Abstract
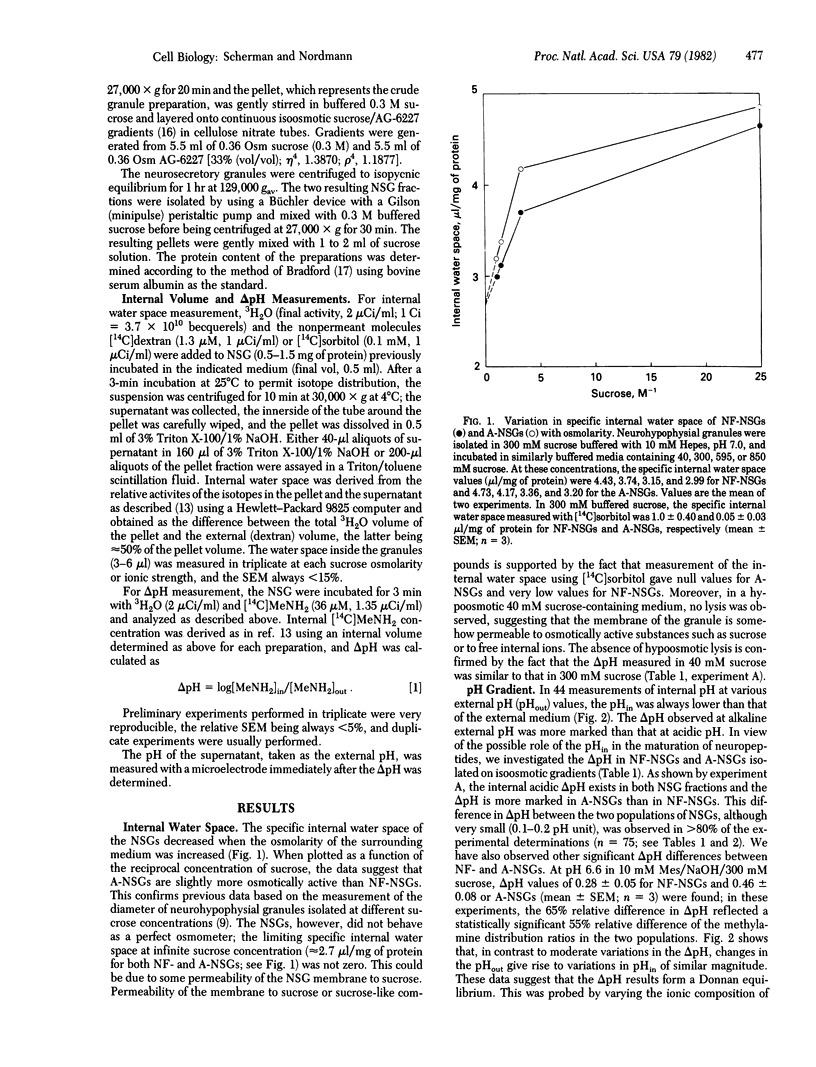
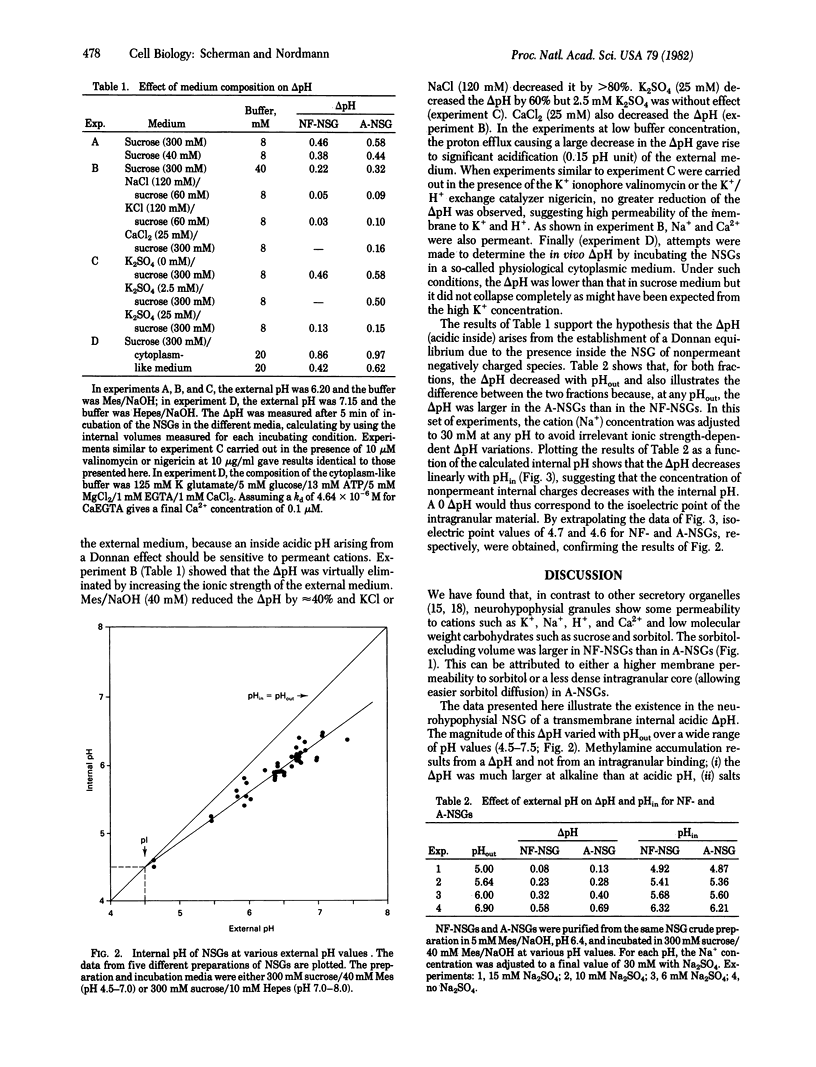
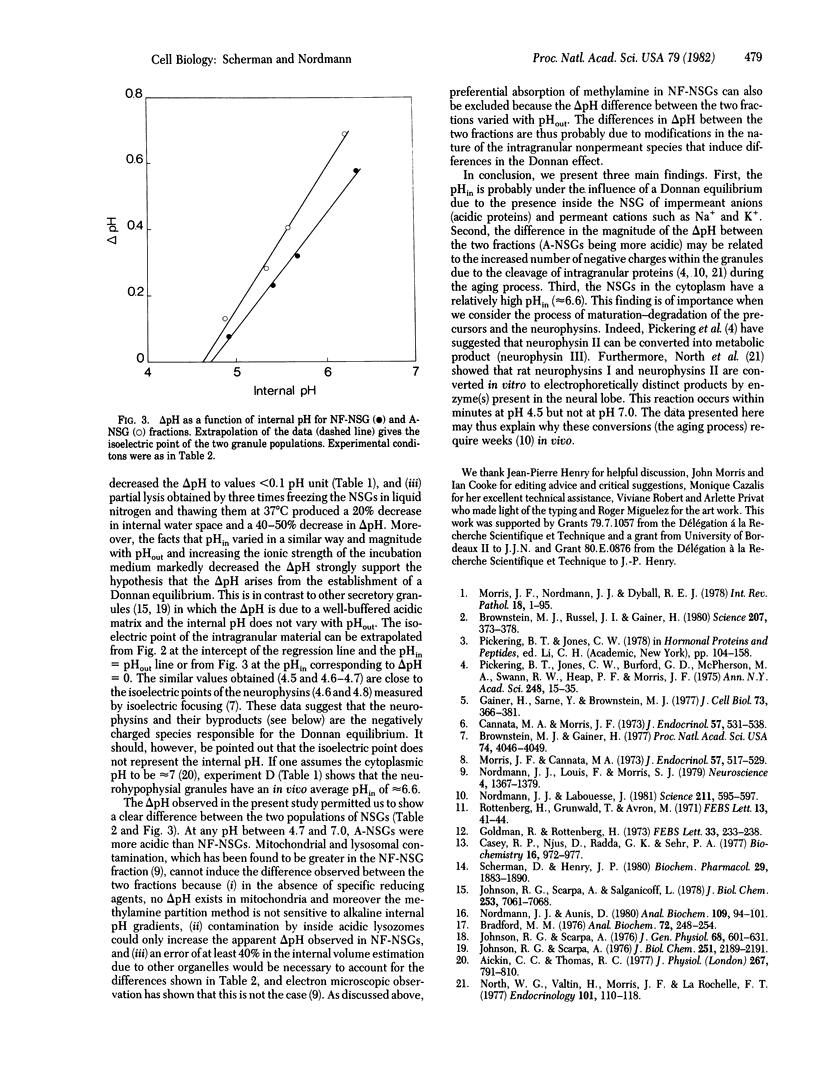
The pH gradient (delta pH) across the membrane of bovine neurohypophysial granules was estimated by using the [14C]methylamine partition technique. The granule membrane showed high permeability to sugars such as sucrose or sorbitol and to cations. Granules suspended in sucrose medium showed an acidic internal delta pH that decreased with external pH from 1.0 pH unit at pH 7.5 to 0 at pH 4.5. High ionic strength of the external medium destroyed the delta pH, indicating that it originated from a Donnan equilibrium. In a medium constituted to simulate cytoplasm, the delta pH was approximately 0.5 pH unit. Aged neurosecretory granules were more acidic inside than were newly formed granules. The results are discussed in relationship to the nature and the duration of maturation and degradation process of the granule matrix.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brownstein M. J., Gainer H. Neurophysin biosynthesis in normal rats and in rats with hereditary diabetes insipidus. Proc Natl Acad Sci U S A. 1977 Sep;74(9):4046–4049. doi: 10.1073/pnas.74.9.4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownstein M. J., Russell J. T., Gainer H. Synthesis, transport, and release of posterior pituitary hormones. Science. 1980 Jan 25;207(4429):373–378. doi: 10.1126/science.6153132. [DOI] [PubMed] [Google Scholar]
- Cannata M. A., Morris J. F. Changes in the appearance of hypothalamo-neurohypophysial neurosecretory granules associated with their maturation. J Endocrinol. 1973 Jun;57(3):531–538. doi: 10.1677/joe.0.0570531. [DOI] [PubMed] [Google Scholar]
