 Therefore, prompt diagnosis and preemptive antibiotic treatment is crucial for the clinical management of these patients.2
Bronchoalveolar lavage (BAL) is a widely performed diagnostic and research procedure, especially in patients with cancer, and remains the cornerstone of microbiologic confirmation in lower respiratory tract infections.2–4 However, there is no consensus regarding the optimal performance of BAL, because wide differences are reported in the mechanical performance of the procedure, diagnostic yield, and complications.2 In fact, diverging recommendations have been made from different societies.5,6 The diversity in BAL protocols performed, especially in clinical trials, limits their capacity for comparison, especially as applied to new diagnostic tests of the BAL effluent. To that end, we examined the mechanics, diagnostic yield, and safety of a standardized BAL protocol in our institution, which is a comprehensive cancer center.
MATERIALS AND METHODS
Patients
Patients at The University of Texas MD Anderson Cancer Center who underwent bronchoscopy with BAL over a 26-month period (from February 2007 to April 2009) for the evaluation of new radiologic infiltrates were enrolled prospectively in a study that was approved by the institutional review board to assess the performance of protocolized BAL after providing informed consent. Clinical, laboratory, and radiologic data were extracted from the electronic medical records. These included age, sex, cancer diagnosis, type and timing of hematologic stem cell transplantation (HSCT), active treatments (chemotherapies, immunosuppressive therapies, and antibiotic therapies), absolute neutrophil count (ANC), platelet count, patterns of radiologic infiltrates, BAL microbiologic and cytologic studies, serum galactomannan antigen detection test, pp65 cytomegalovirus (CMV) antigenemia test, and other pertinent serologic or antigen-detection tests.
The Standardized BAL Protocol
Patients with oxygen saturation < 90% on supplemental oxygen by nasal cannula ≥ 5 L/minute or with unstable respiratory or hemodynamic status were excluded from undergoing bronchoscopy. Thrombocytopenia was not considered a contraindication to BAL, but platelet transfusions frequently were administered in patients who had platelet counts < 20,000/μL before bronchoscopy as long as they were not demonstrably transfusion-refractory. A standardized protocol for BAL performance was adopted collectively by the Department of Pulmonary Medicine. Specifically, after a detailed inspection of the tracheobronchial tree, the bronchoscope was wedged in the segmental or subsegmental bronchus leading to the area of greatest radiologic infiltration; then, a 0.9% sterile saline solution was instilled and retrieved as described in Table 1. A detailed procedure note was dictated for the electronic medical record in each BAL, including bronchoscopic findings, the volume of saline solution instilled and retrieved, the color and consistency of the effluent, and any complications of the procedure.
Table 1.
Bronchoalveolar Lavage Protocol Description
|
Microbiology, Cytopathology, and Cell Counts
BAL effluents were sent for clinically indicated analyses, including microbiologic cultures (bacterial, mycobacterial, fungal, viral), detection of viral antigens, cytopathologic analysis, and determination of total leukocyte counts and leukocyte differential counts. Washings were collected at the discretion of the operating pulmonologist and were submitted for separate microbiologic and cytologic analysis.
Diagnostic Criteria
Bacterial pathogens that were cultured from BAL effluent were considered true pathogens when accompanied by compatible radiographs (segmental or lobar infiltrates) and clinical symptoms (fever, cough, purulent sputum, pleurisy), with or without associated bacteremia or positive cultures of transbronchial lung biopsies. The diagnosis of CMV pneumonia was made after the identification of intracytoplasmic or intranuclear inclusion bodies and/or positive stains for CMV-specific antigens by direct immunofluorescence assays of BAL fluid in the setting of a clinical syndrome consistent with pneumonia. Samples that demonstrated respiratory syncytial virus (RSV), influenza A or B, parainfluenza, Pneumocystis jiroveci, Mycobacterium tuberculosis, or M. avium-Intracellulare (M. avium) were considered diagnostic. Polymicrobial lower respiratory tract infections were defined as the presence of more than 1 pathogen from the same sample in the setting of clinical lung infection. Fungal pneumonia was defined according to standardized criteria of the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group, which consider host immunosuppression/susceptibility factors, microbiologic results, and presenting clinical features, such as fever and compatible radiographs.7 Coagulase-negative staphylococci and Candida spp. were only considered true pathogens in patients who had severe neutropenia (ANC < 500/μL) or active graft-versus-host disease or when positive tissue or blood cultures with the same microorganism were available at the time of BAL and no other pathogen was identified. Positive Gram stains without a congruent positive BAL culture or isolation of typical contaminants, such as Penicillium spp., M. gordonae, herpes simple virus (HSV), or Malbranchea spp., were considered colonization/contamination.8 Cytologic evidence of cancer in the BAL specimen was considered diagnostic. All cases that did not strictly adhere to common clinical diagnostic criteria were adjudicated by an infectious diseases specialist (D.P.K.) and 2 pulmonary specialists (B.F.D. and S.E.E.), and a consensus was reached.
Radiologic and Additional Laboratory Studies
Radiographs that were obtained within 7 days of the procedure were categorized as demonstrating consolidation, ground-glass infiltration, nodules, or interstitial infiltration according to the radiologist’s report. Extracted laboratory tests included neutrophil and platelet counts in peripheral blood at the time of BAL and blood cultures that were obtained within 3 days of the procedure. Aspergillus galactomannan detection (BioRad, Hercules, Calif) was performed at the attending physician’s discretion in patients with a suspicion of invasive fungal infection and required 2 positive results (optical density index, > 0.5) to be scored positive for this study. A serum pp65 CMV-antiginemia test frequently was obtained in patients who underwent HSCT, especially ≤ 100 days after transplantation, and in patients who had pulmonary infiltrates and lymphopenia, and the test was scored positive for the current study when it was obtained within 72 hours of the BAL.
