Abstract
Degenerin/epithelial Na+ channels (DEG/ENaCs) are Na+ channels that are blocked by the diuretic amiloride. In general, they are impermeable for Ca2+ or have a very low permeability for Ca2+. We describe here, however, that a DEG/ENaC from the cnidarian Hydra magnipapillata, the Hydra Na+ channel (HyNaC), is highly permeable for Ca2+ (PCa/PNa = 3.8). HyNaC is directly gated by Hydra neuropeptides, and in Xenopus laevis oocytes expressing HyNaCs, RFamides elicit currents with biphasic kinetics, with a fast transient component and a slower sustained component. Although it was previously reported that the sustained component is unselective for monovalent cations, the selectivity of the transient component had remained unknown. Here, we show that the transient current component arises from secondary activation of the Ca2+-activated Cl− channel (CaCC) of Xenopus oocytes. Inhibiting the activation of the CaCC leads to a simple on–off response of peptide-activated currents with no apparent desensitization. In addition, we identify a conserved ring of negative charges at the outer entrance of the HyNaC pore that is crucial for the high Ca2+ permeability, presumably by attracting divalent cations to the pore. At more positive membrane potentials, the binding of Ca2+ to the ring of negative charges increasingly blocks HyNaC currents. Thus, HyNaC is the first member of the DEG/ENaC gene family with a high Ca2+ permeability.
INTRODUCTION
Ion channels of the degenerin/epithelial Na+ channel (DEG/ENaC) gene family are present in the genome of multicellular organisms ranging from sponges to humans. In unicellular organisms, genes with homology to DEG/ENaCs are absent, suggesting that DEG/ENaC ion channels have evolved in multicellular organisms, where they serve diverse functions (Kellenberger and Schild, 2002), ranging from mechanoreception in Caenorhabditis elegans, detection of salt and pheromones in Drosophila melanogaster, to neurotransmission and Na+ reabsorption in mammals. Correspondingly, gating mechanisms of these channels are similarly diverse (Kellenberger and Schild, 2002) and range from direct mechanical gating to ligand gating, and some DEG/ENaCs are constitutively open. The diverse functions and gating mechanisms raise the question of the primordial function of these channels in the common ancestor of multicellular organisms. Which features are evolutionarily old and which are new?
Comparative analysis of DEG/ENaCs from evolutionarily separated groups of animals can help to answer this question. Animals of the phylum Cnidaria are among the most primitive multicellular animals. They are characterized by a radial symmetry and a primitive nervous system. Thus, the comparative analysis of DEG/ENaCs from cnidarians with those of other animals promises to yield important insights into the evolution of the DEG/ENaC gene family.
Hydra magnipapillata is an important model organism belonging to the class Hydrozoa within the phylum Cnidaria. Four cDNAs coding for DEG/ENaC subunits have been cloned from Hydra and the proteins named Hydra Na+ channels (HyNaCs) (Golubovic et al., 2007; Dürrnagel et al., 2010); a fifth gene apparently encodes a pseudogene (Golubovic et al., 2007). HyNaC subunits 2, 3, and 5 assemble into a heteromeric channel, HyNaC2/3/5, which is directly gated by neuropeptides of the Hydra nervous system (Dürrnagel et al., 2010), Hydra-RFamides I and II (pQWLGGRF-NH2 and pQWFNGRF-NH2, respectively) (Moosler et al., 1996). Because peptide-gated DEG/ENaCs also exist in mollusks (Cottrell et al., 1990; Lingueglia et al., 1995), and because H+-gated acid-sensing ion channels (ASICs) from chordates are closely related to HyNaCs (Golubovic et al., 2007), the common ancestor of DEG/ENaCs from Cnidaria and bilateral organisms was probably a channel gated by an extracellular ligand, perhaps a peptide.
In contrast to their diverse functions and gating mechanisms, all DEG/ENaCs share a secondary structure with two transmembrane domains and a large extracellular domain (Kellenberger and Schild, 2002). Moreover, most DEG/ENaCs share two biophysical characteristics: they are Na+ selective, and they are blocked by the diuretic amiloride.
Some ASICs are an exception to this rule because at prolonged activation, their selectivity changes from Na+ selective to unselective for monovalent cations. This happens at physiological ligand concentrations for shark ASIC1b (Springauf and Gründer, 2010) and at high ligand concentrations (low pH) for ASIC3 (Lingueglia et al., 1997). The Na+-selective state typically is associated with rapidly activating and desensitizing peak currents, whereas the unselective state is associated with small sustained currents. Thus, some ASICs apparently have a dynamic selectivity. When expressed in Xenopus laevis oocytes, HyNaC currents resemble the biphasic ASIC currents: a fast transient current is followed by a comparatively large sustained current, and the sustained current is indeed a relatively unselective current (Golubovic et al., 2007; Dürrnagel et al., 2010).
Here, we show that the transient peptide-activated current is not a Na+ current but rather arises from secondary activation of the Ca2+-activated Cl− channel (CaCC) that is endogenous to Xenopus oocytes (Miledi, 1982; Barish, 1983; Schroeder et al., 2008). Inhibiting activation of the CaCC abolishes transient currents and leads to simple on–off responses of HyNaC without any apparent desensitization. Collectively, our results show that the kinetics of HyNaC currents is simple and that the HyNaC pore is cation unselective with a high Ca2+ permeability (PCa/PNa = 3.8). These results suggest that primordial DEG/ENaCs may have been unselective cation channels and that a highly Na+-selective pore may be an advanced feature of channels from this gene family.
MATERIALS AND METHODS
Electrophysiology
cDNAs for HyNaCs 2, 3, and 5 had been described (Golubovic et al., 2007; Dürrnagel et al., 2010), and mutants of the Ca2+-binding site were constructed as described previously (Paukert et al., 2004). Mutants of the Ca2+-binding site replaced a conserved aspartate residue by a cysteine (D431C in HyNaC2, D433C in HyNaC3, and D438C in HyNaC5). Capped cRNA was synthesized by SP6 RNA polymerase from linearized cDNA using the mMessage mMachine kit (Ambion). Hydra-RFamide I (pQWLGGRF-NH2) was purchased from Genemed Synthesis.
For expression of HyNaC2/3/5, ∼0.8 ng cRNA was injected into defolliculated stage V–VI oocytes of Xenopus and oocytes were kept in oocyte Ringer’s solution 2 (OR-2) 2–4 d before measurements; for expression of HyNaC2/3/5_D-C and P2X4, ∼16 ng cRNA was injected. OR-2 contained (in mM): 82.5 NaCl, 2.5 KCl, 1.0 Na2HPO4, 1.0 MgCl2, 1.0 CaCl2, 5.0 HEPES, 0.5 g/liter PVP, 1,000 U/l penicillin, and 10 mg/l streptomycin, with pH adjusted to 7.3 with NaOH. Whole cell currents were recorded with an amplifier (TurboTec 03X; npi electronic), using an automated pump-driven solution exchange system together with the oocyte-testing carousel controlled by the interface OTC-20 (Madeja et al., 1995). Data acquisition and solution exchange were managed using the software CellWorks (version 5.1.1; npi electronic). Data were filtered at 20 Hz and acquired at 0.1–1 kHz.
Standard bath solution for whole oocyte current measurements contained (in mM): 140 NaCl, 10 HEPES, 1.8 CaCl2, and 1.0 MgCl2, with pH 7.4 adjusted with NaOH. To avoid activation of CaCCs, we injected 50 nl of either EGTA solution (in mM: 20 EGTA and 10 HEPES, pH 7.4) or BAPTA solution (in mM: 20 BAPTA and 10 HEPES, pH 7.4) into oocytes, 15–120 min before recordings. Glass electrodes filled with 3 M KCl were used; they had a resistance of 0.3–1.5 MΩ. If not specified, the membrane potential was clamped at −70 mV.
