Abstract
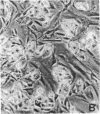
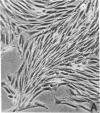
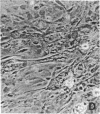
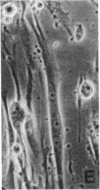
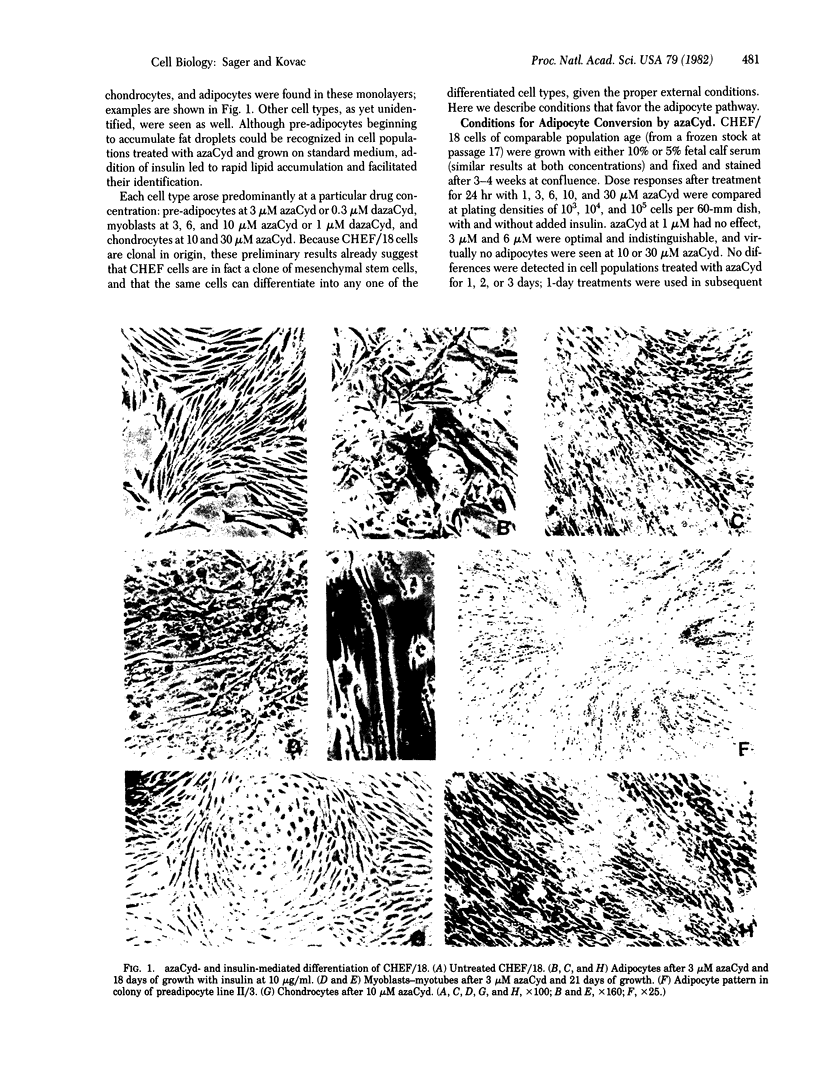
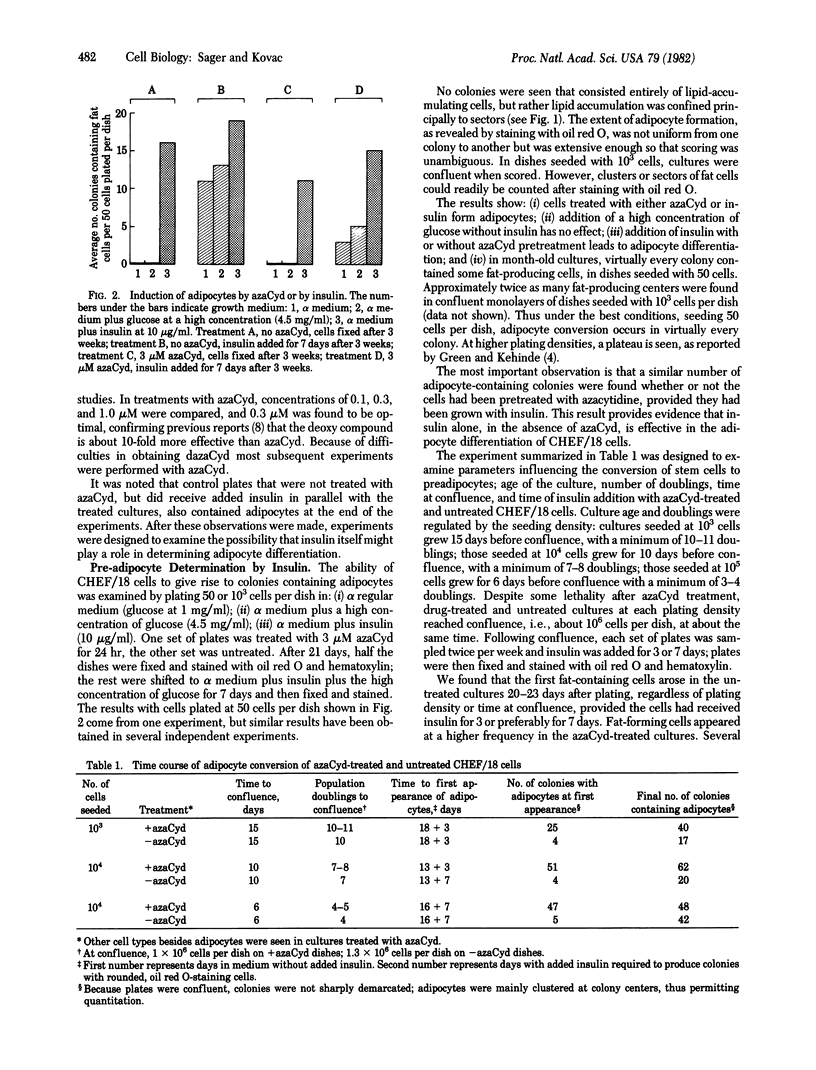
CHEF/18 is a diploid Chinese hamster cell line of embryonic origin, which is fibroblastic in structure, but behaves like a mesenchymal stem cell line in its ability to differentiate into adipocytes, myoblasts, and chondrocytes. With these cells, adipocyte formation has been divided experimentally into two stages: (i) determination of pre-adipocytes, which have lost the ability to form other cell types while retaining their fibroblast structure; and (ii) commitment or terminal differentiation, in which lipids accumulate, adipocyte structure develops, and cells lose the ability to divide. This paper reports that the first stage can be induced by exposure to 5-azacytidine or 2'-deoxy-5-azacytidine, drugs that also induce CHEF cells to form other mesenchymal cell types, or by growth with added insulin. Pre-adipocytes are distinguished from CHEF stem cells by (i) their inability to form other mesenchymal cell types; and (ii) their rapid accumulation of lipid in response to added insulin. The possibility is discussed that both insulin and the cytidine analogs promote differentiation by the same mechanism, namely changes in the pattern of DNA methylation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arber W. Promotion and limitation of genetic exchange. Science. 1979 Jul 27;205(4404):361–365. doi: 10.1126/science.377489. [DOI] [PubMed] [Google Scholar]
- Blundell T. L., Humbel R. E. Hormone families: pancreatic hormones and homologous growth factors. Nature. 1980 Oct 30;287(5785):781–787. doi: 10.1038/287781a0. [DOI] [PubMed] [Google Scholar]
- Burton W. G., Grabowy C. T., Sager R. Role of methylation in the modification and restriction of chloroplast DNA in Chlamydomonas. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1390–1394. doi: 10.1073/pnas.76.3.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christman J. K., Price P., Pedrinan L., Acs G. Correlation between hypomethylation of DNA and expression of globin genes in Friend erythroleukemia cells. Eur J Biochem. 1977 Nov 15;81(1):53–61. doi: 10.1111/j.1432-1033.1977.tb11926.x. [DOI] [PubMed] [Google Scholar]