BAL-Related Complications
Reported complications within 24 hours after the procedure were classified as either major or minor. Major complications were defined prospectively as pneumothorax, hemodynamic instability that required vasopressor support, oxygen desaturation < 90% or > 6% below the pre-procedure level, hypoxemia that required ≥ 2 L per minute of additional oxygen supplementation after discharge from the recovery suite than was required in the preprocedure state, and any event that led to inpatient admission. Mild hypoxemia that required supplemental oxygen therapy during bronchoscopy, transient hemodynamic instability that resolved after the procedure, and self-limited endobronchial bleeding that required no intervention were defined as minor complications.
Data Collection Method and Statistical Analysis
Data were prospectively collected, extracted into a password-protected database, and retrospectively evaluated. For statistical analysis, the PASW Statistics 17 software package (SPSS, Chicago, Ill) was used. Continuous variables were analyzed using the Mann-Whitney U test (non-parametric variables) or analyses of variance (parametric variables), whereas categorical variables were compared using the chi-square test. A P value < .05 was considered statistically significant.
RESULTS
Patient Characteristics
Two hundred eighty-four BAL procedures were performed in 284 consecutive patients. Overall, 187 patients (65.8%) had an underlying hematologic malignancy, and the remaining 90 patients had either a nonhematologic malignancy (31.7%) or a noncancer diagnosis (2.5%), as indicated in Table 2. Patients in the hematologic malignancy group were younger (P < .001) and more frequently were men (P < .001) than the nonhematologic malignancy group. Eighty patients (28.1%) were HSCT recipients (53 allogeneic, 27 autologous). Thirty-two of 80 patients (40%) who were HSCT recipients underwent BAL in the first 100 days after transplantation. Concomitant chemotherapy and broad-spectrum antibiotic administration at the time of BAL were common in the hematologic malignancy group (62.3% and 86.6%, respectively). In contrast, in the nonhematologic malignancy group, only 30.9% of patients were receiving chemotherapy, and 33% were receiving antibiotic therapy (P < .001 vs the hematologic malignancy group). Both neutropenia and thrombocytopenia at the time of BAL were more common in the hematologic malignancy group (Table 2).
Table 2.
Baseline Demographic and Clinical Characteristics of Patients
| Variable | No. of Patients (%) | P | |
|---|---|---|---|
| Heme Group, n = 187 | Non-Heme Group, n = 97 | ||
| Age: Mean ± SD [range], y | 55.3 ± 13.7 [21–85] | 61.5 ± 12.1 [13–46] | <.001 |
| Men | 114 (61) | 35 (36) | <.001 |
| Peripheral blood ANC <500/μLa | 53 (28.3) | 2 (2.1) | <.001 |
| Platelet count: Mean ± SD, ×103/μLa | 113.016 ± 113.717 | 287.022 ± 150.361 | <.001 |
| Immunosuppressive treatmenta | 89 (47.6) | 8 (8.2) | <.001 |
| Active chemotherapya | 116 (62.3) | 30 (30.9) | <.001 |
| Active antibiotic therapya | 162 (86.6) | 32 (33) | <.001 |
| HSCT status | |||
| No HSCT | 107 (57.2) | ||
| Allogeneic HSCT | 53 (28.4) | ||
| <100 d | 18 (9.6) | ||
| ≥100 d | 35 (18.7) | ||
| Autologous HSCT | 27 (14.4) | ||
| <100 d | 11 (5.9) | ||
| ≥100 d | 16 (8.6) | ||
| Solid cancer diagnosis | |||
| Lung cancer | 18 (18.6) | ||
| Nonlung cancerb | 72 (74.2) | ||
| Noncancer diagnosisc | 7 (7.2) | ||
SD, indicates standard deviation; ANC, absolute neutrophil count; Heme, hematologic malignancy diagnosis; Non-Heme, no hematologic malignancy; HSCT, hematopoietic stem cell transplant; SD, standard deviation.
Measured at the time patients underwent bronchoalveolar lavage.
Included 16 patients with metastatic lung disease.
Two patients were screened for breast cancer, 2 were screened for pulmonary nodules, 1 was screened for ovarian fibromas, 1 was screened for sclerosing mesenteritis, and 1 was screened for uterine fibroids, but none had a cancer diagnosis at the time of the current report.
Mechanical Performance of Protocolized BAL in the Study Population
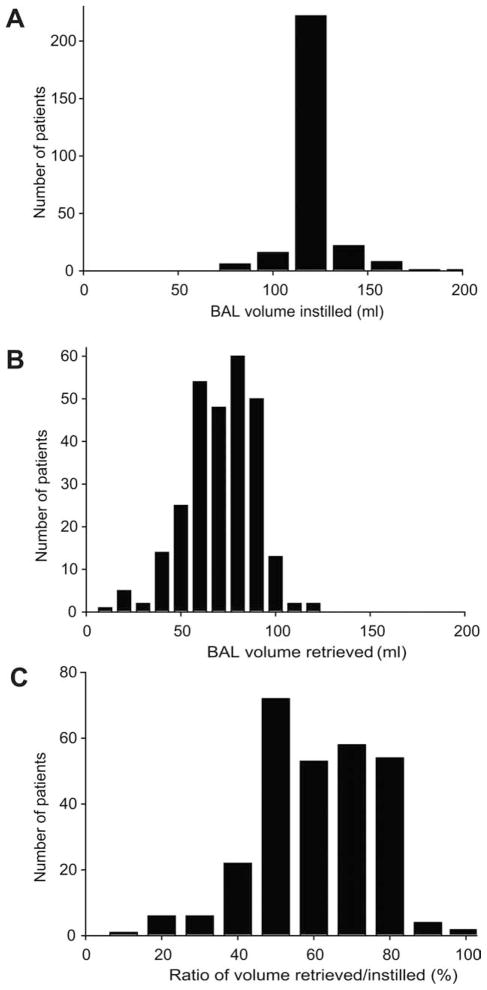
Of the 284 BAL procedures performed, 274 reports (97.5%) provided sufficient data regarding the volume of saline instilled for an analysis of mechanical performance. Of those, exactly 120 mL of sterile saline were instilled in 222 procedures (80.1%), > 120 mL of sterile saline were instilled in 33 procedures (10.5%), and only 22 patients (7.9%) received < 120 mL of sterile saline. The mean volume instilled was 121.5 ± 13.9 mL (range, 80–200 mL) (Fig. 1A). Among the procedures in which < 120 mL saline were instilled, desaturation from baseline oxyhemoglobin concentrations resulted in protocol deviation in 3 procedures, difficulty in wedging the bronchoscope because of anatomic anomalies was reported in 2 procedures, and difficulty managing secretions resulted in protocol deviation in 2 procedures. In 1 procedure, the BAL effluent accidentally was discarded, and a second BAL procedure was repeated with instillation of 80 mL of saline. No specific reason was reported for protocol deviation in the remaining 14 procedures.