Photometric calcium measurements
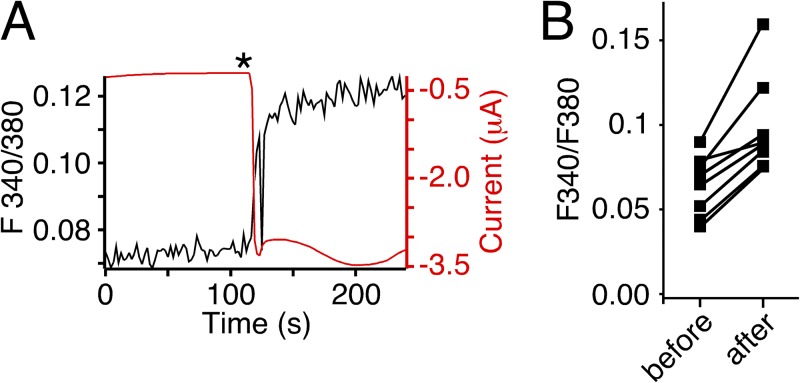
Oocytes were injected with 50 nl Fura-2AM (1 mM) 30–120 min before the recording (the AM ester form was used solely because of availability) and placed with the animal pole facing away from the objective. Measurements used a BX51WI upright microscope (Olympus) with a 40× water-immersion objective (NA 0.8) and a TILL photometry system (TILL Photonics). The region of interest chosen for recording included most of the upper surface of the cell. We do not know how far into the oocyte our optics penetrate but do not expect it to be more than a few micrometers. Fura-2 was excited every 2 s with a 100-ms pulse of 340 nm light and a 50-ms pulse of 380 nm light using a Polychrome 5 light source (TILL Photonics). Emission was collected by a photodiode (TILL Photonics) behind a 535/30-nm bandpass filter, digitized at 5 kHz by a LIH8+8 interface run by Patchmaster software (HEKA). Emission during the 340-nm pulse and the 380-nm pulse was averaged, and the F340/F380 ratio was determined for each time point (emission with 340-nm excitation/emission with 380-nm excitation). Because emission was generally lower with 340-nm than with 380-nm excitation, a longer recording was taken for the 340-nm excitation to increase the signal-to-noise ratio. Simultaneously, currents were recorded with the TurboTec 03X amplifier and digitized at 0.1 kHz using the LIH8+8 interface. To activate HyNaCs, 500 µl Hydra-RFamide I (10 µM) was manually applied to the Petri dish to achieve a final concentration of ∼1 µM. Data were analyzed offline using IGOR (WaveMetrics). The changes induced by HyNaC agonists were reproducible and large compared with the signal-to-noise ratio (see Fig. 5 A). On average, they were 47 ± 9% of the baseline F340/F380 ratio and small compared with the variation between cells in the resting F340/F480 ratio (see Fig. 5 B). We therefore did not aim to calibrate the F340/F380 signal and to convert the obtained numbers into absolute concentrations of Ca2+.
Figure 5.
Activation of HyNaC increases [Ca2+]i in oocytes. (A) Representative trace (black) from photometric Ca2+ measurements illustrating the increase of the F340/380 ratio in a HyNaC2/3/5-expressing oocyte after the application of RFamide I (asterisk). Oocytes had been injected with 50 nl Fura-2AM (1 mM) 30–120 min before the recording. Thus, the increased F340/380 ratio reflects an increase in [Ca2+]i. The red trace represents the current that paralleled the rise in [Ca2+]i. HyNaC was activated by pipetting Hydra-RFamide I into the bath to yield a final concentration of ∼1 µM RFamide I. (B) Changes of the F340/F380 ratio caused by activation of HyNaC for individual measurements (n = 8).
Data analysis
For determination of reversal potentials (Erevs) and calculation of relative permeabilities, oocytes were injected with 20 mM EGTA to minimize the influence of CaCCs. All I-V relationships were corrected for background conductances by subtracting the currents measured at a given voltage without agonist application.
Ionic permeability ratio for monovalent cations PNa/PK was calculated from the shift in Erev when NaCl in the standard bath solution was replaced by an equimolar amount of KCl, according to the following equation derived from the Goldman–Hodgkin–Katz equation:
| (1) |
where ΔErev = ENa − EK, R is the gas constant, F the Faraday constant, and T the temperature. We assumed that the intracellular concentrations of Na+ and K+ are constant within one batch of oocytes.
Assuming additionally that intracellular Ca2+ concentration ([Ca2+]i) is very small, PCa/PNa can be calculated from the shift in Erev when Na+ was replaced by Ca2+ in the application solution, according to the following equation (Lewis, 1979; Bässler et al., 2001):
| (2) |
where ΔErev = ENa − ECa, and R, T, and F have the same meaning as above. The solution used to determine ECa contained (in mM): 10 CaCl2, 126.5 NMDG-Cl, and 10 HEPES, pH 7.4. The solution used to determine ENa contained (in mM): 140 NaCl, 1 CaCl2, and 10 HEPES, pH 7.4; the minor amount of Ca2+ in this solution was considered negligible.
Activity of ions was used in all terms of [c]. Activity coefficients fi of single ions i of valence z were calculated for Na+ and Ca2+ with the Davies equation (Davies, 1962):
| (3) |
where I is the ionic strength, which is defined as:
| (4) |
In all figures showing voltage ramps (Figs. 1 C, 4, 6, and 7, D, F, and G), the individual conditions were measured with different oocytes of the same batch (except measurements for the P2X4 receptor, which were performed on separate batches). Furthermore, batches of oocytes were identical between Figs. 4 and 7 D and between Figs. 6 A and 7 F. The use of separate oocytes was necessary, as leak currents usually increased over time, especially when oocytes were clamped at holding potentials more negative than −70 mV.
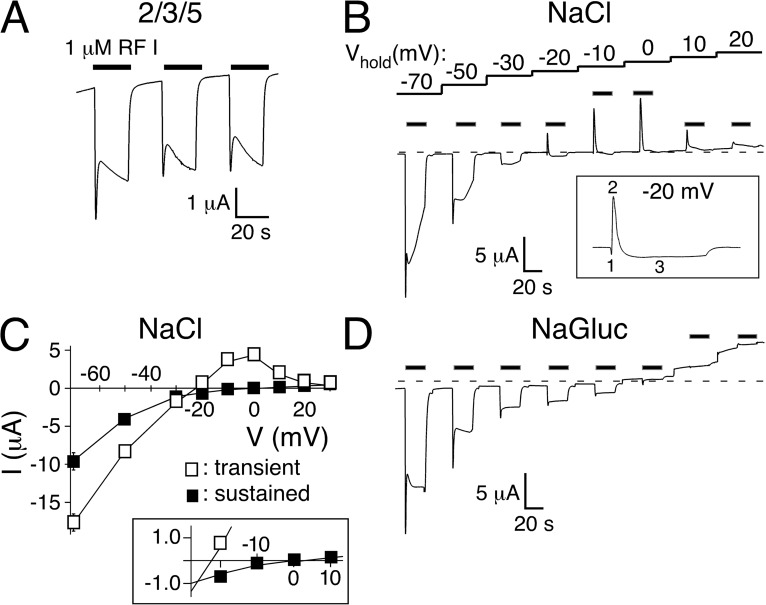
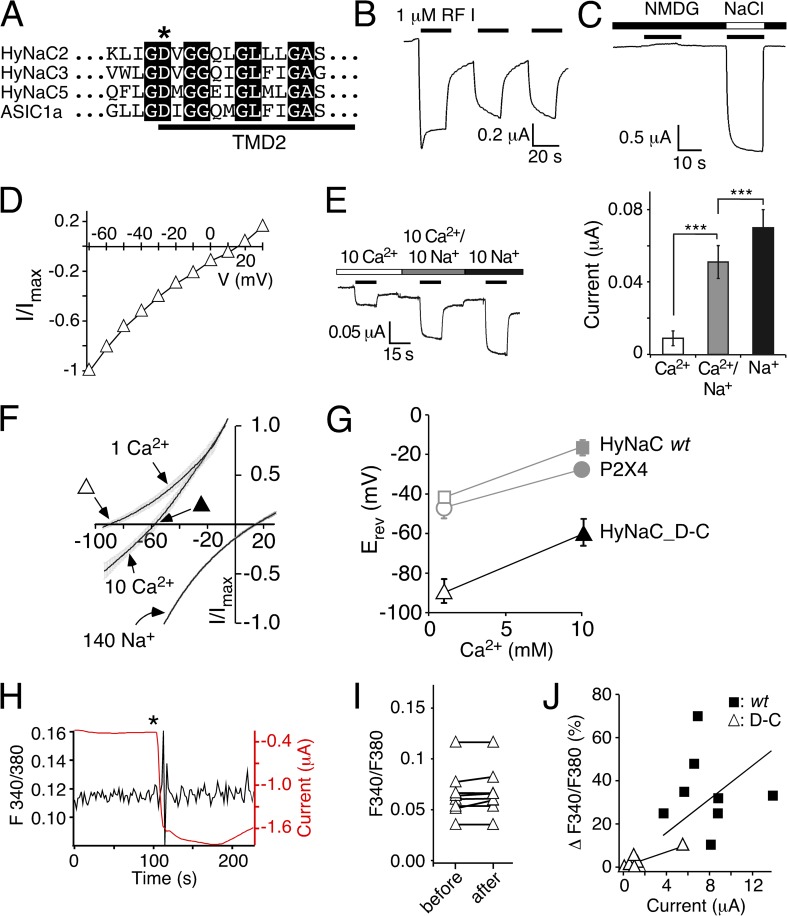
Figure 1.
