Abstract
Safer and more effective oral drugs are required to treat visceral leishmaniasis, a parasitic disease that kills 50-60,000 people each year. Here we report that fexinidazole, a drug currently in phase I clinical trials for treating African trypanosomiasis, shows promise for treating visceral leishmaniasis. This 2-substituted 5-nitroimidazole drug is rapidly oxidized in vivo in mice, dogs and humans to sulfoxide and sulfone metabolites. Both metabolites of fexinidazole were active against Leishmania donovani amastigotes grown in macrophages, whereas the parent compound was inactive. Pharmacokinetic studies with fexinidazole (200 mg kg−1) showed that fexinidazole sulfone achieves blood concentrations in mice above the EC99 value for at least 24h following a single oral dose. A once daily regimen for 5 days at this dose resulted in a 98.4% suppression of infection in a mouse model of visceral leishmaniasis, equivalent to that seen with the drugs miltefosine and Pentostam, which are currently used clinically to treat visceral leishmaniasis. In African trypanosomes, the mode of action of nitro-drugs involves reductive activation via an NADH-dependent bacterial-like nitroreductase. Overexpression of the leishmanial homologue of this nitroreductase in L. donovani increased sensitivity to fexinidazole by 19-fold indicating that a similar mechanism is involved in both parasites. These findings illustrate the potential of fexinidazole as an oral drug therapy for treating visceral leishmaniasis.
Introduction
Nobel prize-winning pharmacologist Sir James Black believed that “the most fruitful basis for the discovery of a new drug is to start with an old drug” (1). This adage is particularly apt in the search for effective drugs to treat neglected tropical diseases such as visceral leishmaniasis. Caused by the protozoan parasites Leishmania donovani and L. infantum, this disease is the second biggest killer in Africa, Asia and Latin America after malaria. Indeed, two anti-leishmanial drugs, miltefosine and amphotericin B, are examples of medicines originally developed for other purposes (anti-cancer and anti-fungal, respectively). Oral miltefosine (2) and a liposomal formulation of amphotericin B (or amphotericin B deoxycholate) (3) form the mainstay of current attempts to eradicate visceral leishmaniasis in India, Bangladesh and Nepal. However, these and other available treatment options are far from ideal. The principal drawbacks of miltefosine are teratogenicity, prolonged treatment, high cost and the rapid development of drug resistance (4,5). Problems associated with amphotericin B include high treatment costs, the need for intravenous administration and unresponsiveness in some Sudanese patients with visceral leishmaniasis (4,6). Thus there is a continuing need for safe and cost-effective drugs suitable for use in resource-poor settings.
There is renewed interest in nitro-heterocyclic compounds for treating infectious disease. The nitric oxide-generating pro-drug PA-824 currently is being tested in phase II clinical trials against tuberculosis (7-9), and the pro-drug nitazoxanide is undergoing clinical trials for the treatment of hepatitis C (8). A nifurtimox–eflornithine combination therapy has recently been approved by WHO for the treatment of the Gambian form of human African trypanosomiasis (10). In the search for a more potent alternative to nifurtimox, the nitroimidazole fexinidazole (Hoe 239) has been re-discovered by the Drugs for Neglected Disease Initiative (DNDi) (11) and is now undergoing testing in phase I clinical trials for treating African sleeping sickness (12).
A bacteria-like nitroreductase has been implicated in both the mode of action and the mechanism of resistance to nitro-drugs in the related trypanosomatids, Trypanosoma brucei and T. cruzi (13-15). Given that the genomes of leishmania parasites contain a homologous nitroreductase gene, we set out to investigate whether fexinidazole could be an effective treatment for visceral leishmaniasis. Here, we describe the leishmanicidal activity and preliminary preclinical profile of fexinidazole as a clinical candidate for visceral leishmaniasis. Our findings suggest that fexinidazole or its metabolites, fexinidazole sulfoxide and fexinidazole sulfone, have the potential to become a safe and effective oral drug therapy for treating the severest form of visceral leishmaniasis.
Results
In vitro sensitivity of L. donovani to fexinidazole and its metabolites
Fexinidazole is currently being tested in pre-clinical and phase I clinical trials as an oral treatment for African trypanosomiasis. This prompted us to investigate the chemotherapeutic potential of this nitroimidazole compound for treating visceral leishmaniasis caused by another protozoan parasite L. donovani. We determined the potency of fexinidazole and its two predominant in vivo metabolites (fexinidazole sulfoxide and sulfone) in vitro against two life cycle stages of L. donovani (strain LdBOB): promastigotes and axenic amastigotes. Fexinidazole showed leishmanicidal activity against both developmental stages of the parasite with EC50 values of 5.6 ± 0.2 and 2.8 ± 0.1 μM against promastigotes and amastigotes, respectively (Table 1). In addition, L. donovani strain LdBOB proved to be just as sensitive to the sulfoxide and sulfone metabolites of fexinidazole as to the unmetabolized form of the drug. The in vitro potency of fexinidazole against L. donovani parasites compared well with that of a current clinically used oral drug miltefosine, which had EC50 values of 6.1 ± 0.3 and 4.4 ± 0.2 μM against promastigotes and amastigotes, respectively. All drugs tested were found to be inactive (EC50 >50 μM) in a counter screen for toxicity using the human fibroblast cell line MRC5.