Figure 1.
These charts illustrate the mechanical performance of a standardized bronchoalveolar lavage (BAL) protocol. The mechanical parameters of a standardized BAL protocol were assessed based on the clinical records of 274 procedures with complete details. Extracted data included (A) the volume of saline instilled for each procedure and (B) the volume of effluent collected. These values were used to calculate (C) the ratio of volume recovered/instilled.
Greater than 40 mL of effluent were retrieved with the protocol in 259 procedures (91.2%); whereas, in 15 procedures (5.2%), < 40 mL of effluent were obtained, as indicted in Figure 1B. The mean volume retrieved was 68.70 ± 18.14 mL (range, 10–120 mL). Reasons for protocol deviation included difficulties in wedging the bronchoscope caused by anatomic abnormalities in 1 procedure, desaturation from baseline oxygenation levels in 2 procedures, early termination of the procedure because of dynamic airway collapse in 2 procedures, and an abundance of copious secretions in 5 procedures. No specific reason was recorded in 5 procedures.
The ratio of fluid instilled to fluid recovered also was determined. For all procedures, the mean ratio was 56.7% ± 14.5% (range, 8%–92%) (Fig. 1C).
Diagnostic Yield, Differential Cell Counts in BAL, and Associated Imaging
Collectively defined as identifying either a likely pathogen or malignant cells in a compatible clinical syndrome, the overall diagnostic yield of BAL was 33.8% (96 of 284 procedures) in our patient population. In the hematologic malignancy group, 55 BAL samples (29.4%) were diagnostic, whereas 110 samples (58.8%) revealed neither a pathogen nor malignant cells, as indicated in Table 3. The remaining 22 samples (11.8%) grew ≥ 1 microorganisms that were considered potential contaminants and/or colonizers. The diagnostic yield was somewhat higher in the nonhematologic malignancy group (P = .021 vs the hematologic malignancy group). In the nonhematologic malignancy group, 41 patients (42.3%) had diagnostic studies, 45 samples (46.8%) yielded neither a pathogen nor malignant cells, and 11 samples (11.3%) grew potential contaminants and/or colonizers (Fig. 2A).
Table 3.
Diagnostic Yield of Bronchoalveolar Lavage by Radiologic Appearance
| BAL Diagnosis | No. of Patients | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Hematologic | Nonhematologic | |||||||||
| Total | Con | Radiology | Int | Total | Con | Radiology | Int | |||
| GG | Nod | GG | Nod | |||||||
| Bacteria | 17 | 11 | 4 | 2 | 9 | 6 | 2 | 1 | ||
| Fungi | 7 | 3 | 4 | 7 | 1 | 1 | 4 | 1 | ||
| Mycobacteria | 1 | 1 | 3 | 3 | ||||||
| Viruses | 13 | 5 | 6 | 2 | 1 | 1 | ||||
| Multiple pathogens | 14 | 10 | 1 | 3 | 7 | 3 | 4 | |||
| Noninfectious | 3a | 2 | 1 | 14b | 8 | 6 | ||||
| Total | 55 | 31 | 12 | 10 | 2 | 41 | 17 | 3 | 19 | 1 |
Con indicates consolidation; GG, ground glass; Nod, nodular; Int, interstitial; BAL, bronchoalveolar lavage.
Included 3 patients with malignancies.
Included 1 patient with alveolar proteinosis and 13 patients with malignancies.
Figure 2.
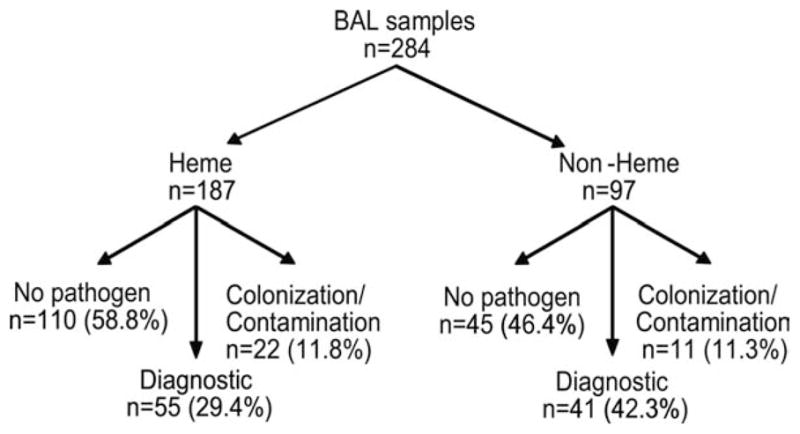
This flow chart illustrates the diagnostic yield of standardized bronchoalveolar lavage (BAL) in a comprehensive cancer center. After allowing for underlying patient malignancy categories (hematologic malignancy [Heme] or other than hematologic malignancy [Non-Heme]), prospectively enrolled BAL procedures were classified as diagnostic if a true pathogen or a malignancy was identified. Nondiagnostic samples were classified further as revealing either no microbial pathogen or revealing a microbe that was not believed likely to cause the presenting syndrome (colonization/contamination).