Transient peptide-activated currents depend on Cl−. (A) Representative current trace showing activation of HyNaC2/3/5 in standard bath. HyNaC was activated with 1 µM Hydra-RFamide I (black bars). (B) Repeated activation of HyNaC at different holding potentials (Vhold) reveals the different Erevs of the transient and the sustained current component. A representative current trace in standard bath solution is shown. Note that the transient current changes to outward at more negative potentials than the sustained current. The inset highlights the three current phases (initial inward, transient outward, and sustained inward) at a holding potential of −20 mV. (C) I/V plot revealing the different Erevs of the transient (open squares) and sustained current components (closed squares) in standard bath (n = 6). Note the strong inward rectification of both components. The inset shows the I/V relations close to the Erevs on an expanded scale. (D) Representative current trace in bath solution lacking Cl− (NaCl replaced by NaGluconate). Note the absence of transient outward currents.
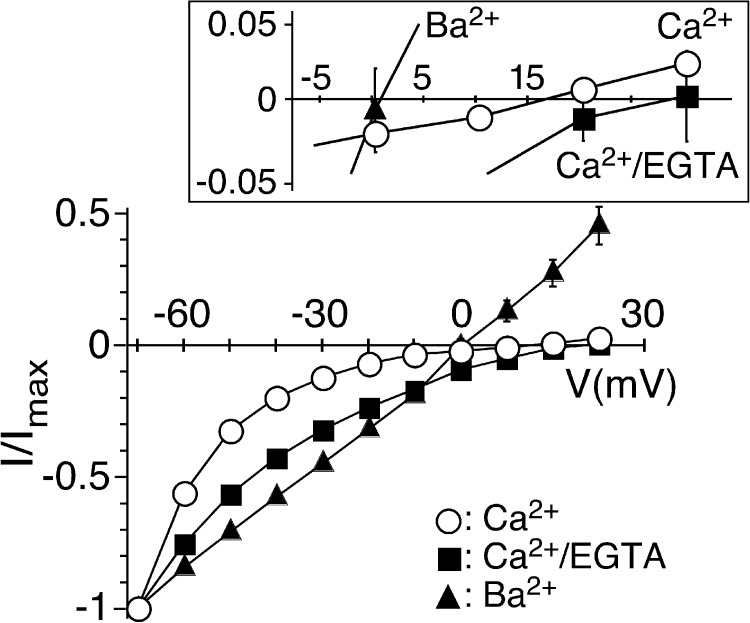
Figure 4.
Preventing a rise in [Ca2+]i changes Erev of sustained peptide-activated currents. I/V relations for HyNaC2/3/5 activated by 1 µM Hydra-RFamide I in standard bath (open circles), in standard bath after injection of EGTA (closed squares), and in standard bath in which CaCl2 had been replaced by BaCl2 (closed triangles). Currents were normalized to the current measured at −70 mV, which had an amplitude of −4.4 ± 1.1 µA (Ca2+), −1.4 ± 0.2 µA (Ca2+/EGTA), or −6.0 ± 0.8 µA (Ba2+) (n = 7 - 8), respectively. The inset shows the I/V relations close to the Erevs on an expanded scale.
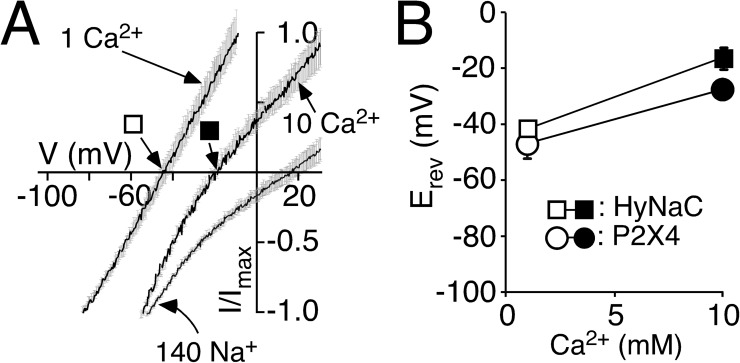
Figure 6.
Permeability ratio PCa/PNa of HyNaC is comparable to P2X4. (A) I/V relations of HyNaC2/3/5 in solutions containing 1 or 10 mM CaCl2 and no NaCl (which had been replaced by 140 or 126.5 mM NMDG-Cl, respectively) and in a solution containing 140 mM NaCl and 1 mM CaCl2. Continuous voltage ramps were run with a speed of 0.46 ms per millivolt (thus, for example, 6 s per 130 mV). Different voltage ranges were chosen for different conditions. Currents were normalized to Imax. Lines are the sum of 8–10 individual measurements. SEM is indicated by gray errors bars. 50 nl EGTA (20 mM) was injected before the recordings. Squares represent the Erevs that are plotted in B. HyNaC was activated by 2 µM RFamide I. (B) Ca2+-dependent shifts of the Erev reveal a high Ca2+ permeability of HyNaC. Erevs of the highly Ca2+-permeable P2X4 receptor are shown for comparison and were similar to HyNaC. P2X4 was activated by 10 µM ATP.
Figure 7.
A conserved aspartate is necessary for the high Ca2+ permeability of HyNaC. (A) Alignment of the amino acid sequences of HyNaC2, 3, and 5 and ASIC1a at the beginning of the second transmembrane domain (TMD2) in the one-letter code. The crucial aspartate is indicated by an asterisk. Completely conserved amino acids are shown as white letters on a black background. (B) Representative current trace for HyNaC_D-C activated by 1 µM RFamide I. The amplitude and shape of the transient current component were more variable than for the wt. Mean maximum current amplitudes were 3.45 ± 1.2 µA for the first activation (n = 5). (C) Representative current trace showing activation of HyNaC_D-C by 1 µM RFamide I in solutions containing 1 mM Ca2+ together with either 140 mM NMDG+ or Na+. No inward current was visible in NMDG+ solutions. (D) I/V plot for HyNaC_D-C activated by 1 µM RFamide I in standard bath solution. Currents were normalized to the current measured at −70 mV, which had an amplitude of −1.1 ± 0.2 µA (n = 6). (E; left) Representative current trace for HyNaC_D-C in solutions containing 10 mM Ca2+, 10 mM Ca2+ and 10 mM Na+, or 10 mM Na+ (nominally free of Ca2+). Conditions were as in Fig. 3 B. (Right) Quantitative comparison of current amplitudes. ***, P < 0.001. (F) I/V relations of HyNaC_D-C in solutions containing 1 or 10 mM CaCl2 and no NaCl (which had been replaced by 140 or 126.5 mM NMDG-Cl, respectively) and in a solution containing 140 mM NaCl and 1 mM CaCl2. Lines are the sum of eight to nine individual measurements. SEM is indicated by gray errors bars. Experimental conditions were as in Fig. 6 A. Triangles represent the Erevs that are plotted in G. (G) Ca2+-dependent shifts of the Erev reveal a significantly lower Ca2+ permeability of HyNaC_D-C compared with HyNaC wt. Erevs of HyNaC wt and P2X4 (gray symbols) are from Fig. 6 B and shown for comparison. (H) Representative trace (black) from photometric Ca2+ measurements illustrating no change of the F340/380 ratio after the application of ∼1 µM RFamide I (asterisk) in a oocyte expressing HyNaC_D-C. The deflections in the F340/380 ratio immediately after the peptide was added are caused by movements of the solution and are unspecific. Oocytes had been injected with 50 nl Fura-2AM (1 mM) 30–120 min before the recording. The red trace represents the current that robustly increased after peptide application. (I) F340/F380 before and after the application of ∼1 µM RFamide I for HyNaC_D-C (n = 8). (J) Plot of the increase of the F340/380 ratio (as percentage) after the application of RFamide I as a function of peptide-activated current amplitudes. Closed squares represent individual measurements for HyNaC wt, and open triangles are for HyNaC_D-C (n = 8). Current amplitudes were smaller for HyNaC_D-C than for HyNaC wt (n = 8; P < 0.001; Fig. 5 B). Solid lines represent linear fits of the fluorescence/current ratio. Note that the slope of this line was larger for wt than for the D-C mutant, indicating a higher relative Ca2+ permeability of the wt.
Results are expressed as mean ± SEM. Statistical significance was determined using Student’s paired or unpaired t test, as appropriate.
RESULTS
The transient current in HyNaC-expressing oocytes is carried by Cl−
Fig. 1 A illustrates the typical biphasic currents elicited by Hydra-RFamide I (Moosler et al., 1996) in oocytes expressing HyNaC2/3/5 (named HyNaC in the remainder of this study, for simplicity). A fast transient current was followed by a slower sustained current; the ratio of transient and sustained currents was variable. Typically, a second peptide application elicited a similar response but of smaller amplitude (72 ± 8% of the first amplitude; n = 11; P < 0.01; Figs. 1 A and 2 A, bottom). A third application elicited a response of similar amplitude as the second (74 ± 9% of the first amplitude; n = 11; P < 0.01; Figs. 1 A and 2 A, bottom). The basis for this inactivation between the first and second application has remained unclear.
