Abstract
In this study, we examined in vitro antibacterial, antifungal, antimalarial, and antileishmanial activities of secondary metabolites (1–8) isolated from the fungus Eurotium repens. All compounds showed mild to moderate antibacterial or antifungal or both activities except 7. The activity of compound 6 was the best of the group tested. The in vitro antimalarial evaluation of these compounds revealed that compounds 1–3, 5, and 6 showed antimalarial activities against both chloroquine-sensitive and chloroquine-resistant strains of Plasmodium falciparum with IC50 values in the range of 1.1–3.0 μg/ml without showing any cytotoxicity to the mammalian cells. Compound 5 displayed the highest antimalarial activity. Antileishmanial activity against Leishmania donovani promastigotes was observed for compounds 1–6 with IC50 values ranging from 6.2 to 23 μg/ml. Antileishmanial activity of compounds 5 and 6 (IC50 values of 7.5 and 6.2 μg/ml, respectively) was more potent than 1–4 (IC50 values ranging from 19–23 μg/ml). Compounds 7 and 8 did not show any antiprotozoal effect. Preliminary structure and activity relationship studies indicated that antibacterial, antifungal, antimalarial, and antileishmanial activities associated with phenol derivates (1–6) seem to be dependent on the number of double bonds in the side chain, which would be important for lead optimization in the future.
Keywords: Antibacterial, Antifungal, Antimalarial, Antileishmanial, Benzyl derivatives, Eurotium repens
Introduction
Diseases caused by bacteria, fungi, and protozoa are common worldwide and major causes of death, disability, and social and economic hindrance for millions of people. According to World Health Organization, over 9.5 million people die each year due to infectious diseases and up to 19% patients are infected from hospital visits throughout the world (Peleg and Hooper, 2010; Gastmeier et al., 2007). Hospital-acquired infections cause a wide range of severe infections including pneumonia, infections of the bloodstream, urinary tract among other organs of the body. Most of the nosocomial pathogens are difficult to treat because they are resistant to many antibiotics (Talbot et al., 2006). The treatment of infections caused by pathogenic fungi also faces enormous challenges. In the past 30 years, the incidence of fungal infections has significantly increased around the world, and current antifungal drugs such as polyene macrolides (amphotericin B), azoles (fluconazole, miconazole, itraconazole, and voriconazole), flucytosine, and the candins (caspofungin acetate and micafungin), are not ideal in terms of efficacy, antifungal spectrum, or safety. Furthermore, recent reports support that invasive candidiasis and aspergillosis has increased dramatically and spread rapidly (Gullo, 2009). Amphotericin B is efficacious against both candidiasis and aspergillosis. However, it exhibits severe side effects such as renal toxicity (Maschmeyer and Ruhnke, 2004). Azoles exhibit drug–drug interactions with other drugs by inhibiting CYP450 isoenzymes. Fluconazole, flucytosine, caspofungin, and micafungin all show narrow antifungal spectrums and are prone to develop drug resistance (Masubuchi et al., 2003). Hence, the urgent need of new agents to combat bacterial and fungal infections is immense. Malaria is a mosquito-borne disease caused by a eukaryotic protist of the genus Plasmodium and is a major global health problem because it kills approximately three million people annually, affects over one hundred countries, and the prevalence of this disease is escalating at an alarming rate, particularly in the last two decades (Wright, 2010). Effective treatment of malaria is limited by the rapid development of resistance of Plasmodium falciparum to conventional drugs such as chloroquine, which necessitates the search for new antimalarial agents (Wells et al., 2009). Leishmaniasis is a tropical disease caused by protozoal parasites of the genus Leishmania (Neuber, 2008) and it affects more than 12 million people in the world with an incidence of about 2 million new cases annually (Santos et al., 2008). The most common treatment of leishmaniasis is the use of antimony (pentavalent antimonials) which includes sodium stibogluconate (Pentostam) and N-methylglucamine antimoniate (Glucantime) (Reithinger et al., 2007). However, this treatment has proved to be inefficient due to drug resistance acquired by the parasite and the high toxicity of these drugs (Croft et al., 2006). Amphotericin B, pentamidine, or paromomycin are commonly used alternative drugs for leishmaniasis. However, these drugs are also considered to be ineffective therapies (Santos et al., 2008). Miltefosine is another well-known drug for the treatment of leishmaniosis. Miltefosine has been approved as an oral drug for visceral and cutaneous leishmaniasis with cure rates of about 98%. However, in vitro studies show that Leishmania can become resistant to Miltefosine (Berman, 2008). Therefore, an increasing number of multidrug-resistant pathogens have become a serious problem particularly during the last decade, and provide the impetus for the search and discovery of novel antimalarial and antileishmanial agents.
Fungi offer a treasure trove for the discovery of structurally unique natural products with potential biomedical applications. Recently, we isolated and identified eight secondary metabolites (1–8) from the fungus Eurotium repens (Gao et al., 2011). In this study, we report the antibacterial, antifungal, and antiprotozoal activities of these compounds.