Of the 55 diagnostic BAL samples from the hematologic malignancy group, 17 samples (30.9%) grew bacterial pathogens (P. aeruginosa, Escherichia coli, Stenotrophomonas maltophilia, Nocardia spp., coagulase-negative staphylococci, Haemophiluss spp.), and 7 patients (12.7%) had isolation of a fungus (Aspergillus spp., non-specified molds, Candida spp.). An atypical mycobacterial infection was identified in 1 patient. Thirteen patients (23.6%) had viral infections (CMV, influenza A, influenza B, RSV). Mixed infections were identified in 14 patients (25.5%). Malignancy was the only noninfectious diagnosis in 3 patients (1 adenocarcinoma, 2 acute leukemia). Diffuse alveolar hemorrhage, defined as BAL fluid with progressively hemorrhagic returns, was not exhibited in any patient. A detailed analysis of the diagnostic yield in the hematologic malignancy group is provided in Table 3.
In the nonhematologic malignancy group, a noninfectious diagnosis (primarily malignancy) was the most frequent (14 patients; 34.1%). Nine samples (22%) grew bacterial pathogens, including P. aeruginosa, Nocardia asteroides, Haemophilus spp., and S. aureus. Seven samples (17%) grew fungi, including Aspergillus spp., Rhizomucor spp., P. jiroveci, and unspecified molds. Viral infection was identified in only 1 sample. Atypical mycobacteria was identified in 3 samples (7.3%). Multiple pathogens were identified in 7 samples (17.1%). A detailed analysis of specific diagnosis in the nonhematologic malignancy group is provided in Table 3.
Consolidation was the predominant radiologic pattern in the hematologic malignancy group (56.4%), particularly when bacterial pathogens were isolated, as indicated in Table 3. Ground-glass opacities (21.8%) were the next most common finding and constituted the predominant radiographic pattern among patients with viral infections. Nodular infiltrates (18.2%) were observed most often in the setting of fungal lung infection (isolated or combined with other pathogens). Interstitial infiltrates were infrequent and were associated only with viral infections.
In contrast, nodular infiltrates constituted the most common radiologic pattern in the nonhematologic malignancy group (46.3%), especially among patients whose BAL fluid revealed either malignant cytology or fungal infection. Consolidations (41.6%) were associated again with bacterial infections but also were encountered in patients who had malignant cells in the BAL fluid. Interstitial infiltrates were identified only in 1 patient with P. jirovecii pneumonia.
Differential cell counts of the leukocytes recovered on BAL for the hematologic malignancy and nonhematologic malignancy groups are reported in Table 4. The ANC tended to be lower in the hematologic malignancy group than the nonhematologic malignancy group for all infectious conditions, as expected. These differences appeared most pronounced when comparing the groups with fungal, mycobacterial, and mixed infections.
Table 4.
Bronchoalveolar Lavage (BAL) Differential Cell Counts (per μL of BAL Effluent)
| Diagnosis | Cell Counts: Mean ± SD | |||||
|---|---|---|---|---|---|---|
| Heme Group | Non-Heme Group | |||||
| WBC, μL | ANC, % | MONO, % | WBC, μL | ANC, % | MONO, % | |
| Bacteria | 393 ± 389 | 13.7 ± 25.8 | 79.6 ± 28.6 | 592 ± 616 | 30.2 ± 36.8 | 65.6 ± 30 |
| Fungi | 175 ± 131 | 2.8 ± 3.4a | 68.5 ± 40.2 | 446 ± 448 | 22 ± 24.1a | 70.2 ± 33.2 |
| Mycobacteria | 250 | 4 | 93 | 528 ± 496 | 28.3 ± 19.7 | 65 ± 16.5 |
| Viruses | 242 ± 226 | 25 ± 32.7 | 70.5 ± 33.7 | 312 | 79 | 15 |
| Multiple pathogens | 932 ± 2019 | 19.1 ± 27.3b | 78.8 ± 26.6c | 954 ± 1801 | 56.7 ± 35.2b | 26 ± 23.9c |
| Malignancy | 527 ± 282 | 22 ± 34.7 | 73.3 ± 32.4 | 463 ± 835 | 18.2 ± 35.6 | 74.1 ± 34.9 |
| Contamination/colonization | 600 ± 945 | 11.8 ± 19.6 | 83.9 ± 20.6 | 372 ± 398 | 24 ± 26.3 | 73.4 ± 25.8 |
| No pathogen | 369 ± 655 | 13.6 ± 20.9 | 80.5 ± 23.1 | 323 ± 637 | 11 ± 20.8 | 86 ± 18.6 |
SD indicates standard deviation; Heme, hematologic malignancy diagnosis; Non-Heme, no hematologic malignancy; WBC, white blood cell count; ANC, absolute neutrophil count; MONO, monocytes.
P= .053.
P= .033.
P= .018.
BAL Mechanics and Diagnostic Yield in Subgroups
The mean volume (± standard error) of fluid instilled in the hematologic malignancy group (121 ± 12.45 mL) was similar to that instilled in the nonhematologic malignancy group (120 ± 17.1 mL). It is noteworthy that the volume of fluid recovered in the hematologic malignancy group was significantly greater than the volume recovered in the nonhematologic malignancy group (70.8 ± 16.8 mL vs 63.8 ± 20 mL, respectively; P = .003). Consequently, the ratio of saline instilled to effluent recovered was greater in the hematologic malignancy group than in nonhematologic malignancy group (58.6 ± 13.3% vs 53 ± 15.9%, respectively; P = .002).