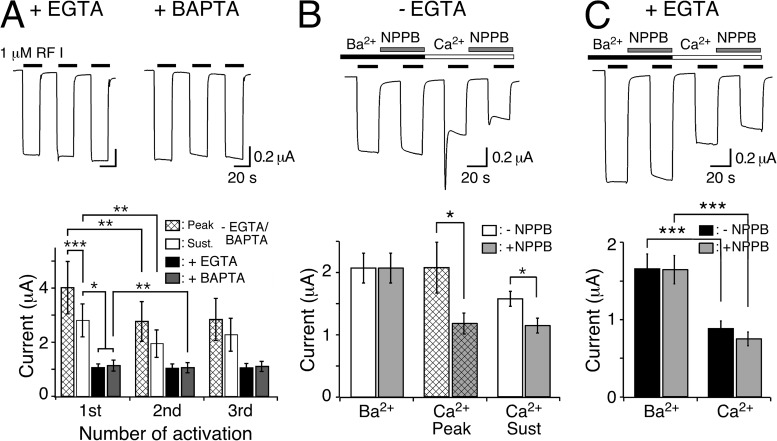
Figure 2.
Chelation of [Ca2+]i abolishes transient peptide-activated currents. (A; top) Representative HyNaC2/3/5 currents in oocytes injected with EGTA or BAPTA. Note the absence of a transient current and the step-like appearance of the currents. (Bottom) Bar graphs comparing the peak (hatched bars) and sustained (open bars) current amplitudes of oocytes expressing HyNaC and of oocytes expressing HyNaC and injected with EGTA (closed bars) or BAPTA (gray bars) (n = 11). (B; top) Representative current trace comparing peptide-activated currents in standard bath and in standard bath in which CaCl2 was replaced by an equimolar amount (1.8 mM) of BaCl2. Currents were measured either in the presence or the absence of 100 µM NPPB. Oocytes were preincubated for 20 s in the corresponding test solutions before activation by 1 µM RFamide I. (Bottom) Bar graph comparing the current amplitudes in the absence of NPPB (open bars) and in the presence of 100 µM NPPB (gray bars) for the indicated conditions. In the presence of Ba2+, no transient current was discernible. The application sequence of the individual solutions was shuffled (n = 8). (C) As in B, for oocytes that had been injected with EGTA. Note the absence of peak currents. (Bottom) Bar graph comparing the current amplitudes in the absence of NPPB (closed bars) and in the presence of 100 µM NPPB (gray bars) for sustained currents. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Previously, we determined Erevs of the sustained current by stepping to different voltages during long agonist applications (Dürrnagel et al., 2010); Erev was around +10 mV, indicating a relatively unselective cation current. Now, we determined Erev of the peptide-activated currents by repeatedly activating HyNaC at different holding potentials between −70 and +20 mV and noticed that transient and sustained components had different Erevs (Fig. 1, B and C). Although at −20 mV the transient current was clearly outward, the sustained current was inward, showing that both currents had different ion selectivity. Although the strong rectification rendered a precise determination of Erev of the sustained current difficult, we estimated it to be 2 ± 3 mV (n = 6; Fig. 1 C), similar to what had been reported previously (Dürrnagel et al., 2010). In contrast, Erev of the transient current was −22 ± 2 mV (n = 6; Fig. 1 C). Thus, the transient current clearly had different ion selectivity than the sustained current (P < 0.001). The leftward shift of the Erev, however, was not compatible with an increased Na+ selectivity of the transient current. In addition, it is notable that at more depolarized potentials, the amplitude of the transient outward current decreased rather than increased in amplitude (Fig. 1, B and C). This decreased amplitude can now be attributed to a block by Ca2+ that inhibits flux of ions at more depolarized potentials (see below).
Assuming an intracellular Cl− concentration of 33 mM, as reported for immature oocytes (Barish, 1983), in our solutions the Cl− equilibrium potential was −38 mV, close to Erev of the transient current. We therefore explored whether Cl− contributed to the transient current and determined Erev in Cl−-free solutions. Under these conditions, outward currents caused by influx of Cl− were abolished (Fig. 1 D). Although Erev of the sustained current was not changed (Erev = 0.6 ± 2.8 mV; n = 5; P = 0.8), no transient outward currents were observed, strongly suggesting that the transient outward current in Cl−-containing solutions was carried by Cl−.
Xenopus oocytes contain a CaCC in their plasma membrane, which is activated by increases in [Ca2+]i (Miledi, 1982; Barish, 1983). Activation of the CaCC leads to biphasic currents: an initial transient Cl− current (Miledi, 1982; Barish, 1983; White and Aylwin, 1990) is followed by sustained Cl− currents (Boton et al., 1990; Wu and Hamill, 1992; Schroeder et al., 2008), resembling the appearance of peptide-activated currents in HyNaC-expressing oocytes. Moreover, repeated activation of the CaCC leads to reduced responses (Boton et al., 1990; Wu and Hamill, 1992) akin to the reduced amplitude of peptide-activated currents with repeated peptide application (Figs. 1 A and 2 A, bottom). We therefore considered that HyNaC is permeable to Ca2+ and that HyNaC activated the CaCC of oocytes. Closer inspection of measurements at a holding potential slightly positive to the Cl− equilibrium potential (−20 mV) indeed revealed a current with three phases (Fig. 1 B, inset): an initial inward current, which could reflect an initial influx of cations through HyNaCs, was followed by a transient outward current, presumably carried by the influx of Cl−, and a third sustained inward current, again dominated by influx of cations through HyNaCs.
The transient current is mediated by the endogenous CaCC
To test for the activation of the CaCC, we injected oocytes with either EGTA or BAPTA to chelate Ca2+ and prevent a rise in intracellular [Ca2+] and activation of the CaCC. Under these conditions, transient currents were indeed completely abolished, and peptide-activated currents had a step-like appearance with no strong desensitization (Fig. 2 A). Moreover, a second peptide application elicited a current of similar amplitude as the first application (for EGTA: 96 ± 5% of the first amplitude, P = 0.7; for BAPTA: 94 ± 2% of the first amplitude, P < 0.01; paired t test; n = 11; Fig. 2 A). In addition to the disappearance of transient currents, sustained currents had significantly smaller amplitudes after injection of EGTA or BAPTA (for EGTA: 43 ± 6% of the sustained current amplitude without EGTA injection, P < 0.01; for BAPTA: 47 ± 6% of the sustained current amplitude without BAPTA injection, P < 0.01; n = 11; Fig. 2 A), suggesting that the CaCC current may contribute not only to the transient but also to the sustained component of the peptide-activated currents. There were no significant differences in oocytes injected with EGTA or BAPTA. Therefore, in the remainder of this study, we used EGTA to prevent a rise in intracellular [Ca2+].
To further test for the activation of the CaCC, we replaced extracellular Ca2+ by Ba2+, which does not activate CaCCs (Barish, 1983). Indeed, in the presence of Ba2+, peptide-activated currents had a step-like appearance like after chelation of intracellular Ca2+ (Fig. 2 B). Furthermore, we pharmacologically blocked CaCC by 5-nitro-2-(3-phenylpropylamino)-benzoate (NPPB) (Wu and Hamill, 1992). 100 µM NPPB reduced the amplitude of transient currents by about half and of sustained currents by 28% (P < 0.05; n = 8; Fig. 2 B), indicating partial block of CaCCs by 100 µM NPPB, which is similar to previous reports (Wu and Hamill, 1992; Schroeder et al., 2008).
We wanted to exclude that NPPB had a direct effect on HyNaC and repeated those experiments with EGTA-injected oocytes. The amplitudes of currents in the presence of Ba2+ were now approximately twofold increased compared with currents in the presence of Ca2+ (n = 8; P < 0.001), suggesting either that Ba2+ potentiated HyNaC currents or that Ca2+ blocked them. NPPB slightly reduced the amplitude of HyNaC currents from 0.88 ± 0.1 to 0.75 ± 0.09 µA (n = 8; P = 0.2; paired t test; Fig. 2 C), indicating that NPPB has no strong direct effect on HyNaC.