- Christman J. K., Weich N., Schoenbrun B., Schneiderman N., Acs G. Hypomethylation of DNA during differentiation of Friend erythroleukemia cells. J Cell Biol. 1980 Aug;86(2):366–370. doi: 10.1083/jcb.86.2.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinides P. G., Jones P. A., Gevers W. Functional striated muscle cells from non-myoblast precursors following 5-azacytidine treatment. Nature. 1977 May 26;267(5609):364–366. doi: 10.1038/267364a0. [DOI] [PubMed] [Google Scholar]
- Constantinides P. G., Taylor S. M., Jones P. A. Phenotypic conversion of cultured mouse embryo cells by aza pyrimidine nucleosides. Dev Biol. 1978 Sep;66(1):57–71. doi: 10.1016/0012-1606(78)90273-7. [DOI] [PubMed] [Google Scholar]
- Darmon M., Serrero G., Rizzino A., Sato G. Isolation of myoblastic, fibro-adipogenic, and fibroblastic clonal cell lines from a common precursor and study of their requirements for growth and differentiation. Exp Cell Res. 1981 Apr;132(2):313–327. doi: 10.1016/0014-4827(81)90107-5. [DOI] [PubMed] [Google Scholar]
- Ehrlich M., Wang R. Y. 5-Methylcytosine in eukaryotic DNA. Science. 1981 Jun 19;212(4501):1350–1357. doi: 10.1126/science.6262918. [DOI] [PubMed] [Google Scholar]
- Green H., Kehinde O. An established preadipose cell line and its differentiation in culture. II. Factors affecting the adipose conversion. Cell. 1975 May;5(1):19–27. doi: 10.1016/0092-8674(75)90087-2. [DOI] [PubMed] [Google Scholar]
- Green H., Kehinde O. Spontaneous heritable changes leading to increased adipose conversion in 3T3 cells. Cell. 1976 Jan;7(1):105–113. doi: 10.1016/0092-8674(76)90260-9. [DOI] [PubMed] [Google Scholar]
- Green H., Meuth M. An established pre-adipose cell line and its differentiation in culture. Cell. 1974 Oct;3(2):127–133. doi: 10.1016/0092-8674(74)90116-0. [DOI] [PubMed] [Google Scholar]