Next, we assessed the correlation of mechanical BAL performance with diagnostic yield. Comparing diagnostic to nondiagnostic procedures in the hematologic malignancy group, the mean volumes instilled (123 ± 12.64 mL vs 120.6 ± 11.9 mL, respectively), the mean volumes retrieved (72.1 ± 14.8 mL vs 70.2 ± 17.4 mL, respectively), and the mean percentage of fluid recovery (58.9% ± 12.1 % vs 58.3% ± 14%, respectively) did not differ significantly. The diagnostic yield of BAL in patients who underwent HSCT ≤ 100 days before BAL was lower than for patients who underwent BAL > 100 days after HSCT (13.8% vs 35.3%; P = .032). The mean percentage of BAL fluid retrieved also was lower in patients who underwent HSCT ≤ 100 days before BAL compared with those who underwent BAL > 100 after HSCT (54.2% vs 59.6%; P = .043). We also observed that the diagnostic yield in neutropenic patients (39 HSCT patients and 14 non-HSCT patients) was significantly higher (41.5%) than the yield in nonneutropenic patients (24.6%; P = .019).
It is noteworthy that an analysis of BAL mechanics in the nonhematologic malignancy group did not reveal an increase in diagnostic yield that corresponded with improved instillation/recovery ratios. Rather, we observed a nonsignificant trend (P = .065) in the opposite direction. When we recovered ≤ 40% of the volume instilled, the diagnostic yield was 64.7%. When 40% to 60% of the instilled volume was retrieved, the BAL was considered diagnostic 40.5% of the time. When > 60% of the instilled volume was collected, only 30.3% of the specimens provided a microbiologic or cytologic diagnosis.
Washings
Collected washings were sent at the performing physician’s discretion for analysis from 177 patients (89.8%) in the hematologic malignancy group and from 92 patients (94.8%) in the nonhematologic malignancy group. Washings from 63 patients (35.6%) in the hematologic malignancy group grew a microorganism that was considered a true pathogen. It is noteworthy that, in 36 of those 63 patients (57.1%), a pathogen was isolated from washings in the setting of a microbiologically negative BAL sample. Additional fungal pathogens were isolated in 11 patients (17.5%), including Aspergillus spp., Candida spp., an unspecified mold, and Rhizomucor spp. CMV was identified in 3 patients (4.8%). Washings grew additional bacterial organisms in 12 patients (19%), including Staphylococcus spp., P. aeruginosa, β-hemolytic Streptococci, Haemophilus spp., Actinomyces spp., and Enterococcus spp. M. avium-Intracellulare was identified in 2 washings (3.2%). Multiple pathogens were recovered in 13 washings (20.6%).
In the nonhematologic malignancy group, washings yielded true pathogens in 23 of 92 samples (25%). Of these 23 washings, 11 (47.8%) revealed a pathogen that was not grown in the BAL sample. The additional pathogens included Staphylococcus spp., E. coli, Haemophilus spp., M. avium, CMV, and parainfluenza type III. Multiple true pathogens were grown in 3 washings (13.0%). A detailed description of the diagnostic yield and the predominant radiologic pattern is provided in Figure 3.
Figure 3.
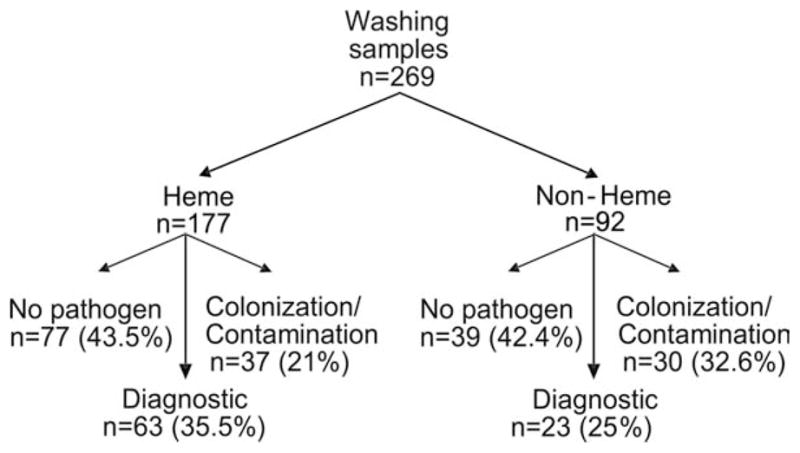
This flow chart illustrates the diagnostic yield of bronchoscopic washings. Washings were sent for microbiologic and cytologic analysis at the discretion of the physicians who performed standardized bronchoalveolar lavage (BAL) After allowing for underlying patient malignancy categories (hematologic malignancy [Heme] or other than hematologic malignancy [Non-Heme]), prospectively enrolled BAL procedures were classified as diagnostic if a true pathogen or a malignancy was identified. Nondiagnostic samples were classified further as revealing either no microbial pathogen or revealing a microbe that was not believed likely to cause the presenting syndrome (colonization/contamination).
Additional Diagnostic Testing
Blood cultures were obtained from 136 patients in the hematologic malignancy group within 3 days of BAL and yielded 9 positive results (6.6%; S. aureus, E. coli, Microsporum spp., Providencia alcalifaciens, Micrococcus spp., coagulase-negative staphylococci). In 2 patients, the pathogen that was isolated in blood cultures also was grown from BAL fluid (S. aureus and coagulase-negative staphylococci specimens). No positive blood cultures were obtained from the nonhematologic malignancy group.
Of 124 Aspergillus serum galactomannan tests that were obtained from the hematologic malignancy group, only 5 (4%) were positive. Aspergillus spp. was isolated in BAL fluid from 1 patient who had a positive serum galactomannan test. In the nonhematologic malignancy group, none of 18 galactomannan tests obtained were positive. Of 131 CMV-antiginemia tests that were performed in the hematologic malignancy group, 20 were positive (15.3%). In 4 of the 20 antigenemic patients, the BAL samples were also positive for CMV.