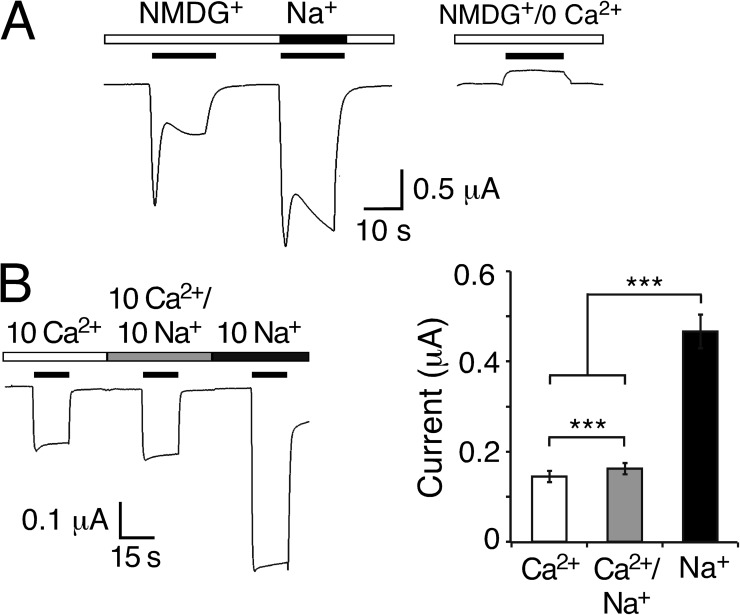
Our results so far strongly argue for an activation of the endogenous CaCC by HyNaCs. Peptide-activated inward currents of HyNaC-expressing oocytes apparently are the sum of a Na+ current flowing through HyNaCs and a Cl− current flowing through CaCCs, with CaCCs being responsible for the biphasic kinetics of the currents. We directly tested this interpretation by activating HyNaCs in the absence of extracellular Na+ (Fig. 3 A). We replaced all Na+ by the large monovalent cation NMDG+ (but kept 1 mM Ca2+), which should abolish most of HyNaC inward currents. Under these conditions, Hydra-RFamide I still elicited a current with biphasic kinetics but of smaller amplitude. Peak current amplitudes were ∼70% (n = 4; P = 0.14) and sustained currents ∼30% (n = 4; P < 0.001) of the currents measured in standard NaCl solution. In control experiments with a solution containing NMDG+ nominally free of Ca2+, no inward current was observed (n = 4; Fig. 3 A). These results are consistent with the idea that Ca2+ influx through HyNaCs secondarily activated CaCCs, greatly amplifying the HyNaC current.
Figure 3.
Peptide-activated currents in the absence of Na+e. (A; left) Representative current trace for HyNaC2/3/5 in solutions containing 1 mM Ca2+ together with 140 mM of either NMDG+ or Na+. 1 µM RFamide I was used for activation. (Right) Control experiment in NMDG+ solution nominally free of Ca2+. Similar results were observed in four out of four oocytes. (B; left) Representative current trace for HyNaC2/3/5 in solutions containing 10 mM Ca2+, 10 mM Ca2+ and 10 mM Na+, or 10 mM Na+ (nominally free of Ca2+). Solutions additionally contained 10 mM HEPES, pH 7.4, and NMDG+ at concentrations to reach similar osmolarity (10 Ca2+: 125 mM NMDGCl; 10 Ca2+/10 Na+: 115 mM NMDGCl; and 10 Na+: 130 mM NMDGCl). Oocytes had been injected with EGTA; 2 µM RFamide I was used for activation. Holding potential was −85 mV. (Right) Quantitative comparison of current amplitudes. ***, P < 0.001.
To directly show Ca2+ influx through HyNaC, we activated HyNaC in the presence of 10 mM Ca2+ as the exclusive extracellular cation and in EGTA-injected oocytes to prevent activation of the CaCC (Fig. 3 B). Under these conditions, Hydra-RFamide I indeed induced a step-like inward current with an amplitude of 0.15 ± 0.01 µA (n = 11; Fig. 3 B), directly demonstrating robust influx of Ca2+ through HyNaC. The addition of an equimolar concentration of Na+ (10 mM) only slightly increased the current amplitude to 0.16 ± 0.01 µA (n = 11; P < 0.01), whereas in the presence of 10 mM Na+ as the exclusive cation, peptide-activated currents were increased approximately threefold (0.47 ± 0.04 µA; n = 11; P < 0.001; Fig. 3 B). The smaller current amplitude with a higher total concentration of permeant cations (10 mM Ca2+/10 mM Na+ compared with 10 mM Na+ alone) suggests that Ca2+ blocks HyNaC in addition to permeating it. This behavior as a permeant blocker prohibits a direct quantitative comparison of the inward Na+ and Ca2+ currents.
HyNaCs are unselective cation channels
Previously, we determined an Erev of sustained peptide-activated currents in HyNaC-expressing oocytes of ∼10 mV and concluded that HyNaCs are unselective cation channels (Golubovic et al., 2007; Dürrnagel et al., 2010). We now show that sustained peptide-activated currents are contaminated by Cl− currents through CaCCs, requiring reevaluation of Erev of HyNaCs. We activated HyNaCs in oocytes for 50 s and stepped the holding potential from −70 to +30 mV in steps of 10 mV every 5 s. We determined Erev in three different solutions: (1) standard bath, (2) standard bath with EGTA-injected oocytes, and (3) standard bath in which Ca2+ had been replaced by Ba2+ (Fig. 4). Like reported previously (Dürrnagel et al., 2010) and as already shown in Fig. 1 B, in standard bath, currents had an Erev of 11.5 ± 2.5 mV (n = 8) and were characterized by strong inward rectification. In EGTA-injected oocytes, Erev was shifted to more positive values (24.0 ± 3.7 mV; n = 6; P < 0.05) and currents were also inwardly rectifying. It should be noted that the strong inward rectification in these two conditions rendered the determination of Erev imprecise. When we replaced Ca2+ by Ba2+, two things changed: Erev was shifted to 3.0 ± 1.9 mV (n = 7; P < 0.05), and I-V relations became linear. These results show, first, that HyNaCs indeed have an Erev indicative of a relatively unselective cation channel and, second, that extracellular Ca2+ is the cause of their inward rectification, presumably by blocking HyNaCs at more positive voltages. This Ca2+ block could explain why currents were enhanced when Ca2+ was replaced by Ba2+ (Fig. 2 C), why transient Cl− currents were smaller at more depolarized potentials in standard bath (Fig. 1 B), and why Na+ currents were strongly reduced by an equimolar amount of Ca2+ (Fig. 3 B). We conclude that Ca2+ is a permeant blocker of HyNaCs and that the block is voltage dependent. Ca2+ strongly blocks HyNaC already at −85 mV (Fig. 3 B) but more strongly as the membrane potential becomes more depolarized, leading to an almost complete block of HyNaC at positive potentials (Fig. 4). At present, we do not have a definite explanation for the shift of Erev when Ca2+ had been replaced by Ba2+ (Fig. 4).
Ca2+ permeability of HyNaC is high
Activation of CaCCs indirectly measures Ca2+ permeation through HyNaC. To directly measure Ca2+ permeation, we additionally performed photometric Ca2+ measurements using Fura-2. The application of Hydra-RFamide I to oocytes that expressed HyNaC and had been injected with Fura-2 led to a robust increase in the F340/F380 ratio, indicating an increase in cytosolic Ca2+ (Fig. 5 A). Simultaneous measurement of currents revealed that the [Ca2+]i increase paralleled the opening of the HyNaC pore (Fig. 5 A). [Ca2+]i quickly rose after channel opening and remained high during channel activation. A similar rise in [Ca2+]i was observed in all oocytes tested (n = 8; P < 0.001; paired t test; Fig. 5 B). The robust signal in photometric Ca2+ measurements after the opening of HyNaCs and the robust activation of CaCCs by HyNaC suggest a high Ca2+ permeability of HyNaCs.
We then estimated the relative Ca2+ permeability of HyNaC by determining Erev in two different Ca2+ concentrations and with EGTA-injected oocytes. To increase the contribution of the Ca2+ current to the total current, we replaced Na+ by the larger impermeant cation NMDG+. In 1 mM Ca2+, Erev was −43 ± 1.5 mV (n = 9), whereas in 10 mM Ca2+, it was significantly shifted to −17 ± 2.6 mV (n = 10; P < 0.001; Fig. 6 A). To assess these values, we made identical measurements with P2X4, a purinergic ion channel of known high Ca2+ permeability (PCa/Pmono = 4.2; Soto et al., 1996). In fact, Erev of P2X4 in 1 and 10 mM Ca2+ was similar to previously published values for P2X4 (Soto et al., 1996) and to Erev of HyNaC (Fig. 6 B), suggesting that PCa/Pmono of HyNaC is on the same order as that of P2X4.
Finally, we calculated the relative Ca2+ permeability PCa/PNa of HyNaC by determining the shift in Erev when the solution contained either Ca2+ or Na+ as the main permeant cation. As mentioned above, Erev with 10 mM Ca2+ was −17 ± 2.6 mV and with 140 mM Na+ it was shifted to 14 ± 2.5 mV (n = 8; Fig. 6 A), allowing us to calculate a permeability ratio PCa/PNa = 3.85 (n = 8; see Materials and methods), which is in good agreement with a PCa/Pmono of P2X4.