Materials and methods
Fungal materials
The fungus used in this study was collected in Tifton, GA in 1978, lyophilized, and stored at −20°C before it was grown on potato-dextrose agar (PDA) at 24°C until discrete fungal colonies appeared. Samples were taken from colonies and kept on PDA slants in test tubes at 24°C, then placed in a 4°C refrigerator until needed. The fungus was identified as E. repens by sequence comparison of its β-tubulin partial gene (Gao et al., 2011) and a voucher specimen (UM-031509) has been deposited in the culture collection of the Department of Medicinal Chemistry at the University of Mississippi. The batch fermentation of the fungus was performed by seeding it on a medium consisting of 100 g shredded wheat, 100 g low-PH mycological broth, 40 g of yeast extract, and 400 g of sucrose in a 2.0 l Fernbach flask followed by incubation at 24°C for 22 days.
Chemistry
The evaluated compounds 1–8 were isolated from the fungus E. repens. Their extraction, isolation, structural elucidation, and the general experimental procedure for CNS activity have been reported (Gao et al., 2011). In brief, the CNS data showed that these metabolites had good affinity for the cannabinoid and opioid receptors.
In vitro antimicrobial assay
All organisms used for the biological evaluation of the metabolites from E. repens were obtained from the American Type Culture Collection (Manassas, VA) and include the fungi Candida albicans ATCC 90028, C. glabrata ATCC 90030, C. krusei ATCC 6258, Cryptococcus neoformans ATCC 90113, and Aspergillus fumigatus ATCC 204305 and the bacteria Staphylococcus aureus ATCC 29213, methicillin-resistant S. aureus ATCC 33591 (MRS), Escherichia coli ATCC 35218, Pseudomonas aeruginosa ATCC 27853, and Mycobacterium intracellulare ATCC 23068. Susceptibility testing was performed using a modified version of the CLSI (formerly NCCLS) methods (Samoylenko et al., 2009 and referenced therein). M. intracellulare was tested using a modified method (Franzblau et al., 1998). Samples were serially diluted in 20% DMSO/saline and transferred in duplicate to 96-well flat bottom microplates. Microbial inocula were prepared by correcting the OD630 of microbe suspensions in incubation broth to afford final target inocula. Ciprofloxacin (ICN Biomedicals, Ohio) for bacteria and amphotericin B (ICN Biomedicals, Ohio) for fungi are included as positive controls in each assay. All organisms were read at either 630 nm using the Biotek Powerwave XS plate reader (Bio-Tek Instruments, Vermont) or 544ex/590em, (M. intracellulare, A. fumigatus) using the Polarstar Galaxy Plate Reader (BMG LabTechnologies, Germany) prior to and after incubation. Percent growth was plotted versus test concentration to afford the IC50.
In vitro antimalarial activity
Antimalarial activity was determined in vitro against chloroquine-sensitive (D6, Sierra Leone) and chloroquine-resistant (W2, Indo China) strains of P. falciparum by measuring plasmodial LDH activity as described earlier (Makler and Hinrichs, 1993). Tested compounds were dissolved in DMSO (2 mg/ml). A 200 μl suspension of P. falciparum culture (2% parasitemia and 2% hematocrit in RPMI 1640 medium supplemented with 10% human serum and 60 μg/ml amikacin) was added to the wells of a 96-well plate containing 10 μl of serially diluted samples. The plate was flushed with a gas mixture of 90% N2, 5% O2, and 5% CO2 and incubated at 37°C for 72 h in a modular incubation chamber. Plasmodial LDH activity was determined by using Malstat™ reagent (Flow Inc., Portland, OR). In brief, 20 μl of the incubation mixture was mixed with 100 μl of the Malstat reagent and incubated for 30 min. Then, 20 μl of a 1:1 mixture of NBT/PES (Sigma, St. Louis, MO) was added and the plate is further incubated for 1 h in dark. The reaction was stopped by adding 100 μl of a 5% acetic acid solution. The plate was read at 650 nm using the EL-340 Biokinetics Reader (Bio-Tek Instruments, Vermont). IC50 values were obtained from the dose–response curves generated by plotting percent growth versus drug concentration. Chloroquine was included in each assay as positive control. DMSO (0.25%) was used as a vehicle control.
In vitro antileishmanial activity
The antileishmanial activity of the compounds was tested in vitro against a culture of L. donovani promastigotes (Ma et al., 2004). The promastigotes were grown in RPMI 1640 medium supplemented with 10% fetal calf serum (Gibco Chem. Co.) at 26°C. A 3-day-old culture was diluted to 5 × 105 promastigotes/ml. Drug dilutions were prepared directly in cell suspension in 96-well plates. Plates were incubated at 26°C for 48 h and growth of leishmania promastigotes was determined by the Alamar blue assay as described earlier. Standard fluorescence was measured on a Fluostar Galaxy plate reader (BMG Lab Technologies) at an excitation wavelength of 544 nm and an emission wavelength of 590 nm. Pentamidine and Amphoterecin B were used as the standard antileishmanial agents. IC50 values were computed from dose–response curves as above.