- Casey R. P., Njus D., Radda G. K., Sehr P. A. Active proton uptake by chromaffin granules: observation by amine distribution and phosphorus-31 nuclear magnetic resonance techniques. Biochemistry. 1977 Mar 8;16(5):972–977. doi: 10.1021/bi00624a025. [DOI] [PubMed] [Google Scholar]
- Gainer H., Sarne Y., Brownstein M. J. Biosynthesis and axonal transport of rat neurohypophysial proteins and peptides. J Cell Biol. 1977 May;73(2):366–381. doi: 10.1083/jcb.73.2.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman R., Rottenberg H. Ion distribution in lysosomal suspensions. FEBS Lett. 1973 Jul 1;33(2):233–238. doi: 10.1016/0014-5793(73)80200-5. [DOI] [PubMed] [Google Scholar]
- Johnson R. G., Scarpa A. Internal pH of isolated chromaffin vesicles. J Biol Chem. 1976 Apr 10;251(7):2189–2191. [PubMed] [Google Scholar]
- Johnson R. G., Scarpa A. Ion permeability of isolated chromaffin granules. J Gen Physiol. 1976 Dec;68(6):601–631. doi: 10.1085/jgp.68.6.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. G., Scarpa A., Salganicoff L. The internal pH of isolated serotonin containing granules of pig platelets. J Biol Chem. 1978 Oct 10;253(19):7061–7068. [PubMed] [Google Scholar]
- Micro-electrode measurement of the intracellular pH and buffering power of mouse soleus muscle fibres. J Physiol. 1977 Jun;267(3):791–810. doi: 10.1113/jphysiol.1977.sp011838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J. F., Cannata M. A. Ultrastructural preservation of the dense core of posterior pituitary neurosecretory granules and its implications for hormone release. J Endocrinol. 1973 Jun;57(3):517–529. doi: 10.1677/joe.0.0570517. [DOI] [PubMed] [Google Scholar]
- Morris J. F., Nordmann J. J., Dyball R. E. Structure-function correlation in mammalian neurosecretion. Int Rev Exp Pathol. 1978;18:1–95. [PubMed] [Google Scholar]