Table 1. Key physicochemical properties and in vitro leishmanicidal activity of fexinidazole and its metabolites.
| Compound | Structure | Molecular weight† |
cLogP† | Polar surface area (Å2)† |
Unbound fraction (Fu)‡ |
L. donovani EC50*, μM (Hill slope) |
||
|---|---|---|---|---|---|---|---|---|
| Promastigote | Axenic amastigote |
Amastigote (in macrophages) |
||||||
| Fexinidazole |
|
279 | 2.5 | 73 | 0.15 | 5.6 ± 0.2 (4.1) | 2.8 ± 0.1 (4.5) | >50 |
| Fexinidazole sulfoxide |

|
295 | 1.3 | 90 | 0.86 | 3.1 ± 0.1 (4.4) | 4.5 ± 0.3 (3.5) | 5.3 ± 0.1 (2.4) |
| Fexinidazole sulfone |

|
311 | 1.0 | 107 | 0.73 | 4.8 ± 0.1 (5.0) | 1.6 ± 0.1 (3.8) | 5.3 ± 0.2 (2.4) |
| Miltefosine |
|
366 | 6.0 | 56 | 0.002 | 6.1 ± 0.3 (4.0) | 4.4 ± 0.2 (3.1) | 3.3 ± 0.3 (1.8) |
Results are the weighted mean and standard error of at least three independent experiments
Calculated using the StarDrop software package
Unbound fraction in mouse plasma (14)
The leishmanicidal activity of fexinidazole and its metabolites was evaluated against intracellular L. donovani (LV9) amastigotes in peritoneal mouse macrophages in vitro. Although fexinidazole sulfoxide and sulfone remained just as potent against intracellular amastigotes (EC50s of 5.3 ± 0.1 and 5.3 ± 0.2 μM, respectively), fexinidazole itself had little effect on their viability at concentrations up to and including 50 μM (Table 1). Nevertheless, the sensitivity of intracellular amastigotes to the major metabolites of fexinidazole compared favorably with that of the current frontline drug miltefosine (EC50 - 3.3 ± 0.3 μM). Indeed, when Hill slopes are taken into account, the calculated EC99 values for the sulfoxide and sulfone (41.2 and 45.2 μM) are somewhat better than for miltefosine (52.2 μM)
Physicochemical properties of fexinidazole and its metabolites
The discrepancy between the potency of fexinidazole and that of its major metabolites against intracellular amastigotes is not easily explained, but may account for the failure so far to identify the anti-leishmanial potential of this compound. Given that fexinidazole is just as potent as its metabolites against extracellular parasites, its lack of activity against amastigotes within macrophages cannot be due to differential activity against a cellular target. Rather, these findings may be explained by the failure of the parent drug to enter or accumulate to therapeutic concentrations within the host macrophage. Analysis of the physicochemical properties of fexinidazole revealed that it binds more readily to plasma proteins than to either the sulfoxide or sulfone consistent with its higher cLogP (i.e. 16-32-fold difference in arithmetic terms, Table 1). However, these and other parameters are still within an acceptable range for a drug-like molecule (16).
In vivo sensitivity of L. donovani to fexinidazole and its metabolites
The efficacy of fexinidazole was evaluated in a mouse model of visceral leishmaniasis. Seven days following infection with L. donovani strain LV9 ex vivo amastigotes, groups of BALB/c mice were dosed orally, once daily, with a range of fexinidazole concentrations for five consecutive days. Fourteen days after infection, parasite burden in the liver was determined. As in previous studies (14), fexinidazole was well tolerated by mice and proved to be an extremely effective, dose-dependent inhibitor of L. donovani infection, with five single daily doses of 200 mg kg−1 suppressing infection by 98.4% (Fig. 1A). Lower doses of drug were also effective in treating the murine model of infection, with the ED50 and ED90 estimated at 12 and 57 mg kg−1, respectively (Fig. 1A, insert). This compares well with the current anti-leishmanial drugs miltefosine (ED50, 4 mg kg−1 and ED90, 27 mg kg−1) and Pentostam (ED50, 20 mg kg−1 and ED90, 57 mg kg−1) in similar in vivo studies (17).
Fig. 1. Pharmacodynamic and pharmacokinetic properties of fexinidazole and its metabolites.
(A) Effects of drug treatment on the parasite burden of mice infected with L. donovani. Groups of mice (five per group) infected with L. donovani (strain LV9) were dosed with drug vehicle (orally), Pentostam (subcutaneously), miltefosine (orally) or fexinidazole (orally) on day 7 post-infection and for the following four days. On day 14 post-infection, all animals were humanely euthanized and parasite burdens were determined microscopically by examining Giemsa-stained liver smears. Parasite load is expressed in Leishman Donovan Units (LDU): mean number of amastigotes per liver cell × mg liver (29). The inset shows the dose response curve for fexinidazole (ED50 = 11.9 ± 2.3 mg kg−1). (B) Blood concentration of fexinidazole and its metabolites following oral dosing with fexinidazole (200 mg kg−1). The EC99 values of fexinidazole sulfoxide (10,500 ng ml−1) and sulfone (11,500 ng ml−1) for L. donovani (strain LV9) cultured in ex vivo mouse macrophages are shown as dotted lines. Fexinidazole, black circles; sulfoxide, teal; sulfone, maroon. Data are the mean and standard deviation from 3 mice.