In the hematologic malignancy group, 8 patients underwent transbronchial biopsies at the time of their BAL. Five biopsies were nondiagnostic, 1 revealed malignant cells, 1 suggested Erdheim-Chester disease, and 1 revealed a noncaseating granulomata. No pathogens were identified on BAL to suggest an infectious cause of granulomata, and the patient was diagnosed clinically with sarcoidosis. In the nonhematologic malignancy group, 15 patients underwent transbronchial biopsies. Nine of those biopsies were nondiagnostic, 3 revealed malignant cells, 2 demonstrated fungal invasion, and 1 specimen grew alpha-hemolytic streptococci on culture of homogenized biopsy tissue. No BAL cultures grew pathogens for any of the biopsied patients. One patient who had fungal invasion noted on biopsy also had malignant cells and galactomannan positivity identified on BAL analysis. No patients underwent surgical lung biopsy during the study period. Nasal washes were positive for RSV in 2 patients, for parainfluenza in 1 patient (who also had a BAL sample that was positive for parainfluenza), and for influenza B in 1 patient (who also had a BAL sample that was positive for influenza B).
BAL-Related Complications
Even with our extremely liberal definition, only 10 BAL-related complications were encountered (3.6%). Nine of those were minor complications and included transient hypoxemia (n = 6), sinus tachycardia (n = 1), hemodynamic instability that resolved with discontinuation of the procedure (n = 1), and mild alveolar hemorrhage (n = 1). The single major complication required intensive care unit admission for the delivery of supplemental oxygen for > 24 hours. There were no procedure-attributable deaths.
Mortality
The 30-day mortality rate was 18.8% (35 of 187 patients) in the hematologic malignancy group and 7.2% (7 of 97 patients) in the nonhematologic malignancy group (P < .05). In contrast to previously reported associations between pathogen identification and improved outcomes,2,9,10 the 30-day mortality rate among patients with hematologic malignancies was greater for patients who had a diagnostic BAL compared with patients who had a nondiagnostic BAL (31% vs 13.6%; P = .006). In the nonhematologic malignancy group, the 30-day mortality rate did not differ significantly according to diagnostic versus nondiagnostic status.
DISCUSSION
Bronchoscopy with BAL remains the diagnostic tool of choice for the evaluation of new lung infiltrates because of its historic safety and superior diagnostic yield compared with other modalities. This is particularly relevant in the cancer population because of issues of immunocompromise, frequent exposure to the healthcare setting and nosocomial pathogens, and susceptibility to unusual opportunistic pathogens. BAL has been performed for more than 3 decades with mean diagnostic yields reported between 15% and > 55%.10–15 This variability largely is attributed to diversities in the patient population characteristics,16 differences on BAL effluent analysis, and, more recently, the availability of new diagnostic tools.17,18 Although the American Thoracic Society has provided recommendations regarding BAL technique,6 to our knowledge, there have been no analyses of a standardized protocol or the association of BAL mechanics to safety and/or diagnostic yield in cancer patients.
The objective of the current study was to evaluate the mechanical properties of a standardized BAL protocol and its efficacy in determining the etiology of new lung infiltrates in patients with cancer. The overall diagnostic yield in the hematologic malignancy group was 29.4%. The finding that 87% of patients with hematologic malignancies were receiving antibiotics at the time of BAL may have accounted for the lower diagnostic yield.10 However, the rate of microbiologically diagnostic BALs in the hematologic malignancy group (52 of 187 patients; 27.8%) was identical to that observed in the nonhematologic malignancy group (27 of 97 patients; 27.8%), although only 33% of patients were receiving antibiotics in the latter group. The most common pathogens in the hematologic malignancy group were bacteria. Among these were several coagulase-negative Staphylococci that often are considered contaminants/colonizers.1 However, Inai et al demonstrated in an autopsy study that coagulase-negative Staphylococci constituted the predominant Gram positive cocci isolated from lung tissue in neutropenic patients with pneumonia.19 Multiple pathogens were recovered in 14 of 187 procedures (9.6%). This is consistent with recent studies reporting that polymicrobial infections account for 15% of the microbiologically documented bloodstream infections in this patient population.1 The somewhat lower diagnostic yield for multiple pathogens in our study may be attributed to the finding that the majority of samples that demonstrated yeast growth were designated as colonizers, whereas other groups have included Candida spp. among their diagnostic samples.20 Our incidence of viral lower respiratory tract infections, especially CMV, was comparable the incidence reported in some studies9 but higher that that reported by others.14 A high incidence of microbiologically proven, invasive fungal infections (eg, Aspergillus spp.) was not observed in our study, probably because of the widespread use of mold-active prophylaxis in our institution.21,22 Mycobacteria also rarely were isolated, which is congruent with other studies.12
The protocolized approach to the BAL procedure is an important feature of these data. We attempted to ensure that each sample was collected similarly (which is not done rigorously in most studies) so that we could compare performance across time and operators. We selected a required minimum effluent volume that would allow the performance of all typical clinical analyses based on previously published data suggesting that a 40-mL recovery volume should be sufficient to sample the alveolar space rather than the airway lining fluid.23,24 The minimum BAL volume retrieved that led to a diagnosis was 20 mL (ratio, 56.7%). Our protocol resulted in higher volumes instilled, volumes recovered, and percentage recovered than were reported in other series,25 but these differences were not associated with a higher diagnostic yield. Although they may be attributable to population differences, we suspect that our stringent diagnostic criteria are the most likely explanation for these differences. In addition, even if our diagnostic yield did not exceed previously published rates, our mean fluid recovery exceeded the 50% of volume instilled, which reportedly is consistent with satisfactory BAL performance, as observed previously.25
The standardization of sample-collection techniques like BAL is important to allow for interpretable investigations of clinical effectiveness as new diagnostic technologies become available. For example, galactomannan detection enzyme-linked immunosorbent assay kits and real-time polymerase chain reaction assays for pathogen genomic material in BAL samples are promising complementary diagnostic tools for the detection of opportunistic mold infections in the lung.17,26–29 However, reports from clinical trials of the diagnostic accuracy of these tests vary widely.17,26,28,29 If the specimens are collected differently at different centers or even by different physicians, then it becomes difficult to determine whether diagnostic variability arises from problems with the new technology, differences in patient populations, or the inconsistent mechanics of specimen collection. Consequently, adoption of a standardized BAL protocol may facilitate comparisons between series and could optimize the development of novel diagnostic strategies.