We also calculated the permeability ratio PNa/PK of HyNaC by determining Erev with 140 mM of either Na+ or K+, yielding a relative permeability PNa/PK = 3.0 (n = 8; not depicted), which is in agreement with previous results (Dürrnagel et al., 2010). The higher relative permeability for Na+ over K+ of HyNaC compared with P2X4 (PNa/PK of ∼1; Soto et al., 1996) can explain the slightly more positive Erev of HyNaC in 10 mM Ca2+ (−17 ± 2.6 mV compared with −27 ± 1 mV; P < 0.01) in the experiment shown in Fig. 6 B.
In summary, we obtained consistent evidence for a high relative Ca2+ permeability of HyNaC.
Negative charges at the outer entrance to the ion pore are necessary for the high Ca2+ permeability of HyNaC
ASIC1a is a DEG/ENaC that is slightly permeable for Ca2+ (Waldmann et al., 1997; Bässler et al., 2001; Samways et al., 2009) and at the same time blocked by Ca2+ (Paukert et al., 2004). A ring of conserved aspartates at the outer mouth of the ASIC1a pore is crucial for Ca2+ block, presumably by constituting a Ca2+-binding site (Paukert et al., 2004). These aspartates are conserved in HyNaC subunits 2, 3, and 5 (Fig. 7 A). Therefore we asked whether they contribute to Ca2+ permeability of HyNaCs. Substitution of the conserved aspartate by cysteine in all three HyNaC subunits (2/3/5; see Materials and methods) resulted in functional channels (HyNaC_D-C). Activation of HyNaC_D-C in oocytes evoked currents similar to HyNaC wild type (wt) (Fig. 7 B), but the presence of a peak current component was more variable, suggesting reduced activation of CaCCs by HyNaC_D-C. Furthermore, current amplitudes were smaller than those for HyNaC wt, as would be expected when CaCCs were less efficiently activated. To compensate for the smaller current amplitudes, we injected a 20-fold greater amount of cRNA for HyNaC_D-C. When we replaced Na+ by NMDG+ (keeping 1 mM Ca2+) to abolish most of HyNaC_D-C inward currents, Hydra-RFamide I no longer elicited robust currents (Fig. 7 C), whereas it elicited robust inward currents in standard bath (I = 1 ± 0.25 µA; n = 5; Fig. 7 C). As we have shown above that the currents in the absence of Na+ are CaCC currents, these findings indicate that activation of HyNaC_D-C did indeed not efficiently activate CaCCs and support reduced Ca2+ influx through HyNaC_D-C. In standard bath, Erev of HyNaC_D-C was +16 ± 2 mV (n = 6; Fig. 7 D), indicative of a relatively unselective cation current, similar to HyNaC wt. Of note, HyNaC_D-C currents were not inwardly rectifying (Fig. 7 D), showing that the conserved Asp is necessary for inward rectification and in line with the requirement of Ca2+ for inward rectification. In EGTA-injected oocytes, HyNaC_D-C currents in the presence of 10 mM Ca2+ as the sole extracellular cation were tiny and approximately sevenfold smaller than in the presence of 10 mM Na+ (0.01 ± 0.004 µA compared with 0.07 ± 0.01 µA; n = 11; Fig. 7 E); the addition of 10 mM Ca2+ to the Na+-containing solution only slightly decreased the current amplitude by 29 ± 3% (0.05 ± 0.01 µA; n = 11; P < 0.001; Fig. 7 E), whereas for HyNaC wt, it decreased the current amplitude by 74 ± 2% (P < 0.001; Fig. 3 B). These results are consistent with the interpretation that HyNaC_D-C had a lower Ca2+ permeability and was less blocked by Ca2+ than HyNaC wt.
With 1 and 10 mM Ca2+ in EGTA-injected oocytes, the Erev of HyNaC_D-C was significantly shifted to more negative potentials compared with HyNaC wt (n = 7–8; P < 0.001; Fig. 7, F and G), confirming a reduced Ca2+ permeability of HyNaC_D-C. The relative Ca2+ permeability of HyNaC_D-C, calculated by determining the shift in Erev when the solution contained either Ca2+ or Na+ as the main permeant cation (Fig. 7 F; see Materials and methods), was PCa/PNa = 0.44 (n = 8), thus ninefold smaller than for HyNaC wt. In contrast, the permeability ratio for monovalent cations was comparable to HyNaC wt (PNa/PK = 2.9; n = 8; not depicted). These results suggest that the inefficient activation of CaCCs by HyNaC_D-C is caused by its strongly reduced Ca2+ permeability.
In photometric Ca2+ measurements, activation of HyNaC_D-C did not significantly increase the F340/F380 ratio (n = 8; P = 0.12; paired t test, Fig. 7 I; example in Fig. 7 H), consistent with a reduced Ca2+ influx in HyNaC_D-C–expressing oocytes. As expected, the increase in the F340/F380 ratio correlated with the current amplitude and could be fitted reasonably well with a line (Fig. 7 J). This linear regression analysis for the F340/F380 ratio change versus current showed a shallower slope for the D-C mutant than for HyNaC wt (P < 0.01; Fig. 7 J), which is also consistent with a reduced Ca2+ permeability of HyNaC_D-C.
DISCUSSION
HyNaCs are highly Ca2+ permeable
In a previous study (Dürrnagel et al., 2010), we described biphasic currents after the application of Hydra-RFamides in oocytes expressing HyNaC and entirely attributed these currents to currents flowing through the HyNaC pore. We concluded that HyNaC partially desensitizes and shows a tachyphylaxis from first to second peptide application. Moreover, we noted a variable ratio between transient and sustained currents (Dürrnagel et al., 2010).
Our new results show that those previous conclusions were wrong. HyNaCs are highly Ca2+-permeable members of the DEG/ENaC gene family. In Xenopus oocytes, this high Ca2+ permeability leads to robust activation of the endogenous CaCC, and the Cl− current adds to the cation current flowing through HyNaC itself. This secondary activation of the CaCC is entirely responsible for the biphasic appearance of peptide-activated currents in HyNaC-expressing oocytes. This conclusion is based on several different observations: (a) Erev of the transient current is Cl− dependent; (b) intracellular chelation of Ca2+ abolishes the transient current and leads to step-like HyNaC currents; (c) Hydra-RFamides elicit biphasic inward currents also in the absence of permeant monovalent cations; (d) Ca2+ measurements reveal an increase in [Ca2+]i that parallels the opening of the HyNaC pore; and (e) Erev of HyNaC is Ca2+ dependent. These results consistently show that HyNaCs are Ca2+ permeable. Moreover, our results show that HyNaC currents have no strong time dependence; in the presence of the intracellular Ca2+ chelators EGTA or BAPTA, Hydra-RFamides elicit step-like currents that do not desensitize. The apparent biphasic HyNaC current and tachyphylaxis from first to second peptide application (Golubovic et al., 2007; Dürrnagel et al., 2010) arise from the secondary activation of the CaCC. Consequently, tachyphylaxis was abolished in cells injected with EGTA (Fig. 2 A).
Several ligand-gated channels share a high Ca2+ permeability with HyNaCs and activate CaCCs when expressed in Xenopus oocytes, among them NMDA receptors (Leonard and Kelso, 1990), kainate receptor GluR6 (Egebjerg and Heinemann, 1993), α7 and α9 nicotinic acetylcholine receptors (Galzi et al., 1992; Vernino et al., 1992; Séguéla et al., 1993; Katz et al., 2000), and purinergic P2X4 receptors (Soto et al., 1996). Our estimates of the Ca2+ permeability of HyNaCs, PCa/PNa = 3.85, revealed a Ca2+ permeability similar to that of P2X4 (Soto et al., 1996), PCa/Pmono = 4.2, placing HyNaCs among the highly Ca2+-permeable ligand-gated ion channels. We note, however, that PCa/PNa ratios obtained by measurements of the shift in Erevs generally have to be regarded with caution, as the Goldman–Hodgkin–Katz constant field voltage equation, which was used to calculate permeability ratios, requires that there is no interaction among permeating ions (Burnashev et al., 1995; Hille, 2001). Like for most ion channels, this is not the case for HyNaCs, implying that PCa/PNa is not constant but depends in a complex manner on the voltage and the individual concentrations of divalent and monovalent ions on both sides of the membrane. For example, for HyNaC we estimated a ratio of PCa/PNa ≈ 10, when using Erev in a solution containing 1 mM (instead of 10 mM) Ca2+.
The FMRFamide-activated Na+ channel, FaNaC, is another peptide-gated ion channel from the DEG/ENaC gene family (Lingueglia et al., 1995). When expressed in oocytes, FMRFamide elicits currents that develop comparatively slowly over a few seconds and that partially desensitize. Moreover, FaNaC shows an Erev indicative of a highly Na+-selective ion channel (Lingueglia et al., 1995). Thus, kinetics and ion selectivity are considerably different between the only two known peptide-gated ion channels, both from the DEG/ENaC gene family.