In vivo pharmacokinetic properties of fexinidazole
In our previous study on T. brucei, we demonstrated that the sulfur group of fexinidazole is rapidly metabolized in vivo to a sulfone or sulfoxide group and that these metabolites remain at high concentrations in the blood for up to 8h following oral dosing (14). As one of the principal goals of anti-trypanosomatid drug discovery is to identify an orally available drug that can be given once daily (18), we re-evaluated blood concentrations of fexinidazole sulfoxide and sulfone over 48h. Total blood concentrations of both the sulfoxide and sulfone comfortably exceeded their respective EC99 levels shortly after oral dosing (Fig. 1B). Although the sulfoxide accumulated rapidly in the blood, its concentration dropped below the EC99 after 8h. In contrast, blood concentrations of the sulfone were slower to accumulate, but remained above therapeutic levels for more than 24h. Given that fexinidazole sulfoxide and sulfone are equipotent and additive in combination (Fig. 2), their cumulative blood concentrations exceed the EC99 for ~30h, underlining the potential of fexinidazole as a once daily, oral treatment for treating visceral leishmaniasis.
Fig. 2. EC50 for combinations of fexinidazole sulfoxide and sulfone against promastigotes.

Isobologram shows the EC50 values obtained with combinations of fexinidazole sulfoxide and sulfone against promastigotes of the LdBOB strain of L. donovani. Promastigotes in mid-log growth were incubated with combinations of drug relative to their individual EC50 values. The EC50 values of each combination were determined after 72h. Data are the mean ± SD of triplicate measurements.
Activation of fexinidazole by Leishmania major nitroreductase
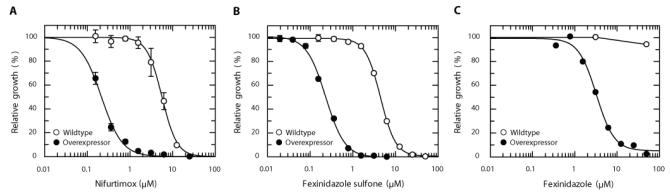
Nitroheterocyclic compounds, such as fexinidazole, are believed to act as prodrugs that require enzyme-mediated reduction by nitroreductases to generate cytotoxic species that cause DNA, lipid and protein damage (19). To determine whether reduction by a nitroreductase is central to the mechanism of action of fexinidazole, the L. major nitroreductase was overexpressed in L. donovani (strain LdBOB) amastigotes. Increased concentrations of NTR in these transgenic parasites was confirmed by a 27-fold shift in their sensitivity to nifurtimox (5.6 ± 0.2 and 0.2 ± 0.1 μM for wildtype parasites and parasites overexpressing nitroreductase, respectively), a nitrofuran drug known to undergo two-electron reduction by NTR (Fig. 3A) (13). This shift in nifurtimox sensitivity was accompanied by a similar increase in susceptibility to fexinidazole sulfone, with axenic amastigotes expressing L.major nitroreductase showing 19-fold greater sensitivity (EC50 = 0.23 ± 0.1 μM) to the nitroimidazole compound than wildtype parasites (EC50 = 4.4 ± 0.1 μM) (Fig. 3B). Fexinidazole and its sulfoxide metabolite also demonstrated a similar increase in potency against axenic amastigotes overexpressing the nitroreductase. In contrast, there was no concomitant shift in the sensitivity of axenic amastigotes to the alkyl phospholipid miltefosine (EC50 values 4.4 ± 0.1 and 4.9 ± 0.2 μM for wildtype and transgenic parasites, respectively). Thus, nitroreductase plays a crucial role in activation of fexinidazole and its metabolites in L. donovani. Interestingly, amastigotes overexpressing nitroreductase were >15-fold more susceptible to fexinidazole (EC50, 3.2 ± 0.3 μM) than wildtype amastigotes (EC50 > 50 μM) in macrophages (Figure 3C). These findings suggest that fexinidazole does not accumulate within host macrophages to the same extent as its metabolites.
Fig. 3. Drug susceptibility of amastigotes overexpressing nitroreductase.
Panels A and B show Leishmania donovani (strain LdBOB) amastigotes cultured under axenic conditions, whereas panel C shows amastigotes cultured in macrophages. Data are the mean ± SD of triplicate measurements for panels A and B and duplicate measurements for panel C. Some standard deviations are within the data points. Symbols: open circles, wildtype; closed circles, parasites overexpressing nitroreductase.