Although the volumes instilled were somewhat higher in our study than in other reported series, in our study of protocolized BAL, we observed a very low complication rate that was consistent with existing reports.30 Only 1 patient required an escalation from the preprocedure level of care. It is noteworthy that, even among our very severely thrombocytopenic cancer patient population, we observed no significant bleeding events.
Another interesting aspect of our experience was the contribution of bronchial washings to the diagnostic evaluation. Bronchial washings often are disregarded as potential sources of contamination from outside the lung areas of interest. However, by analyzing the washings, we identified a likely pathogen in 17% of patients that would not have been identified by BAL alone. This is consistent with limited existing data from a noncancer population31 and suggests that there may be a diagnostic role for bronchial washings, particularly among our patients with hematologic malignancies. Because these samples were not collected in a standardized manner, it is the authors’ position that additional investigations will be required to confirm the broader diagnostic utility of bronchial washings, but it seems clear that the isolation of definite pathogens should prompt further evaluation and treatment, even if they are not identified on BAL.32
Taken together, the current data provide a framework for protocolized BAL to allow investigation of future diagnostic technologies across providers and institutions, and our results offer insight into the diagnostic performance of this protocol in a comprehensive cancer care center. We demonstrate high protocol compliance in a “real-life,” high-volume clinical environment and reveal a very low rate of complications, even among high-risk patients. Adoption of this or similar standardized BAL techniques may improve clinical and laboratory comparisons between clinical trials. This may result in more prompt and accurate diagnosis of pneumonia and, ultimately, should translate into improved patient outcomes.
Acknowledgments
We thank the members of the University of Texas MD Anderson Cancer Center Department of Pulmonary Medicine for their participation in this study and willingness to adopt a protocolized BAL strategy.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
This work was supported by grant KL2-RR02419 (S.E.E.) from the National Center for Research Resources, National Institutes of Health, and by a Physician-Scientist Award (S.E.E.) that is supported by Cancer Center Support Grant P30-CA016672 to the University of Texas MD Anderson Cancer Center from the National Cancer Institute, National Institutes of Health.
References
- 1.Rolston KV, Bodey GP, Safdar A. Polymicrobial infection in patients with cancer: an underappreciated and underreported entity. Clin Infect Dis. 2007;45:228–233. doi: 10.1086/518873. [DOI] [PubMed] [Google Scholar]
- 2.Maschmeyer G, Beinert T, Buchheidt D, et al. Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Oncology (DGHO) Diagnosis and antimicrobial therapy of pulmonary infiltrates in febrile neutropenic patients—guidelines of the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Oncology (DGHO) Ann Hematol. 2003;82(suppl 2):S118–S126. doi: 10.1007/s00277-003-0765-3. [DOI] [PubMed] [Google Scholar]
- 3.Joos L, Tamm M. Breakdown of pulmonary host defense in the immunocompromised host: cancer chemotherapy. Proc Am Thorac Soc. 2005;2:445–448. doi: 10.1513/pats.200508-097JS. [DOI] [PubMed] [Google Scholar]
- 4.Elting LS, Rubenstein EB, Rolston KV, Bodey GP. Outcomes of bacteremia in patients with cancer and neutropenia: observations from 2 decades of epidemiological and clinical trials. Clin Infect Dis. 1997;25:247–259. doi: 10.1086/514550. [DOI] [PubMed] [Google Scholar]
- 5.Klech H, Pohl W. Technical recommendations and guidelines for bronchoalveolar lavage (BAL) Eur Res J. 1989;2:561–585. [PubMed] [Google Scholar]
- 6.Goldstein RA, Rohatgi PK, Bergofsky EH. Clinical role of bronchoalveolar lavage in adults with pulmonary disease. Am Rev Respir Dis. 1990;142:481–486. doi: 10.1164/ajrccm/142.2.481. [DOI] [PubMed] [Google Scholar]
- 7.Ascioglu S, Rex JH, de Pauw B, et al. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin Infect Dis. 2002;34:7–14. doi: 10.1086/323335. [DOI] [PubMed] [Google Scholar]
- 8.Lionakis MS, Kontoyiannis DP. The significance of isolation of saprophytic molds from the lower respiratory tract in patients with cancer. Cancer. 2004;100:165–172. doi: 10.1002/cncr.11876. [DOI] [PubMed] [Google Scholar]
- 9.Kuehnhardt D, Hannemann M, Schmidt B, Heider U, Possinger K, Eucker J. Therapeutic implication of BAL in patients with neutropenia. Ann Hematol. 2009;88:1249–1256. doi: 10.1007/s00277-009-0747-1. [DOI] [PubMed] [Google Scholar]
- 10.Von Eiff M, Zuhlsdorf M, Roos N, Thomas M, Buchner T, van de Loo J. Pulmonary infiltrates in patients with haematologic malignancies: clinical usefulness of non-invasive bronchoscopic procedures. Eur J Haematol. 1995;54:157–162. doi: 10.1111/j.1600-0609.1995.tb00207.x. [DOI] [PubMed] [Google Scholar]
- 11.Cordonnier C, Bernaudin JF, Fleury J, et al. Diagnostic yield of bronchoalveolar lavage in pneumonitis occurring after allogeneic bone marrow transplantation. Am Rev Respir Dis. 1985;132:1118–1123. doi: 10.1164/arrd.1985.132.5.1118. [DOI] [PubMed] [Google Scholar]