In vitro cytotoxicity
The in vitro cytotoxicity was also determined against mammalian kidney fibroblasts (VERO cells). The assay was performed in 96-well tissue culture-treated plates as described earlier (Mustafa et al., 2004). In brief, cells were seeded in the wells of a 96-well plate (25,000 cells/well) and incubated for 24 h. Samples were added and plates were again incubated for 48 h. The number of viable cells was determined by neutral red assay. IC50 values were determined from dose curves as described above. Doxorubicin was used as a positive control, while DMSO was used as vehicle control.
Results and discussion
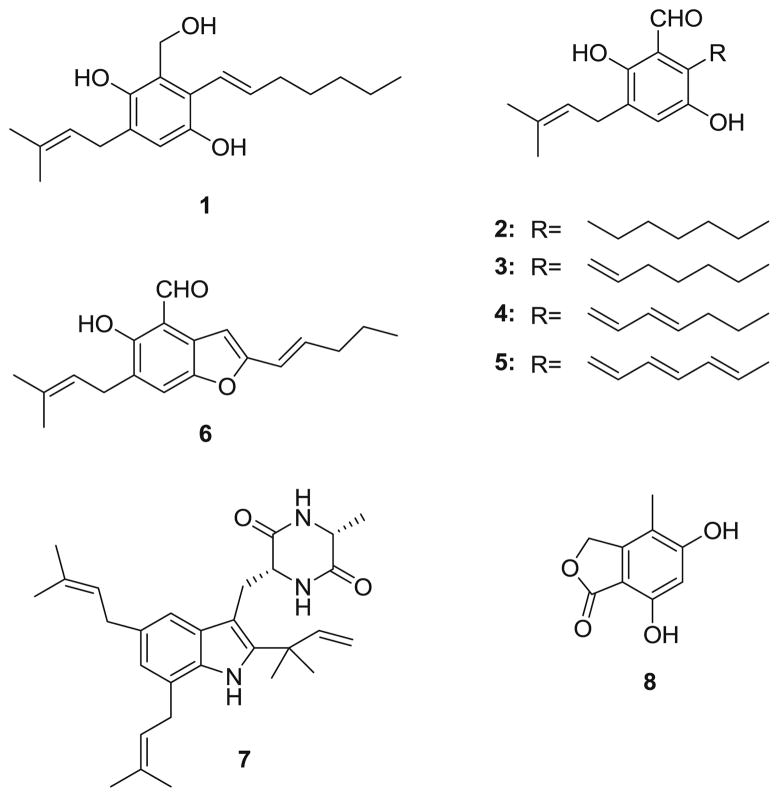
In a high-throughput screening employing a receptor binding assay to explore natural products with selective affinity for specific opioid receptors and specific cannabinoid receptors, which could provide novel drug leads for neuropathic pain, we isolated and identified eight compounds 1–8 (Fig. 1) from the fungus E. repens, (E)-2-(hept-1-enyl)-3-(hydroxymethyl)-5-(3-methylbut-2-enyl)benzene-1,4-diol (1), flavoglaucin (2), tetrahydroauroglaucin (3), dihydroauroglaucin (4), auroglaucin (5), 2-(2′,3-epoxy-1′,3′-heptadienyl)-6-hydroxy-5-(3-methyl-2-butenyl)benzaldehyde (6), one diketopiperazine alkaloid echinulin (7), and 5,7-dihydroxy-4-methylphthalide (8) (Gao et al., 2011). Most of them exhibited good binding affinities on opioid or cannabinoid receptors. Furthermore, all these compounds have been assayed for specific activities by a variety of groups. For example, 5,7-dihydroxy-4-methylphthalide (8) exhibited immunosuppressive activity (Fujimoto et al. 1999). tetrahydroauroglaucin (3) showed cytotoxic activity against sex cells of the sea urchin Strongylocentrotus intermedius at a concentration of 0.5 mg/l (Smetanina et al., 2007). The toxicity of echinulin has been studied by several groups. Echinulin (7) exhibited cytotoxicity to HeLa cells at the concentration of 100 mg/l (Umeda et al., 1974; Wang et al., 2006) and caused severe damage of alveolar organization and thickening of alveolar walls and liver damage (Ali et al., 1989). However, echinulin (7) was not genotoxic (Mori et al., 1984). Furthermore, swine refused to eat food containing 8 μg/l and drinking refusal in mice was attributed to echinulin (7) in the water at 90 mg/l (Vesonder et al., 1988). Flavoglaucin (2) was cytotoxic to HeLa cells (Umeda et al., 1974), caused hepatic damage in rabbits (Nazar et al., 1984), inhibited mitochondrial respiration and induced mitochondrial swelling (Kawai et al., 1986). Furthermore, flavoglaucin (2), tetrahydroauroglaucin (3), dihydroauroglaucin (4), auroglaucin (5), and 2-(2′,3-epoxy-1′,3′-heptadienyl)-6-hydroxy-5-(3-methyl-2-butenyl)benzaldehyde (6) exhibited antioxidant activity and radical scavenging activity against DPPH (Mikaye et al., 2009; Ishikawa et al., 1984; Li et al., 2006). A recent study indicated that flavoglaucin (2), tetrahydroauroglaucin (3), dihydroauroglaucin (4), and auroglaucin (5) all inhibited mouse skin tumor promotion in a two-stage carcinogenesis (Mikaye et al., 2010).