- Jakob H., Buckingham M. E., Cohen A., Dupont L., Fiszman M., Jacob F. A skeletal muscle cell line isolated from a mouse teratocarcinoma undergoes apparently normal terminal differentiation in vitro. Exp Cell Res. 1978 Jul;114(2):403–408. doi: 10.1016/0014-4827(78)90499-8. [DOI] [PubMed] [Google Scholar]
- Jones P. A., Taylor S. M. Cellular differentiation, cytidine analogs and DNA methylation. Cell. 1980 May;20(1):85–93. doi: 10.1016/0092-8674(80)90237-8. [DOI] [PubMed] [Google Scholar]
- King G. L., Kahn C. R. Non-parallel evolution of metabolic and growth-promoting functions of insulin. Nature. 1981 Aug 13;292(5824):644–646. doi: 10.1038/292644a0. [DOI] [PubMed] [Google Scholar]
- Kitchin R. M., Sager R. Genetic analysis of tumorigenesis: V. Chromosomal analysis of tumorigenic and nontumorigenic diploid chinese hamster cell lines. Somatic Cell Genet. 1980 Jan;6(1):75–87. doi: 10.1007/BF01538697. [DOI] [PubMed] [Google Scholar]
- Lapeyre J. N., Becker F. F. 5-Methylcytosine content of nuclear DNA during chemical hepatocarcinogenesis and in carcinomas which result. Biochem Biophys Res Commun. 1979 Apr 13;87(3):698–705. doi: 10.1016/0006-291x(79)92015-1. [DOI] [PubMed] [Google Scholar]
- Razin A., Riggs A. D. DNA methylation and gene function. Science. 1980 Nov 7;210(4470):604–610. doi: 10.1126/science.6254144. [DOI] [PubMed] [Google Scholar]
- Reznikoff C. A., Brankow D. W., Heidelberger C. Establishment and characterization of a cloned line of C3H mouse embryo cells sensitive to postconfluence inhibition of division. Cancer Res. 1973 Dec;33(12):3231–3238. [PubMed] [Google Scholar]
- Rosen O. M., Rubin C. S., Cobb M. H., Smith C. J. Insulin stimulates the phosphorylation of ribosomal protein S6 in a cell-free system derived from 3T3-L1 adipocytes. J Biol Chem. 1981 Apr 25;256(8):3630–3633. [PubMed] [Google Scholar]
- Rosen O. M., Smith C. J., Hirsch A., Lai E., Rubin C. S. Recent studies of the 3T3-L1 adipocyte-like cell line. Recent Prog Horm Res. 1979;35:477–499. doi: 10.1016/b978-0-12-571135-7.50015-1. [DOI] [PubMed] [Google Scholar]