Fexinidazole-mediated parasite killing
Another desirable feature of an anti-leishmanial drug is cytocidal rather than cytostatic activity against the parasite (18). To gauge the speed of fexinidazole-mediated parasite death, mid-log axenic amastigotes were incubated with fexinidazole sulfone at a concentration equivalent to 10 times its EC50 value (Fig. 4). Growth of drug-treated cultures ceased almost immediately with cell numbers declining after 10h with no intact cells visible by 30h. To determine the actual point where treated cells completely lose viability, parasites were washed and sub-cultured at defined intervals without drug. Cells remained viable for 24h in the presence of fexinidazole sulfone; however, at 30h no viable cells could be recovered from culture. Incubation of amastigotes with fexinidazole sulfoxide resulted in a similar cytotoxic profile with no viable parasites recovered following a 30h exposure to drug. The fact that fexinidazole sulfoxide and sulfone are leishmanicidal rather than cytostatic is advantageous from a drug discovery perspective because drug therapy does not then need to be dependent on a fully functional patient immune response (20).
Fig. 4. The cytocidal effect of fexinidazole sulfone on L. donovani axenic amastigotes.

Fexinidazole sulfone (36 μM, equivalent to 10 times the determined EC50 value) was added to an early-log growth culture of axenic amastigotes from the LdBOB strain of L. donovani (~1 × 106 ml−1). At intervals, the cell density was determined and then samples of culture (500 μl) were removed, washed and resuspended in fresh culture medium in the absence of drug. The viability of washed parasites was monitored for up to 72h following removal from drug exposure and the point of irreversible drug toxicity was determined. Symbols: open circle: no inhibitor; filled circles: fexinidazole sulfone; double dagger, no viable parasites recovered from culture.
Discussion
Finding new uses for existing drugs (21) is a highly attractive strategy for drug discovery for 3 neglected diseases such as visceral leishmaniasis. As preclinical studies have already been completed on such compounds, the cost, time and risk involved in developing drugs for an alternative use are greatly reduced. Bearing in mind the lack of resources available for drug development against diseases such as visceral leishmaniasis, this strategy can greatly expedite the drug discovery process. In the case of fexinidazole, comprehensive preclinical pharmacological and safety studies have already been completed as a precursor to clinical development of the drug for the treatment of African sleeping sickness (11). Notably, our study indicates that fexinidazole is a safe, orally available drug candidate with no major side effects that would preclude its evaluation in humans. We provide strong evidence for the potential of fexinidazole as an oral drug for treating visceral leishmaniasis. In both in vitro and murine models of L. donovani infection, fexinidazole and its major metabolites demonstrated leishmanicidal activity. Indeed, fexinidazole and its metabolites performed as well as miltefosine, the only available anti-leishmanial oral drug. The specificity and potency of fexinidazole bodes well for future clinical development of this 2-substituted 5-nitroimidazole. Using a dose translation formula (22), an effective daily dose of 25 mg/kg in our in vivo mouse model equates to a human equivalent dose of 2 mg/kg, in keeping with the current treatment regimen for oral miltefosine (2.5 mg/kg/day for 28 days). Such a dose is likely to be easily achievable in humans as single oral doses of 1200 mg fexinidazole (equivalent to ~ 20 mg/kg) have been successfully given to male volunteers in the bioavailability arm of a DNDi-sponsored phase I clinical trial (http://clinicaltrials.gov/ct2/show/NCT01340157). In addition, pharmacokinetic studies in dog (11) suggest that fexinidazole could also be used to treat canine visceral leishmaniasis and offer a more acceptable alternative to canine culling in the control of zoonotic (L. infantum) disease (23).
The in vivo pharmacokinetic profile of fexinidazole strongly supports its use as an oral drug that can be given once daily. As we reported in our previous study (14), fexinidazole is rapidly oxidized in vivo to fexinidazole sulfoxide and sulfone metabolites. The speed of this metabolism combined with the leishmanicidal properties of the sulfoxide and sulfone suggest that they, and not the parent compound, are likely to be the therapeutically relevant species. Taking this into consideration, the cumulative blood concentrations of both metabolites remain well above their EC99 (determined in vitro) for the entire 24h following dosing supporting the potential of this drug as a once daily treatment. Following oral administration, fexinidazole and its metabolites are readily distributed throughout the body and well absorbed (11). Of particular relevance to our current studies with visceral leishmaniasis is the accumulation in the liver and spleen (11), the major target organ for viscerotropic Leishmania species. Collectively, these data suggest that the drug metabolism and pharmacokinetic profile of fexinidazole may make it suitable for use in the treatment of visceral leishmaniasis. The next stage in development would be to confirm the activity of fexinidazole in the chronic hamster model of infection that is thought to more accurately reflect human disease. In addition, a variety of recent clinical isolates (including parasite lines resistant to current drugs) from Asia, Africa and Latin America need to be tested for sensitivity to fexinidazole.