- Nordmann J. J., Aunis D. Distribution of secretory granules from adrenal medulla and neurohypophysis on continuous isoosmotic density gradients formed with a new ioxaglic derivative AG-6227. Anal Biochem. 1980 Nov 15;109(1):94–101. doi: 10.1016/0003-2697(80)90015-9. [DOI] [PubMed] [Google Scholar]
- Nordmann J. J., Labouesse J. Neurosecretory granules: evidence from an aging process within the neurohypophysis. Science. 1981 Feb 6;211(4482):595–597. doi: 10.1126/science.7455700. [DOI] [PubMed] [Google Scholar]
- Nordmann J. J., Louis F., Morris S. J. Purification of two structurally and morphologically distinct populations of rat neurohypophysial secretory granules. Neuroscience. 1979;4(9):1367–1379. doi: 10.1016/0306-4522(79)90164-7. [DOI] [PubMed] [Google Scholar]
- North W. G., Valtin H., Morris J. F., La Rochelle F. T., Jr Evidence for metabolic conversions of rat neurophysins within neurosecretory granules of the hypothalamo-neurohypophysial system. Endocrinology. 1977 Jul;101(1):110–118. doi: 10.1210/endo-101-1-110. [DOI] [PubMed] [Google Scholar]
- Pickering B. T., Jones C. W., Burford G. D., McPherson M., Swann R. W., Heap P. F., Morris J. F. The role of neurophysin proteins: suggestions from the study of their transport and turnover. Ann N Y Acad Sci. 1975 Feb 21;248:15–35. doi: 10.1111/j.1749-6632.1975.tb34174.x. [DOI] [PubMed] [Google Scholar]
- Rottenberg H., Grunwald T., Avron M. Direct determination of DeltapH in chloroplasts, and its relation to the mechanisms of photoinduced reactions. FEBS Lett. 1971 Feb 12;13(1):41–44. doi: 10.1016/0014-5793(71)80659-2. [DOI] [PubMed] [Google Scholar]
- Scherman D., Henry J. P. Effect of drugs on the ATP-induced and pH-gradient-driven monoamine transport by bovine chromaffin granules. Biochem Pharmacol. 1980 Jul 1;29(13):1883–1890. doi: 10.1016/0006-2952(80)90098-2. [DOI] [PubMed] [Google Scholar]