- Royer H. D., Sager R. Methylation of chloroplast DNAs in the life cycle of Chlamydomonas. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5794–5798. doi: 10.1073/pnas.76.11.5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sager R., Grabowy C., Sano H. The mat-1 gene in Chlamydomonas regulates DNA methylation during gametogenesis. Cell. 1981 Apr;24(1):41–47. doi: 10.1016/0092-8674(81)90499-2. [DOI] [PubMed] [Google Scholar]
- Sager R., Kovac P. E. Genetic analysis of tumorigenesis: I. Expression of tumor-forming ability in hamster hybrid cell lines. Somatic Cell Genet. 1978 May;4(3):375–392. doi: 10.1007/BF01542849. [DOI] [PubMed] [Google Scholar]
- Sager R., Kovac P. E. Genetic analysis of tumorigenesis: IV. Chromosome reduction and marker segregation in progeny clones from Chinese hamster cell hybrids. Somatic Cell Genet. 1979 Jul;5(4):491–502. doi: 10.1007/BF01538883. [DOI] [PubMed] [Google Scholar]
- Sano H., Grabowy C., Sager R. Differential activity of DNA methyltransferase in the life cycle of Chlamydomonas reinhardi. Proc Natl Acad Sci U S A. 1981 May;78(5):3118–3122. doi: 10.1073/pnas.78.5.3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. J., Rubin C. S., Rosen O. M. Insulin-treated 3T3-L1 adipocytes and cell-free extracts derived from them incorporate 32P into ribosomal protein S6. Proc Natl Acad Sci U S A. 1980 May;77(5):2641–2645. doi: 10.1073/pnas.77.5.2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. O. Nucleotide sequence specificity of restriction endonucleases. Science. 1979 Aug 3;205(4405):455–462. doi: 10.1126/science.377492. [DOI] [PubMed] [Google Scholar]
- Spiegelman B. M., Green H. Control of specific protein biosynthesis during the adipose conversion of 3T3 cells. J Biol Chem. 1980 Sep 25;255(18):8811–8818. [PubMed] [Google Scholar]
- Taylor S. M., Jones P. A. Multiple new phenotypes induced in 10T1/2 and 3T3 cells treated with 5-azacytidine. Cell. 1979 Aug;17(4):771–779. doi: 10.1016/0092-8674(79)90317-9. [DOI] [PubMed] [Google Scholar]
- Walker M. S., Becker F. F. DNA methylase activity of normal liver, regenerating liver, and a transplantable hepatocellular carcinoma. Cancer Biochem Biophys. 1981;5(3):169–173. [PubMed] [Google Scholar]