Overexpression of the L. major nitroreductase resulted in a marked increase in parasite sensitivity to fexinidazole and its metabolites suggesting that, as with most other nitroheterocyclic compounds, fexinidazole acts as a prodrug that must be activated by nitroreduction. Recent studies have shown that trypanosomal nitroreductases catalyze the sequential two-electron reduction of nifurtimox resulting in the generation of a cytotoxic, unsaturated open-chain nitrile derivative (15). It remains to be seen if fexinidazole is activated by the Leishmania nitroreductase in a similar manner. Genetic studies indicate that nitroreductase is essential for survival in vitro for the African trypanosome (13). However, modulation of the nitroreductase levels within the trypanosomatids directly affects sensitivity to nitro-compounds in vitro, with reduced concentrations of the enzyme leading to nitro-drug resistance (13). Reliance on a single enzyme for pro-drug activation may leave drugs such as fexinidazole vulnerable to the emergence of drug resistance, a possibility that would seem to be supported by the ease with which nitro-drug resistance can be generated in the trypanosomatids in vitro (13,14). One strategy to circumvent this problem would be to use fexinidazole as part of a combination therapy, with the rationale that the likelihood of resistance developing to a single agent is relatively high, but the likelihood of resistance developing to two compounds is much lower (24). Work to identify an appropriate partner drug for fexinidazole is currently underway. However, unlike the nifurtimox-eflornithine combination therapy introduced for the treatment of African trypanosomiasis, eflornithine is unlikely to be suitable for treating visceral leishmaniasis (25).
In conclusion, the data presented in this study underlines the potential of fexinidazole as a much needed additional oral therapy for visceral leishmaniasis. The biological and pharmacokinetic properties of this 2-substituted 5-nitroimidazole compound appear to be ideally suited for use against the severest form of leishmaniasis. With comprehensive preclinical pharmacological and safety studies already completed for fexinidazole, there is every reason to hope that fexinidazole can progress rapidly into clinical development for the treatment of this devastating parasitic disease.
Materials and Methods
Ethics statement
All animal experiments were approved by the Ethical Review Committee at the University of Dundee and performed under the Animals (Scientific Procedures) Act 1986 (UK Home Office Project Licence PPL 60/4039) in accordance with the European Communities Council Directive (86/609/EEC).
Cell lines and culture conditions
The clonal Leishmania donovani cell line LdBOB (derived from MHOM/SD/62/1S-CL2D) was grown as either promastigotes or axenic amastigotes in media specific for each developmental stage (26). Amastigotes were cultivated at 37 °C in 5% CO2 and promastigotes were grown at 26 °C. Parasites were cycled between developmental stages after a maximum of 7 passages.
L. donovani (LV9 strain; WHO designation: MHOM/ET/67/HU3) ex vivo amastigotes were used in both in vitro and in vivo drug sensitivity assays. Amastigotes were derived from hamster spleens, as previously described (27).
In vitro drug sensitivity assays
To examine the effects of test compounds on growth, triplicate cultures were seeded with 1 × 105 parasites ml−1. Parasites were grown in the presence of drug for 72h, after which 50 μM resazurin was added to each well and fluorescence (excitation of 528 nm and emission of 590 nm) measured after a further 4-h incubation (28). Data were processed using GRAFIT (version 5.0.4; Erithacus software) and fitted to a 4-parameter equation to obtain the effective concentration inhibiting growth by 50% (EC50):
In this equation [i] represents inhibitor concentration and m is the slope factor. Experiments were repeated at least three times and data are presented as the weighted mean plus weighted standard deviation (28). For isobologram determinations, fexinidazole sulfoxide and sulfone were tested in fixed combinations relative to their respective EC50 values. The EC50 values of each combination were determined after 72h and plotted as an isobologram.
In macrophage drug sensitivity assays
Mouse peritoneal macrophages were harvested from BALB/c mice by lavage with ice-cold phosphate buffered saline, 24h following an intraperitoneal injection of 2% (w/v) soluble starch (Sigma). Harvested cells were pelleted by centrifugation (350 × g, 10 min, 4°C), resuspended in 0.5 ml of Red Cell Lysis Buffer (Sigma) and incubated for 2 min at room temperature. Serum-free RPMI 1640 (Sigma) was then added up to 20 ml and cells pelleted (350 × g, 5 min, 4°C) prior to two further washes in RPMI. Macrophages were then plated in Lab-tek 8–well chamber slides (VWR International, UK) at a density of 1 × 105 cells per well and left to adhere for 30 min at 37°C and in 5% CO2. Serum-free media was then replaced with RPMI 1640 containing 20% (v/v) fetal calf serum (FCS) and cells incubated for a further 1h prior to infection with ex vivo amastigotes (1 × 106 per well). Amastigotes were left to infect host cells for 4h at 37°C and in 5% CO2. Non-phagocytosed amastigotes were removed by washing adherent macrophages with Hanks Balanced Salt solution (Gibco, UK) and drug dilutions were then added in RPMI containing 20% FCS. Infected macrophages were incubated in the presence of drug for 72h at 37°C and 5% CO2. At the end-point of each assay, chamber slides were fixed with 100% methanol, stained with Giemsa and examined microscopically. Numbers of intracellular amastigotes in one hundred macrophages (per well) were determined and the percentage infection established, as compared to an untreated control (100%). EC50 values were then determined for each drug, as described above.