Fig. 1.
The structures of compounds 1–8
The good biological activities spectrum of the compounds 1–8 made us realize that they all have strong potentials in drug discovery, especially the treatment of diseases caused by bacteria, fungi, and protozoa, which became serious and common worldwide. Furthermore, there are not any reports on the antibacterial, antifungal and antimalarial activities of these compounds. We decided to evaluate the compounds 1–8 for their antibacterial, antifungal, and antimalarial activities and assess the structure– activity relationships (SARs) of these compounds. The antibacterial activities were evaluated against S. aureus, methicillin-resistant S. aureus (MRS), E. coli, P. aeruginosa, and M. intracellulare. None of these compounds showed in vitro antibacterial activity against E. coli, P. aeruginosa, and M. intracellulare (data not shown). Flavoglaucin (2), tetrahydroauroglaucin (3), and 2-(2′,3-epoxy-1′,3′-heptadienyl)-6-hydroxy-5-(3-methyl-2-butenyl)benzaldehyde (6) exhibited good antibacterial activity against S. aureus with IC50 values of 14.32, 13.51, and 7.75 μg/ml, respectively, and (E)-2-(hept-1-enyl)-3-(hydroxymethyl)-5-(3-methylbut-2-enyl) benzene-1,4-diol (1), flavoglaucin (2), and 2-(2′,3-epoxy-1′,3′-heptadienyl)-6-hydroxy-5-(3-methyl-2-butenyl)-benzaldehyde (6) were active against methicillin-resistant S. aureus (MRS) with IC50 values of 11.97, 10.41, and 5.40 μg/ml, respectively, while the rest of the compounds were inactive. Ciprofloxacin was included as a positive control for antibacterial activity (Table 1). The antifungal activities were evaluated against a panel of pathogenic fungi (C. albicans, C. glabrata, C. krusei, C. neoformans, and A. fumigatus) associated with opportunistic infections. Amphotericin B was included as a standard antifungal drug for comparison. None of the compounds showed in vitro antifungal activity against C. albicans and A. fumigatus (data not shown). (E)-2-(hept-1-enyl)-3-(hydroxymethyl)-5-(3-methylbut-2-enyl)benzene-1,4-diol (1), tetrahydroauroglaucin (3), dihydroauroglaucin (4), auroglaucin (5), and 2-(2′,3-epoxy-1′,3′-heptadienyl)-6-hydroxy-5-(3-methyl-2-butenyl) benzaldehyde (6) showed antifungal activity against C. glabrata with IC50 values of 7.17, 6.15, 2.39, 7.33, and 1.13 μg/ml, respectively. 2-(2′,3-epoxy-1′,3′-heptadienyl)-6-hydroxy-5-(3-methyl-2-butenyl)benzaldehyde (6) and 5,7-dihydroxy-4-methylphthalide (8) also showed activity against C. neoformans with IC50 values of 5.31 and 18.08 μg/ml, respectively. Among these compounds, only auroglaucin (5) exhibited moderate antifungal activity against C. krusei with an IC50 value of 10.93 μg/ml (Table 1).
Table 1.
In vitro antimicrobial activity of 1–8 (IC50 in μg/ml)
| Compounds | Antifungal
|
Antibacterial
|
|||
|---|---|---|---|---|---|
| C. glabrata | C. krusei | C. neoformansa | S. aureus | MRS | |
| 1 | 7.17 | na | na | na | 11.97 |
| 2 | na | na | na | 14.32 | 10.41 |
| 3 | 6.15 | na | na | 13.51 | na |
| 4 | 2.39 | na | na | na | na |
| 5 | 7.33 | 10.93 | na | na | na |
| 6 | 1.13 | na | 5.31 | 7.75 | 5.40 |
| 7 | na | na | na | na | na |
| 8 | na | na | 18.08 | na | na |
| Amphotericin B | 0.32 | 0.36 | 1.23 | nt | nt |
| Ciprofloxacin | nt | nt | nt | 0.10 | 0.10 |
IC50 = the test concentration that affords 50% inhibition of growth. MRS methicillin-resistant S. aureus, na not active at the highest test concentration of 20 μg/ml, nt not tested
Cryptococcus neoformans
Antimalarial activity was determined in vitro against chloroquine-sensitive (D6, Sierra Leone) and chloroquine-resistant (W2, Indo China) strains of P. falciparum by measuring plasmodial LDH activity. (E)-2-(hept-1-enyl)-3-(hydroxymethyl)-5-(3-methylbut-2-enyl)benzene-1,4-diol (1), flavoglaucin (2), tetrahydroauroglaucin (3), auroglaucin (5), and 2-(2′,3-epoxy-1′,3′-heptadienyl)-6-hydroxy-5-(3-methyl-2-butenyl)benzaldehyde (6) exhibited moderate antimalarial activities with IC50 values ranging from 1.1 to 3.0 μg/ml (Table 2). Antimalarial activity of auroglaucin (5) was stronger than others auroglaucin (5) (IC50 1.8 and 1.1 μg/ml against D6 and W2, respectively). Although dihydroauroglaucin (4) is structurally similar to (E)-2-(hept-1-enyl)-3-(hydroxymethyl)-5-(3-methylbut-2-enyl) benzene-1,4-diol (1), flavoglaucin (2), tetrahydroauroglaucin (3), and auroglaucin (5); 2-(2′,3-epoxy-1′,3′-heptadienyl)-6-hydroxy-5-(3-methyl-2-butenyl)benzaldehyde (6) and dihydroauroglaucin (4) were found to be inactive.