- 12.Jain P, Sandur S, Meli Y, Arroliga AC, Stoller JK, Mehta AC. Role of flexible bronchoscopy in immunocompromised patients with lung infiltrates. Chest. 2004;125:712–722. doi: 10.1378/chest.125.2.712. [DOI] [PubMed] [Google Scholar]
- 13.Shannon VR, Andersson BS, Lei X, Champlin RE, Kontoyiannis DP. Utility of early versus late fiberoptic bronchoscopy in the evaluation of new pulmonary infiltrates following hematopoietic stem cell transplantation. Bone Marrow Transplant. 2010;45:647–655. doi: 10.1038/bmt.2009.203. [DOI] [PubMed] [Google Scholar]
- 14.Hummel M, Rudert S, Hof H, Hehlmann R, Buchheidt D. Diagnostic yield of bronchoscopy with bronchoalveolar lavage in febrile patients with hematologic malignancies and pulmonary infiltrates. Ann Hematol. 2008;87:291–297. doi: 10.1007/s00277-007-0391-6. [DOI] [PubMed] [Google Scholar]
- 15.Azoulay E, Mokart D, Rabbat A, et al. Diagnostic bronchoscopy in hematology and oncology patients with acute respiratory failure: prospective multicenter data. Crit Care Med. 2008;36:100–107. doi: 10.1097/01.CCM.0000295590.33145.C4. [DOI] [PubMed] [Google Scholar]
- 16.Cornillet A, Camus C, Nimubona S, et al. Comparison of epidemiological, clinical, and biological features of invasive aspergillosis in neutropenic and nonneutropenic patients: a 6-year survey. Clin Infect Dis. 2006;43:577–584. doi: 10.1086/505870. [DOI] [PubMed] [Google Scholar]
- 17.Maertens J, Maertens V, Theunissen K, et al. Bronchoalveolar lavage fluid galactomannan for the diagnosis of invasive pulmonary aspergillosis in patients with hematologic diseases. Clin Infect Dis. 2009;49:1688–1693. doi: 10.1086/647935. [DOI] [PubMed] [Google Scholar]
- 18.Aquino VR, Goldani LZ, Pasqualotto AC. Update on the contribution of galactomannan for the diagnosis of invasive aspergillosis. Mycopathologia. 2007;163:191–202. doi: 10.1007/s11046-007-9010-2. [DOI] [PubMed] [Google Scholar]
- 19.Inai K, Iwasaki H, Noriki S, et al. Frequent detection of multidrug-resistant pneumonia-causing bacteria in the pneumonia lung tissues of patients with hematological malignancies. Int J Hematol. 2007;86:225–232. doi: 10.1532/IJH97.07233. [DOI] [PubMed] [Google Scholar]
- 20.Kontoyiannis DP, Reddy BT, Torres HA, et al. Pulmonary candidiasis in patients with cancer: an autopsy study. Clin Infect Dis. 2002;34:400–403. doi: 10.1086/338404. [DOI] [PubMed] [Google Scholar]
- 21.Leventakos K, Lewis RE, Kontoyiannis DP. Fungal infections in leukemia patients: how do we prevent and treat them? Clin Infect Dis. 2010;50:405–415. doi: 10.1086/649879. [DOI] [PubMed] [Google Scholar]
- 22.Chandrasekar PH, Ramesh M. Challenges in the management of invasive aspergillosis in hematopoietic stem cell transplantation. Expert Rev Anti Infect Ther. 2009;7:1151–1153. doi: 10.1586/eri.09.105. [DOI] [PubMed] [Google Scholar]
- 23.Haslam PL, Baughman RP. Report of ERS Task Force: guidelines for measurement of acellular components and standardization of BAL. Eur Respir J. 1999;14:245–248. doi: 10.1034/j.1399-3003.1999.14b01.x. [DOI] [PubMed] [Google Scholar]
- 24.Meyer KC. Bronchoalveolar lavage as a diagnostic tool. Semin Respir Crit Care Med. 2007;28:546–560. doi: 10.1055/s-2007-991527. [DOI] [PubMed] [Google Scholar]
- 25.Rosell A, Xaubet A, Agusti C, et al. RASTA Study Group. A new BAL fluid instillation and aspiration technique: a multi-center randomized study. Respir Med. 2006;100:529–535. doi: 10.1016/j.rmed.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 26.Bergeron A, Belle A, Sulahian A, et al. Contribution of galactomannan antigen detection in BAL to the diagnosis of invasive pulmonary aspergillosis in patients with hematologic malignancies. Chest. 2010;137:410–415. doi: 10.1378/chest.09-0701. [DOI] [PubMed] [Google Scholar]
- 27.Francesconi A, Kasai M, Petraitiene R, et al. Characterization and comparison of galactomannan enzyme immunoassay and quantitative real-time PCR assay for detection of Aspergillus fumigatus in bronchoalveolar lavage fluid from experimental invasive pulmonary aspergillosis. J Clin Microbiol. 2006;44:2475–2480. doi: 10.1128/JCM.02693-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hsu LY, Ding Y, Phua J, et al. Galactomannan testing of bronchoalveolar lavage fluid is useful for diagnosis of invasive pulmonary aspergillosis in hematology patients. BMC Infect Dis. 2010;10:44–49. doi: 10.1186/1471-2334-10-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klont RR, Mennink-Kersten MASH, Verweij PE. Utility of Aspergillus antigen detection in specimens other than serum specimens. Clin Infect Dis. 2004;39:1467–1474. doi: 10.1086/425317. [DOI] [PubMed] [Google Scholar]
- 30.Rano A, Agusti C, Jimenez P, et al. Pulmonary infiltrates in non-HIV immunocompromised patients: a diagnostic approach using non-invasive and bronchoscopic procedures. Thorax. 2001;56:379–387. doi: 10.1136/thorax.56.5.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pinckard JK, Kollef M, Dunne WM. Culturing bronchial washings obtained during bronchoscopy fails to add diagnostic utility to culturing the bronchoalveolar lavage fluid alone. Diagn Microbiol Infect Dis. 2002;43:99–105. doi: 10.1016/s0732-8893(02)00372-3. [DOI] [PubMed] [Google Scholar]
- 32.Vandewoude KH, Blot SI, Depuydt P, et al. Clinical relevance of Aspergillus isolation from respiratory tract samples in critically ill patients [serial online] Crit Care. 2006;10:R31. doi: 10.1186/cc4823. [DOI] [PMC free article] [PubMed] [Google Scholar]