In vivo drug sensitivity
Groups of female BALB/c mice (5 animals per group) were inoculated with L. donovani LV9 amastigotes harvested from the spleen of an infected hamster (27) via intravenous injection (tail vein). Each mouse was infected with a 0.2 ml bolus (equivalent to 2 × 107 amastigotes) on day 0 of the study. From day 7 post-infection, groups of mice were treated with either drug vehicle only (orally), with Pentostam (15 mg kg−1 subcutaneously), miltefosine, (12 mg kg−1 orally) or with fexinidazole (25, 50, 100 or 200 mg kg−1 orally) once daily and for 5 days. Drug dosing solutions were prepared fresh each day. On day 14 post-infection, three days after the completion of all treatments, all animals were humanely euthanized and parasite burdens determined microscopically by examining Giemsa-stained liver smears (Rapi Diff II, Biotech Sciences Ltd). Numbers of amastigotes/500 liver cells were counted and the parasite burden expressed in Leishman Donovan Units (LDU): mean number of amastigotes per liver cell × mg weight of liver (29). The LDU of drug-treated samples are compared to that of untreated samples and the % inhibition calculated. ED50 values were determined using GRAFIT (version 5.0.13; Erithacus software) by fitting data to a 4-parameter equation, as described above.
Cloning and expression of L. major nitroreductase in LdBOB
The L. major nitroreductase gene (LmjF.05.0660) was amplified by PCR from L. major Friedlin genomic DNA using the sense primer 5′-GGATCCATGCTTCGCCGCAGCCCCCGCT-3′ and the antisense primer 5′-GGATCCCTAGAACTTGTTCCACCGCACGGTG-3′ both with additional BamHI sites (underlined). The PCR product was then cloned into the pCR-Blunt II-TOPO vector (Invitrogen) and sequenced. The pCR-Blunt II-TOPO-LmNTR construct was then digested with BamHI and the fragment cloned into the pIR1SAT expression vector (30) resulting in a pIR1SAT-LmNTR construct. Mid-log L. donovani promastigotes (WT, LdBOB) were transfected with pIR1SAT-LmNTR using the Human T-Cell Nucleofector kit and nucleofector (Amaxa, program V-033). Following transfection, cells were allowed to grow for 16-24h in modified M199 medium (26) with 10 % fetal calf serum prior to drug selection with nourseothricin (100 μg ml−1, Jena Bioscience, Germany). Cloned cell lines were generated by limiting dilution, maintained in selective medium and removed from drug selection for one passage prior to experiments.
Chemical synthesis of fexinidazole and fexinidazole metabolites
Fexinidazole, fexinidazole sulfoxide and fexinidazole sulfone were prepared in two steps from (1-methyl-5-nitro-1H-imidazol-2-yl)methanol (31), according to published procedures (32,33). Alternatively, fexinidazole sulfone was prepared by reacting (1-methyl-5-nitro-1H-imidazol-2-yl)methanol and 4-(methylsulfonyl)phenol under Mitsunobu reaction conditions (34). Compound purity was determined by liquid chromatography-mass spectrometry (LC-MS), with all compounds found to be of ≥95% purity. For in vivo experiments compound purity was further analyzed by ultraperformance liquid chromatography-mass spectrometry (UPLC-MS), with all compounds found to be of ≥99% purity. Full details of synthetic methods and analytical data are available from the authors.
Determination of fexinidazole exposure in mice after acute oral dosing
Fexinidazole (200 mg kg−1) was orally administered to BALB/c mice. The dose solution was prepared on the day of dosing and the vehicle was 10% (v/v) dimethyl sulfoxide (DMSO) in peanut oil. Blood samples (10 μl) were collected from the tail vein of each animal into Micronic tubes (Micronic BV) containing deionised water (20 μl) at defined intervals post-dose and stored at −80°C until analysis. Blood levels of fexinidazole and major metabolites in mouse blood were determined by UPLC-MSMS (14).
Physicochemical properties
The software package StarDrop by Optibrium was used to calculate physical parameters including LogP, molecular weight and polar surface area (PSA) for fexinidazole and its metabolites. The plasma protein binding of miltefosine, and fexinidazole and its oxidized metabolites were determined by the equilibrium dialysis method (28).
Single Sentence Summary: Fexinidazole, a drug currently in clinical trials for African sleeping sickness, shows potential as an oral treatment for another neglected tropical disease.