Table 2.
In vitro antiprotozoal activity and cytotoxicity of 1–8 (IC50 in μg/ml)
| Compounds | Antileishmanial | Antimalarial
|
Cytotoxicity vero cells | |
|---|---|---|---|---|
| L. donovani |
P. falciparum
|
|||
| D6 | W2 | |||
| 1 | 19 | 3.0 | 2.8 | nc |
| 2 | 23 | 3.0 | 2.7 | nc |
| 3 | 22 | 2.8 | 2.3 | nc |
| 4 | 20 | na | na | nc |
| 5 | 7.5 | 1.8 | 1.1 | nc |
| 6 | 6.2 | 3.0 | 2.8 | nc |
| 7 | na | na | na | nc |
| 8 | na | na | na | nc |
| Pentamidine | 1 | nt | nt | nt |
| Chloroquine | nt | 0.016 | 0.155 | nt |
| Doxorubicin | nt | nt | nt | 7.5 |
IC50 = the test concentration that affords 50% inhibition of growth compared to the solvent control. na not active, nt not tested, nc not cytotoxic
Antileishmanial activity against Leishmania donovani promastigotes was determined by Alamar BlueTM assay. Most of these compounds showed moderate to mild activities with IC50 values ranging from 6.2 to 23 μg/ml (Table 2). Auroglaucin (5) and 2-(2′,3-epoxy-1′,3′-heptadienyl)-6-hydroxy-5-(3-methyl-2-butenyl)benzaldehyde (6) were more active than others with IC50 values of 7.5 and 6.2 μg/ml, respectively.
None of these compounds showed any toxicity toward mammalian kidney fibroblast (Vero cells). Echinulin (7) was inactive in all bioassays.
From the results above, we find that all the active compounds, (E)-2-(hept-1-enyl)-3-(hydroxymethyl)-5-(3-methylbut-2-enyl)benzene-1,4-diol (1), flavoglaucin (2), tetrahydroauroglaucin (3), auroglaucin (5), and 2-(2′,3-epoxy-1′,3′-heptadienyl)-6-hydroxy-5-(3-methyl-2-butenyl) benzaldehyde (6), have common structures. They all are benzyl derivatives with a dimethyl allyl and a seven carbon chain moiety. Concise structure and activity relationships of this type of compounds are summarized. For antibacterial activity, the number of double bonds in the seven carbon chain is important for maintaining this kind of activity. Compounds lacking a double bond or possessing only one double bond are active while compounds possessing two or three double bonds have a loss of antibacterial activity (dihydroauroglaucin (4), auroglaucin (5) exhibited no antibacterial activity). Furthermore, an aldehyde functionality is crucial for selectivity because (E)-2-(hept-1-enyl)-3-(hydroxymethyl)-5-(3-methylbut-2-enyl) benzene-1,4-diol (1) showed antibacterial activity against methicillin-resistant S. aureus (MRS) and tetrahydroauroglaucin (3) exhibited antibacterial activities against S. aureus. A double bond is also crucial for maintaining antifungal activity. The lack of a double bond in the seven carbon chain can result in the loss of antifungal activity against C. glabrata, C. krusei, C. neoformans. Aldehyde functionality is not important for antifungal activity because (E)-2-(hept-1-enyl)-3-(hydroxymethyl)-5-(3-methylbut-2-enyl)benzene-1,4-diol (1), and tetrahydroauroglaucin (3) have similar antifungal activities. For antimalarial activity, two double bonds can lead to the loss of antimalarial activity. However, three double bonds allows for the most potent antimalarial activity. Aldehyde functionality is not important for this type of activity because (E)-2-(hept-1-enyl)-3-(hydroxymethyl)-5-(3-methylbut-2-enyl)benzene-1,4-diol (1) and tetrahydroauroglaucin (3) have similar antimalarial activities. (E)-2-(hept-1-enyl)-3-(hydroxymethyl)-5-(3-methylbut-2-enyl)benzene-1,4-diol (1), flavoglaucin (2), tetrahydroauroglaucin (3), dihydroauroglaucin (4), auroglaucin (5), and 2-(2′,3-epoxy-1′,3′-heptadienyl)-6-hydroxy-5-(3-methyl-2-butenyl)benzaldehyde (6) exhibited good antileishmanial activity. 2-(2′,3-epoxy-1′,3′-heptadienyl)-6-hydroxy-5-(3-methyl-2-butenyl) benzaldehyde (6) showed the most potent and the double bonds in the chain maintained a high activity (IC50 is 6.2 μg/ml).