A conserved Asp confers high Ca2+ permeability
In addition, we show that a conserved aspartate is necessary for the high Ca2+ permeability of HyNaCs. Again, several observations support this conclusion: (a) when the conserved aspartate was substituted by a cysteine (HyNaC_D-C), Hydra-RFamides elicit no strong inward currents in the absence of permeant monovalent cations; (b) Erev with Ca2+ as main charge carrier was significantly shifted compared with wt; (c) the relative Ca2+ permeability was reduced to PCa/PNa = 0.44; and (d) photometric Ca2+ measurements revealed no increase in [Ca2+]i when channels opened. The aspartate is conserved in ASICs and is crucial for Ca2+ block of ASICs (Paukert et al., 2004) and for coordinating monovalent cations at the outer ASIC pore (Gonzales et al., 2009). Thus, it is likely that these aspartates form a ring of negative charges at the outer entry of the HyNaC pore that attracts cations, in particular Ca2+. Removing the aspartate abolished inward rectification, suggesting that the aspartate is a determinant of a Ca2+-binding site, which mediates Ca2+ block and thus inward rectification of HyNaC.
Because ASICs also have the crucial Asp but are impermeable for Ca2+, with the exception of ASIC1a that has a low Ca2+ permeability (Bässler et al., 2001; Samways et al., 2009), the Asp is necessary but not sufficient for Ca2+ permeability of HyNaCs. Reminiscent of this situation, for NMDA receptor channels it has been proposed that Ca2+ interacts with “external” and “deep” sites, corresponding to regions at the external mouth and central in the pore of the channel, respectively (Watanabe et al., 2002). The conserved aspartate of HyNaCs and ASICs could represent an external Ca2+-interaction site, and the putative internal site would also be necessary for Ca2+ permeation. In fact, for ASIC1, amino acids at the intracellular N terminus determine Ca2+ permeability (Bässler et al., 2001). Future experiments will show whether the high Ca2+ permeability of HyNaCs also depends on their intracellular N termini or other regions along the axis of their pore.
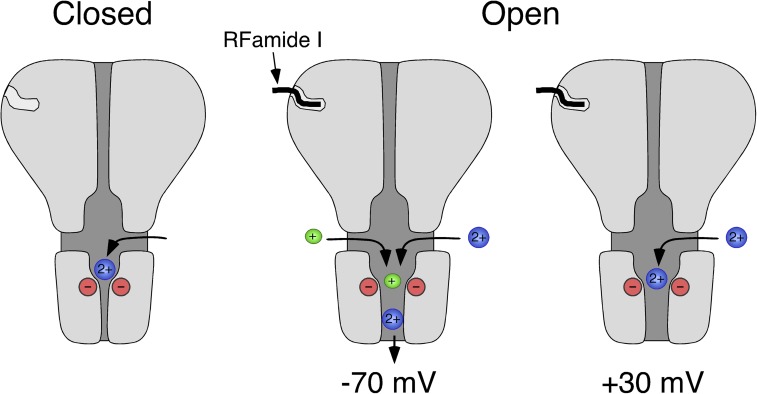
Collectively, our results suggest the following scenario (Fig. 8). Ca2+ gets attracted to the outer mouth of the HyNaC pore by the negative charges of the conserved ring of aspartates. At positives potentials (for example, +30 mV), Ca2+ inefficiently permeates the channel and blocks the open pore, presumably by tightly binding to the aspartates. At more negative potentials (for example, −70 mV) Ca2+ more efficiently permeates the channel, liberating the pore for the passage also of monovalent cations.
Figure 8.
Scheme illustrating Ca2+ permeation through HyNaC. In the closed conformation (left), Ca2+ can probably access the extracellular vestibule through three lateral fenestrations (Jasti et al., 2007; Li et al., 2011) and bind to the conserved aspartates at the outer mouth of the ion pore. After binding of the ligand, HyNaC opens and, at negative membrane potentials, Ca2+ permeates the channel (−70 mV, middle), liberating the ion pore. At depolarized potentials (+30 mV, right), however, Ca2+ remains bound to the ring of negative charges and blocks the open pore. Based on the crystal structure of chicken ASIC1 (Jasti et al., 2007).
HyNaCs are the first DEG/ENaCs with a high Ca2+ permeability
So far, all DEG/ENaCs have been found to be more or less Na+ selective; in fact, Na+ selectivity is a defining hallmark of these channels. In the few exceptions, where DEG/ENaCs are associated with unselective ion pores, the unselective state coexists with a Na+-selective state, as in the case of some ASICs (Lingueglia et al., 1997; Springauf and Gründer, 2010) and rat brain liver intestine Na+ channel (rBLINaC, now named bile acid–sensitive ion channel, or BASIC) (Sakai et al., 1999; Wiemuth and Gründer, 2010; Wiemuth et al., 2012). In these cases, the Na+-selective state probably carries most of the current under physiological conditions. MEC-4, a subunit of a mechanosensitive DEG/ENaC from C. elegans (Driscoll and Chalfie, 1991), can be constitutively activated by mutation (MEC-4(d)) and then carries a Ca2+ component with a permeability ratio of Ca2+ versus Na+ (PCa/PNa) of 0.22 (Bianchi et al., 2004), >10 times lower than for HyNaCs. Moreover, the physiologically relevant current carried by Mec-4 is a Na+ current (Goodman et al., 2002; O’Hagan et al., 2005). Thus, HyNaCs are the first DEG/ENaCs that are unselective cation channels with a high Ca2+ permeability.
At present, we can only speculate on the role of Ca2+ permeability for the physiological function of HyNaCs. HyNaCs are expressed at the base of the tentacles, perhaps in epitheliomuscular cells, and the preprohormone gene that encodes their ligands (Darmer et al., 1998), Hydra-RFamides I and II, is expressed in adjacent nerve cells of the hypostome and upper gastric region (Hansen et al., 2000). Therefore, it has been speculated that HyNaCs contribute to coordinate the Hydra feeding response (Golubovic et al., 2007; Dürrnagel et al., 2010). In agreement with this hypothesis, Antho-RFamide (pQGRF-NH2), a related RFamide peptide from anthozoans, another class within the phylum Cnidaria that contains sea anemones, has excitatory actions on sea anemone muscle preparations and increases the frequency of spontaneous tentacle contractions (McFarlane et al., 1991). Thus, the action of Antho-RFamide is compatible with the idea that Hydra-RFamides are transmitters at neuromuscular junctions and that HyNaCs are their postsynaptic receptors. Of note, another anthozoan neuropeptide, Antho-RWamide I (pQSLRW-NH2), increases an inward Ca2+ current in endodermal myoepithelial cells of sea anemones (Cho and McFarlane, 1996). Whatever the exact physiological function of HyNaCs is, it is likely that Ca2+ influx through open HyNaCs supports their function. The block by Ca2+ of HyNaC will limit influx of Ca2+, especially at more depolarized membrane potentials.
In summary, we show that HyNaC has simple kinetics and does not desensitize in the prolonged presence of its ligands, and that HyNaC is an unselective cation channel with a high Ca2+ permeability, which is an uncommon feature of DEG/ENaCs. Therefore, it is possible that the highly selective Na+ pore of DEG/ENaCs that is a characterizing feature of these channels is in fact a feature that was not shared by the common ancestor and developed during evolution.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft (grant GR 1771/7-1 to S. Gründer) and the Interdisciplinary Centre for Clinical Research within the Faculty of Medicine at RWTH Aachen University (grant N5-1 to B.H. Falkenburger).
Lawrence G. Palmer served as editor.