Lay Summary: Kala azar (visceral leishmaniasis) is a parasitic disease afflicting 500,000 people living in parts of Asia, Africa and Latin America, killing about 50-60,000 patients per year. Current drug treatments are unsatisfactory for reasons such as high cost, drug resistance or the need for hospitalization. The mode of action of the nitro-drug fexinidazole, currently undergoing clinical trials for a related disease (African sleeping sickness) is thought to involve metabolic activation by a bacteria-like nitroreductase. Because we had identified a homologous nitroreductase gene in the leishmania genome, we tested fexinidazole for anti-leishmanial activity against the insect (promastigote) and mammalian (amastigote) stages of the parasite. Although fexinidazole was active against promastigotes, it was found to be inactive against amastigotes cultured in mammalian macrophages. However, we had established previously that the sulfur group of fexinidazole is oxidized in mice to form fexinidazole sulfone and sulfoxide. Notably, these compounds were found to be active. Our pharmacological studies on fexinidazole and its metabolites in mice predicted that therapeutic levels could be obtained by once daily oral dosing. Indeed, fexinidazole was found to be extremely effective in a mouse model of visceral leishmaniasis such that five single daily doses of 200 mg/kg suppressed infection by more than 98%. The potency of this compound in mice was comparable to two other clinical drugs, miltefosine and Pentostam. A transgenic parasite line that overproduces the leishmania nitroreductase was found to become hypersensitive to fexinidazole and its sulfoxide, suggesting that the mode of action involves reduction of the drug to form toxic metabolites. Together with the currently available results of Phase I clinical trials carried out by the drugs for Neglected Disease initiative, our findings suggest that fexinidazole has potential for use as a much needed cheap, safe and efficacious oral therapy for visceral leishmaniasis.
Acknowledgements
Funding: This work was supported by grants to AHF from the Wellcome Trust (079838, 077705 and 083481) http://www.wellcome.ac.uk.
Footnotes
Competing interests: The authors declare that they have no competing interests.
REFERENCES
- 1.Raju TNK. The Nobel chronicles. Lancet. 2000;355:1022. doi: 10.1016/s0140-6736(05)74775-9. [DOI] [PubMed] [Google Scholar]
- 2.Matlashewski G, Arana B, Kroeger A, Battacharya S, Sundar S, Das P, Sinha PK, Rijal S, Mondal D, Zilberstein D, Alvar J. Visceral leishmaniasis: elimination with existing interventions. Lancet Infect. Dis. 2011;11:322–325. doi: 10.1016/S1473-3099(10)70320-0. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization . Control of the Leishmaniases 949. World Health Organization; Geneva: 2010. [PubMed] [Google Scholar]
- 4.Croft SL, Sundar S, Fairlamb AH. Drug Resistance in Leishmaniasis. Clin. Microbiol. Rev. 2006;19:111–126. doi: 10.1128/CMR.19.1.111-126.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.den Boer ML, Alvar J, Davidson RN, Ritmeijer K, Balasegaram M. Developments in the treatment of visceral leishmaniasis. Expert Opinion on Emerging Drugs. 2009;14:395–410. doi: 10.1517/14728210903153862. [DOI] [PubMed] [Google Scholar]
- 6.Mueller M, Ritmeijer K, Balasegaram M, Koummuki Y, Santana MR, Davidson R. Unresponsiveness to AmBisome in some Sudanese patients with kala-azar. Trans. R. Soc. Trop. Med. Hyg. 2007;101:19–24. doi: 10.1016/j.trstmh.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 7.Barry CE, Boshoff HIM, Dowd CS. Prospects for clinical introduction of nitroimidazole antibiotics for the treatment of tuberculosis. Curr. Pharm. Des. 2004;10:3239–3262. doi: 10.2174/1381612043383214. [DOI] [PubMed] [Google Scholar]
- 8.Ginsberg AM, Laurenzi MW, Rouse DJ, Whitney KD, Spigelman MK. Safety, tolerability, and pharmacokinetics of PA-824 in healthy subjects, Antimicrob. Agents Chemother. 2009;53:3720–3725. doi: 10.1128/AAC.00106-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nuermberger E, Tyagi S, Tasneen R, Williams KN, Almeida D, Rosenthal I, Grosset JH. Powerful bactericidal and sterilizing activity of a regimen containing PA-824, moxifloxacin, and pyrazinamide in a murine model of tuberculosis. Antimicrob. Agents Chemother. 2008;52:1522–1524. doi: 10.1128/AAC.00074-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Priotto G, Kasparian S, Mutombo W, Ngouama D, Ghorashian S, Arnold U, Ghabri S, Baudin E, Buard V, Kazadi-Kyanza S, Ilunga M, Mutangala W, Pohlig G, Schmid C, Karunakara U, Torreele E, Kande V. Nifurtimox-eflornithine combination therapy for second-stage African Trypanosoma brucei gambiense trypanosomiasis: a multicentre, randomised, phase III, non-inferiority trial. Lancet. 2009;374:56–64. doi: 10.1016/S0140-6736(09)61117-X. [DOI] [PubMed] [Google Scholar]