The antibacterial, antifungal, and antimicrobial potency of benzyl derivatives usually depend on the substitutions. First, previous literature mentioned that the aldehyde (–CHO) group of benzaldehyde could lead to more antibacterial activity than the carboxyl (–COOH) group. Our results showed that the methoxy (–CH2OH) group caused the loss of activity against S. aureus. It had no affect on the activity against MRSA, C. glabrata, L. donovani and P. falciparum. One possible mechanism proposed to explain why benzaldehydes exhibited antibacterial activities was that the benzaldhydes became covalently attached to surface SH groups of the cells (Ramos-Nino et al., 1998). However, the methoxy (–CH2OH) group incapable of forming a covalent bond with SH groups, which does not support the proposed mechanism. Second, the biological activity was affected by the unsaturation of the carbon chain substitution. Previous reports have confirmed that the unsaturation of the fatty acids can affect the biological activity (Sun et al., 2003). For example, the potency of (9Z)-hexadecenoic acid (palmitoleic acid; C16:1N-7) was about twice as much as (6Z, 9Z, 12Z)-hexadecatrienoic acid (HTA; C16:3N-4) against S. aureus (Desbois et al., 2008). The mechanism of action remains unknown but suggestions have been made that the cell membranes could be as the main target. Our results confirmed that the number and position of double bonds of long carbon chain substitution in these compounds have an important role in antimicrobial and antiprotozoal activities. We proposed that the fatty acid substitution of these compounds may interact with cell membranes leading to leakage, reduction of nutrient uptake or inhibition of cellular respiration and the number and position of the double bond can change the lipophilicity of the compounds, then affecting their interaction with cell membranes.
In conclusion, we have reported the antibacterial, antifungal, antimalarial, and antileishmanial activities of eight benzyl derivatives against seven human pathogens. The results have developed a better understanding of SARs of benzyl derivatives which have good potential in drug discovery.
Acknowledgments
This study was supported by a Grant Number 5P20RR021929 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH). NIAID, Division of AIDS, Grant No. AI 27094 and USDA Agricultural Research Service Specific Cooperative Agreement No. 58-6408-2-0009 are acknowledged for partial support of the study. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health. Furthermore, this investigation was conducted in a facility constructed with support from research facilities improvement program C06 RR-14503-01 from the NIH National Center for Research Resources.
Contributor Information
Jiangtao Gao, Department of Medicinal Chemistry, School of Pharmacy, The University of Mississippi, Faser Hall 417, University, MS 38677, USA.
Mohamed M. Radwan, National Center for Natural Products Research, Research Institute of Pharmaceutical Sciences, School of Pharmacy, University of Mississippi, University Mississippi 38677, USA. Department of Pharmacognosy, Faculty of Pharmacy, University of Alexandria, Alexandria, Egypt
Francisco León, Department of Medicinal Chemistry, School of Pharmacy, The University of Mississippi, Faser Hall 417, University, MS 38677, USA.
Xiaoning Wang, Department of Medicinal Chemistry, School of Pharmacy, The University of Mississippi, Faser Hall 417, University, MS 38677, USA.
Melissa R. Jacob, National Center for Natural Products Research, Research Institute of Pharmaceutical Sciences, School of Pharmacy, University of Mississippi, University Mississippi 38677, USA
Babu L. Tekwani, National Center for Natural Products Research, Research Institute of Pharmaceutical Sciences, School of Pharmacy, University of Mississippi, University Mississippi 38677, USA. Department of Pharmacology, University of Mississippi, University, Mississippi 38677, USA
Shabana I. Khan, National Center for Natural Products Research, Research Institute of Pharmaceutical Sciences, School of Pharmacy, University of Mississippi, University Mississippi 38677, USA
Shari Lupien, USDA-ARS Western Regional Plant Introduction Station, Washington State University, Pullman WA 99164, USA.
Robert A. Hill, Bio-Protection Research Centre, Lincoln University, Lincoln 7647, New Zealand
Frank M. Dugan, USDA-ARS Western Regional Plant Introduction Station, Washington State University, Pullman WA 99164, USA
Horace G. Cutler, Natural Products Discovery Group, College of Pharmacy and Health Sciences, Mercer University, Atlanta, GA 30341, USA
Stephen J. Cutler, Email: cutler@olemiss.edu, Department of Medicinal Chemistry, School of Pharmacy, The University of Mississippi, Faser Hall 417, University, MS 38677, USA. National Center for Natural Products Research, Research Institute of Pharmaceutical Sciences, School of Pharmacy, University of Mississippi, University Mississippi 38677, USA
References
- Ali M, Mohammed N, Alnaqeeb M, Hassan R, Ahmad H. Toxicity of echinulin from Aspergillus chevalieri in rabbits. Toxicol Lett. 1989;48:235–241. doi: 10.1016/0378-4274(89)90049-0. [DOI] [PubMed] [Google Scholar]
- Berman J. Treatment of leishmaniasis with miltefosine: 2008 status. Expert Opin Drug Metab Toxicol. 2008;4:1209–1216. doi: 10.1517/17425255.4.9.1209. [DOI] [PubMed] [Google Scholar]
- Croft SL, Sundar S, Fairlamb AH. Drug resistance in leishmaniasis. Clin Microbiol Rev. 2006;19:111–126. doi: 10.1128/CMR.19.1.111-126.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desbois AP, Lebl T, Yan LM, Smith VJ. Isolation and structural characterization of two antibacterial free fatty acids from the marine diatom, Phaeodactylum tricornutum. Appl Microbiol Biotechnol. 2008;81:755–764. doi: 10.1007/s00253-008-1714-9. [DOI] [PubMed] [Google Scholar]
- Franzblau SG, Witzig RS, McLaughlin JC, Torres P, Madico G, Hernandez A, Degnan MT, Cook MB, Quenzer VK, Ferguson RM, Gilman RH. Rapid, low-technology MIC determination with clinical Mycobacterium tuberculosis isolates by using the microplate Alamar Blue assay. J Clin Microbiol. 1998;36:362–366. doi: 10.1128/jcm.36.2.362-366.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto H, Fujimaki T, Okuyama E, Yamazaki M. Immunomodulatory constituents from an ascomycete, Microascus tardifaciens. Chem Pharm Bull. 1999;47:1426–1432. doi: 10.1248/cpb.47.1426. [DOI] [PubMed] [Google Scholar]
- Gao J, León F, Radwan MM, Dale OR, Gemelli CA, Manly SP, Lupien S, Wang X, Hill RA, Dugan FM, Cutler HG, Cutler SJ. Benzyl derivatives with in Vitro binding affinity for human opioid receptors and cannabinoid Receptors from the Fungus Eurotium repens. J Nat Prod. 2011;74:1636. doi: 10.1021/np200147c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gastmeier P, Sohr D, Geffers C, Behnke M, Ruden H. Risk factors for death due to nosocomial infection in intensive care unit patients: findings from the krankenhaus infektions surveillance system. Infect Control Hosp Epidemiol. 2007;28:466–472. doi: 10.1086/510810. [DOI] [PubMed] [Google Scholar]
- Gullo A. Invasive fungal infections the challenge continues. Drugs. 2009;69:65–73. doi: 10.2165/11315530-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Ishikawa Y, Morimoto K, Hamasaki T. Flavoglaucin, a metabolite of Eurotium chevalieri, its antoxidation and synergism with tocopherol. J Am Oil Chem Soc. 1984;61:1864–1868. [Google Scholar]
- Kawai K, Hisada K, Nakamaru T, Nozawa Y, Kitamura J. Inhibition of mitochondrial electron transport system by flavoglaucin from Eurotium chevalieri. II. The interaction with complex III. Mycotoxins. 1986;24:13–18. [Google Scholar]
- Li Y, Li XF, Lee U, Kang JS, Choi HD, Sona BW. A new radical scavenging anthracene glycoside, asperflavin ribofuranoside, and polyketides from a marine isolate of the fungus Microsporum. Chem Pharm Bull. 2006;54:882–883. doi: 10.1248/cpb.54.882. [DOI] [PubMed] [Google Scholar]
- Ma GY, Khan SI, Jacob MR, Tekwani BL, Li ZQ, Pasco DS, Walker LA, Khan LA. Antimicrobial and antileishmanial activities of hypocrellins A and B. Antimicrob Agents Chemother. 2004;48:4450–4452. doi: 10.1128/AAC.48.11.4450-4452.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makler MT, Hinrichs DJ. Measurement of the lactatede-hydrogenase activity of Plasmodium falciparum as an assessment of parasitemia. Am J Trop Med Hyg. 1993;48:205–210. doi: 10.4269/ajtmh.1993.48.205. [DOI] [PubMed] [Google Scholar]
- Maschmeyer G, Ruhnke M. Update on antifungal treatment of invasive Candida and Aspergillus infections. Mycoses. 2004;47:263–276. doi: 10.1111/j.1439-0507.2004.01003.x. [DOI] [PubMed] [Google Scholar]
- Masubuchi M, Ebiike H, Kawasaki E, Sogabe S, Morikami K, Shiratori Y, Tsujii S, Fujii T, Sakata K, Hayase M, Shindoh H, Aoki Y, Ohtsuka T, Shimma N. Synthesis and biological activities of benzofuran antifungal agents targeting fungal N-myristoyl transferase. Bioorg Med Chem. 2003;11:4463–4478. doi: 10.1016/s0968-0896(03)00429-2. [DOI] [PubMed] [Google Scholar]