- 11.Torreele E, Trunz BB, Tweats D, Kaiser M, Brun R, Mazue G, Bray MA, Pecoul B. Fexinidazole - a new oral nitroimidazole drug candidate entering clinical development for the treatment of sleeping sickness. PLoS Negl. Trop. Dis. 2010;4:e923. doi: 10.1371/journal.pntd.0000923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DNDi . Delivering innovation and building a robust pipeline DNDi Annual Report 2008-2009. SRO-Kundig; Geneva, Switzerland: 2009. [Google Scholar]
- 13.Wilkinson SR, Taylor MC, Horn D, Kelly JM, Cheeseman I. A mechanism for cross-resistance to nifurtimox and benznidazole in trypanosomes. Proc. Natl. Acad. Sci. USA. 2008;105:5022–5027. doi: 10.1073/pnas.0711014105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sokolova AY, Wyllie S, Patterson S, Oza SL, Read KD, Fairlamb AH. Cross-resistance to nitro drugs and implications for treatment of human African trypanosomiasis. Antimicrob. Agents Chemother. 2010;54:2893–2900. doi: 10.1128/AAC.00332-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hall BS, Bot C, Wilkinson SR. Nifurtimox activation by trypanosomal type I nitroreductases generates cytotoxic nitrile metabolites. J. Biol. Chem. 2011;286:13088–13095. doi: 10.1074/jbc.M111.230847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Advanced Drug Delivery Reviews. 2001;46:3–26. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 17.Escobar P, Yardley V, Croft SL. Activities of hexadecylphosphocholine (miltefosine), AmBisome, and sodium stibogluconate (Pentostam) against Leishmania donovani in immunodeficient scid mice. Antimicrob. Agents Chemother. 2001;45:1872–1875. doi: 10.1128/AAC.45.6.1872-1875.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wyatt PG, Gilbert IH, Read KD, Fairlamb AH. Target validation: linking target and chemical properties to desired product profile. Curr. Top. Med. Chem. 2011;11:1275–1283. doi: 10.2174/156802611795429185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raether W, Hänel H. Nitroheterocyclic drugs with broad spectrum activity. Parasitol. Res. 2003;90:S19–S39. doi: 10.1007/s00436-002-0754-9. [DOI] [PubMed] [Google Scholar]
- 20.Frearson JA, Wyatt PA, Gilbert IH, Fairlamb AH. Target assessment for antiparasitic drug discovery. Trends Parasitol. 2007;23:589–595. doi: 10.1016/j.pt.2007.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cavalla D. Therapeutic switching: A new strategic approach to enhance R&D productivity. Idrugs. 2005;8:914–918. [PubMed] [Google Scholar]
- 22.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 23.Romero GA, Boelaert M. Control of visceral leishmaniasis in Latin America - a systematic review. PLoS Negl. Trop. Dis. 2010;4:e584. doi: 10.1371/journal.pntd.0000584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.White NJ, Olliaro PL. Strategies for the prevention of antimalarial drug resistance: Rationale for combination chemotherapy for malaria. Parasitol. Today. 1996;12:399–401. doi: 10.1016/0169-4758(96)10055-7. [DOI] [PubMed] [Google Scholar]
- 25.Olenyik T, Gilroy C, Ullman B. Oral putrescine restores virulence of ornithine decarboxylase-deficient Leishmania donovani in mice. Mol. Biochem. Parasitol. 2011;176:109–111. doi: 10.1016/j.molbiopara.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goyard S, Segawa H, Gordon J, Showalter M, Duncan R, Turco SJ, Beverley SM. An in vitro system for developmental and genetic studies of Leishmania donovani phosphoglycans. Mol. Biochem. Parasitol. 2003;130:31–42. doi: 10.1016/s0166-6851(03)00142-7. [DOI] [PubMed] [Google Scholar]
- 27.Wyllie S, Fairlamb AH. Refinement of techniques for the propagation of Leishmania donovani in hamsters. Acta Trop. 2006;97:364–369. doi: 10.1016/j.actatropica.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 28.Jones DC, Hallyburton I, Stojanovski L, Read KD, Frearson JA, Fairlamb AH. Identification of a kappa-opioid agonist as a potent and selective lead for drug development against human African trypanosomiasis. Biochem. Pharmacol. 2010;80:1478–1486. doi: 10.1016/j.bcp.2010.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bradley DJ, Kirkley J. Regulation of Leishmania populations within the host. I. the variable course of Leishmania donovani infections in mice. Clin. Exp. Immunol. 1977;30:119–129. [PMC free article] [PubMed] [Google Scholar]
- 30.Gaur U, Showalter M, Hickerson S, Dalvi R, Turco SJ, Wilson ME, Beverley SM. Leishmania donovani lacking the Golgi GDP-Man transporter LPG2 exhibit attenuated virulence in mammalian hosts. Exp. Parasitol. 2009;122:182–191. doi: 10.1016/j.exppara.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chauviere G, Bouteille B, Enanga B, de Albuquerque C, Croft SL, Dumas M, Perie J. Synthesis and biological activity of nitro heterocycles analogous to megazol, a trypanocidal lead. J. Med. Chem. 2003;46:427–440. doi: 10.1021/jm021030a. [DOI] [PubMed] [Google Scholar]
- 32.Hoff DR, Henry DW. Patent (International Publication Number: 3,299,090, International Publication Date: 17-1-1967)
- 33.Winkelmann E, Raether W. Patent (International Publication Number: 4,042,705, International Publication Date: 16-8-1977)
- 34.Swamy KCK, Kumar NNB, Balaraman E, Kumar KVPP. Mitsunobu and related reactions: advances and applications. Chem. Rev. 2009;109:2551–2651. doi: 10.1021/cr800278z. [DOI] [PubMed] [Google Scholar]