- Mikaye Y, Ito C, Itoigawa M, Osawa T. Antioxidants produced by eurotium herbariorum of filamentous fungi used for the manufacture of karebushi, dried bonito (katsuobushi) Biosci Biotechnol Biochem. 2009;73:1323–1327. doi: 10.1271/bbb.80887. [DOI] [PubMed] [Google Scholar]
- Mikaye Y, Ito C, Tokuda H, Osawa T, Itoigawa M. Evaluation of flavoglaucin, its derivatives and pyranonigrins produced by molds used in fermented foods for inhibiting tumor promotion. Biosci Biotechnol Biochem. 2010;74:1120–1122. doi: 10.1271/bbb.90955. [DOI] [PubMed] [Google Scholar]
- Mori H, Kawai K, Ohbayashi F, Kuniyasu T, Yamazaki M, Hamasaki T, Williams G. Genotoxicity of a variety of mycotoxins in the hepatocyte primary culture/DNA repair test using rat and mouse hepatocytes. Cancer Res. 1984;44:2918–2923. [PubMed] [Google Scholar]
- Mustafa J, Khan SI, Ma GY, Walker LA, Khan IA. Synthesis and anticancer activities of fatty acid analogs of podophyllotoxin. Lipids. 2004;39:167–172. doi: 10.1007/s11745-004-1215-5. [DOI] [PubMed] [Google Scholar]
- Nazar M, Ali M, Fatima T, Gubler C. Toxicity of flavoglaucin from Aspergillus chevalieri in rabbits. Toxicol Lett. 1984;23:233–237. doi: 10.1016/0378-4274(84)90132-2. [DOI] [PubMed] [Google Scholar]
- Neuber H. Leishmaniasis. J Dtsch Dermatol Ges. 2008;6:754–764. doi: 10.1111/j.1610-0387.2008.06809.x. [DOI] [PubMed] [Google Scholar]
- Peleg AY, Hooper DC. Current concepts hospital-acquired infections due to gram-negative bacteria. N Engl J Med. 2010;362:1804–1813. doi: 10.1056/NEJMra0904124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Nino ME, Ramirez-Rodriguez CA, Clifford MN, Adams MR. QSARs for the effect of benzaldehydes on foodborne bacteria and the role of sulfhydryl groups as targets of their antibacterial activity. J Appl Microbil. 1998;84:207–212. doi: 10.1046/j.1365-2672.1998.00324.x. [DOI] [PubMed] [Google Scholar]
- Reithinger R, Dujardin JC, Louzir H, Pirmez C, Alexander B, Brooker S. Cutaneous leishmaniasis. Lancet Infect Dis. 2007;7:581–596. doi: 10.1016/S1473-3099(07)70209-8. [DOI] [PubMed] [Google Scholar]
- Samoylenko V, Jacob MR, Khan SI, Zhao J, Tekwani BL, Midiwo JO, Walker LA, Muhammad I. Antimicrobial, antiparasitic and cytotoxic spermine alkaloids from Albizia schimperiana. Nat Prod Commun. 2009;4:791–796. [PMC free article] [PubMed] [Google Scholar]
- Santos DO, Coutinho CER, Madeira MF, Bottino CG, Vieira RT, Nascimento SB, Bernardino A, Bourguignon SC, Corte-Real S, Pinho RT, Rodrigues CR, Castro HC. Leishmaniasis treatment—a challenge that remains: a review. Parasitol Res. 2008;103:1–10. doi: 10.1007/s00436-008-0943-2. [DOI] [PubMed] [Google Scholar]
- Smetanina OF, Kalinovskii AI, Khudyakova YV, Slinkina NN, Pivkin MV, Kuznetsova TA. Metabolites from the marine fungus Eurotium repens. Chem Nat Compd. 2007;43:395–398. [Google Scholar]
- Sun CQ, O’Connor CJ, Roberton AM. Antibacterial actions of fatty acids and monoglycerides against Helicobacter pylori. FEMS Immunol Med Microbiol. 2003;36:9–17. doi: 10.1016/S0928-8244(03)00008-7. [DOI] [PubMed] [Google Scholar]
- Talbot GH, Bradley J, Edwards JE, Gilbert D, Scheld M, Bartlett JG. Bad bugs need drugs: an update on the development pipeline from the Antimicrobial Availability Task Force of the Infectious Diseases Society of America. Clin Infect Dis. 2006;42:657–668. doi: 10.1086/499819. [DOI] [PubMed] [Google Scholar]
- Umeda M, Yamashita T, Saito M, Skeita S, Takahashi C, Yoshihira K, Natori S, Kurata H, Udagawa S. Chemical and cytotoxicity survey on the metabolites of toxic fungi. Jpn J Exp Med. 1974;44:83–96. [PubMed] [Google Scholar]
- Vesonder RF, Lambert R, Wicklow DT, Biehl ML. Eurotium spp. and echinulin in feed refused by swine. Appl Environ Microbiol. 1988;54:830–831. doi: 10.1128/aem.54.3.830-831.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Li XM, Teuscher F, Li DL, Diesel A, Ebel R, Proksch P, Wang BG. Chaetopyranin, a benzaldehyde derivative, and other related metabolites from Chaetomium globosum an endophytic fungus derived from the marine red alga Polysiphonia urceolata. J Nat Prod. 2006;69:1622–1625. doi: 10.1021/np060248n. [DOI] [PubMed] [Google Scholar]
- Wells TNC, Alonso PL, Gutteridge WE. New medicines to improve control and contribute to the eradication of malaria. Nat Rev Drug Discov. 2009;8:879–891. doi: 10.1038/nrd2972. [DOI] [PubMed] [Google Scholar]
- Wright CW. Recent developments in research on terrestrial plants used for the treatment of malaria. Nat Prod Rep. 2010;27:961–968. doi: 10.1039/c002196m. [DOI] [PubMed] [Google Scholar]