Footnotes
Abbreviations used in this paper:
- ASIC
- acid-sensing ion channel
- [Ca2+]i
- intracellular Ca2+ concentration
- CaCC
- Ca2+-activated Cl− channel
- DEG/ENaC
- degenerin/epithelial Na+ channel
- Erev
- reversal potential
- HyNaC
- Hydra Na+ channel
- NPPB
- 5-nitro-2-(3-phenylpropylamino)-benzoate
- wt
- wild type
References
- Barish M.E. 1983. A transient calcium-dependent chloride current in the immature Xenopus oocyte. J. Physiol. 342:309–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bässler E.L., Ngo-Anh T.J., Geisler H.S., Ruppersberg J.P., Gründer S. 2001. Molecular and functional characterization of acid-sensing ion channel (ASIC) 1b. J. Biol. Chem. 276:33782–33787 10.1074/jbc.M104030200 [DOI] [PubMed] [Google Scholar]
- Bianchi L., Gerstbrein B., Frøkjaer-Jensen C., Royal D.C., Mukherjee G., Royal M.A., Xue J., Schafer W.R., Driscoll M. 2004. The neurotoxic MEC-4(d) DEG/ENaC sodium channel conducts calcium: implications for necrosis initiation. Nat. Neurosci. 7:1337–1344 10.1038/nn1347 [DOI] [PubMed] [Google Scholar]
- Boton R., Singer D., Dascal N. 1990. Inactivation of calcium-activated chloride conductance in Xenopus oocytes: roles of calcium and protein kinase C. Pflugers Arch. 416:1–6 10.1007/BF00370214 [DOI] [PubMed] [Google Scholar]
- Burnashev N., Zhou Z., Neher E., Sakmann B. 1995. Fractional calcium currents through recombinant GluR channels of the NMDA, AMPA and kainate receptor subtypes. J. Physiol. 485:403–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho K., McFarlane I.D. 1996. The anthozoan neuropeptide Antho-RWamide I modulates Ca2+ current in sea anemone myoepithelial cells. Neurosci. Lett. 209:53–56 10.1016/0304-3940(96)12597-0 [DOI] [PubMed] [Google Scholar]
- Cottrell G.A., Green K.A., Davies N.W. 1990. The neuropeptide Phe-Met-Arg-Phe-NH2 (FMRFamide) can activate a ligand-gated ion channel in Helix neurones. Pflugers Arch. 416:612–614 10.1007/BF00382698 [DOI] [PubMed] [Google Scholar]
- Darmer D., Hauser F., Nothacker H.P., Bosch T.C., Williamson M., Grimmelikhuijzen C.J.P. 1998. Three different prohormones yield a variety of Hydra-RFamide (Arg-Phe-NH2) neuropeptides in Hydra magnipapillata. Biochem. J. 332:403–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies C.W. 1962. Ion Association. Butterworths, London: 190 pp [Google Scholar]
- Driscoll M., Chalfie M. 1991. The mec-4 gene is a member of a family of Caenorhabditis elegans genes that can mutate to induce neuronal degeneration. Nature. 349:588–593 10.1038/349588a0 [DOI] [PubMed] [Google Scholar]
- Dürrnagel S., Kuhn A., Tsiairis C.D., Williamson M., Kalbacher H., Grimmelikhuijzen C.J., Holstein T.W., Gründer S. 2010. Three homologous subunits form a high affinity peptide-gated ion channel in Hydra. J. Biol. Chem. 285:11958–11965 10.1074/jbc.M109.059998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egebjerg J., Heinemann S.F. 1993. Ca2+ permeability of unedited and edited versions of the kainate selective glutamate receptor GluR6. Proc. Natl. Acad. Sci. USA. 90:755–759 10.1073/pnas.90.2.755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galzi J.L., Devillers-Thiéry A., Hussy N., Bertrand S., Changeux J.P., Bertrand D. 1992. Mutations in the channel domain of a neuronal nicotinic receptor convert ion selectivity from cationic to anionic. Nature. 359:500–505 10.1038/359500a0 [DOI] [PubMed] [Google Scholar]
- Golubovic A., Kuhn A., Williamson M., Kalbacher H., Holstein T.W., Grimmelikhuijzen C.J., Gründer S. 2007. A peptide-gated ion channel from the freshwater polyp Hydra. J. Biol. Chem. 282:35098–35103 10.1074/jbc.M706849200 [DOI] [PubMed] [Google Scholar]
- Gonzales E.B., Kawate T., Gouaux E. 2009. Pore architecture and ion sites in acid-sensing ion channels and P2X receptors. Nature. 460:599–604 10.1038/nature08218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman M.B., Ernstrom G.G., Chelur D.S., O’Hagan R., Yao C.A., Chalfie M. 2002. MEC-2 regulates C. elegans DEG/ENaC channels needed for mechanosensation. Nature. 415:1039–1042 10.1038/4151039a [DOI] [PubMed] [Google Scholar]
- Hansen G.N., Williamson M., Grimmelikhuijzen C.J.P. 2000. Two-color double-labeling in situ hybridization of whole-mount Hydra using RNA probes for five different Hydra neuropeptide preprohormones: evidence for colocalization. Cell Tissue Res. 301:245–253 10.1007/s004410000240 [DOI] [PubMed] [Google Scholar]
- Hille B. 2001. Ion Channels of Excitable Membranes. Third edition. Sinauer Associates, MA. 814 pp [Google Scholar]
- Jasti J., Furukawa H., Gonzales E.B., Gouaux E. 2007. Structure of acid-sensing ion channel 1 at 1.9 Å resolution and low pH. Nature. 449:316–323 10.1038/nature06163 [DOI] [PubMed] [Google Scholar]
- Katz E., Verbitsky M., Rothlin C.V., Vetter D.E., Heinemann S.F., Elgoyhen A.B. 2000. High calcium permeability and calcium block of the alpha9 nicotinic acetylcholine receptor. Hear. Res. 141:117–128 10.1016/S0378-5955(99)00214-2 [DOI] [PubMed] [Google Scholar]
- Kellenberger S., Schild L. 2002. Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol. Rev. 82:735–767 [DOI] [PubMed] [Google Scholar]
- Leonard J.P., Kelso S.R. 1990. Apparent desensitization of NMDA responses in Xenopus oocytes involves calcium-dependent chloride current. Neuron. 4:53–60 10.1016/0896-6273(90)90443-J [DOI] [PubMed] [Google Scholar]
- Lewis C.A. 1979. Ion-concentration dependence of the reversal potential and the single channel conductance of ion channels at the frog neuromuscular junction. J. Physiol. 286:417–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T., Yang Y., Canessa C.M. 2011. Outlines of the pore in open and closed conformations describe the gating mechanism of ASIC1. Nat Commun. 2:399 10.1038/ncomms1409 [DOI] [PubMed] [Google Scholar]
- Lingueglia E., Champigny G., Lazdunski M., Barbry P. 1995. Cloning of the amiloride-sensitive FMRFamide peptide-gated sodium channel. Nature. 378:730–733 10.1038/378730a0 [DOI] [PubMed] [Google Scholar]
- Lingueglia E., de Weille J.R., Bassilana F., Heurteaux C., Sakai H., Waldmann R., Lazdunski M. 1997. A modulatory subunit of acid sensing ion channels in brain and dorsal root ganglion cells. J. Biol. Chem. 272:29778–29783 10.1074/jbc.272.47.29778 [DOI] [PubMed] [Google Scholar]
- Madeja M., Musshoff U., Speckmann E.J. 1995. Improvement and testing of a concentration-clamp system for oocytes of Xenopus laevis. J. Neurosci. Methods. 63:211–213 10.1016/0165-0270(95)00094-1 [DOI] [PubMed] [Google Scholar]
- McFarlane I.D., Anderson P.A., Grimmelikhuijzen C.J. 1991. Effects of three anthozoan neuropeptides, Antho-RWamide I, Antho-RWamide II and Antho-RFamide, on slow muscles from sea anemones. J. Exp. Biol. 156:419–431 [DOI] [PubMed] [Google Scholar]
- Miledi R. 1982. A calcium-dependent transient outward current in Xenopus laevis oocytes. Proc. R. Soc. Lond. B Biol. Sci. 215:491–497 10.1098/rspb.1982.0056 [DOI] [PubMed] [Google Scholar]
- Moosler A., Rinehart K.L., Grimmelikhuijzen C.J.P. 1996. Isolation of four novel neuropeptides, the hydra-RFamides I-IV, from Hydra magnipapillata. Biochem. Biophys. Res. Commun. 229:596–602 10.1006/bbrc.1996.1849 [DOI] [PubMed] [Google Scholar]
- O’Hagan R., Chalfie M., Goodman M.B. 2005. The MEC-4 DEG/ENaC channel of Caenorhabditis elegans touch receptor neurons transduces mechanical signals. Nat. Neurosci. 8:43–50 10.1038/nn1362 [DOI] [PubMed] [Google Scholar]
- Paukert M., Babini E., Pusch M., Gründer S. 2004. Identification of the Ca2+ blocking site of acid-sensing ion channel (ASIC) 1: Implications for channel gating. J. Gen. Physiol. 124:383–394 10.1085/jgp.200308973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai H., Lingueglia E., Champigny G., Mattei M.G., Lazdunski M. 1999. Cloning and functional expression of a novel degenerin-like Na+ channel gene in mammals. J. Physiol. 519:323–333 10.1111/j.1469-7793.1999.0323m.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samways D.S., Harkins A.B., Egan T.M. 2009. Native and recombinant ASIC1a receptors conduct negligible Ca2+ entry. Cell Calcium. 45:319–325 10.1016/j.ceca.2008.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder B.C., Cheng T., Jan Y.N., Jan L.Y. 2008. Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell. 134:1019–1029 10.1016/j.cell.2008.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Séguéla P., Wadiche J., Dineley-Miller K., Dani J.A., Patrick J.W. 1993. Molecular cloning, functional properties, and distribution of rat brain alpha 7: a nicotinic cation channel highly permeable to calcium. J. Neurosci. 13:596–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto F., Garcia-Guzman M., Gomez-Hernandez J.M., Hollmann M., Karschin C., Stühmer W. 1996. P2X4: an ATP-activated ionotropic receptor cloned from rat brain. Proc. Natl. Acad. Sci. USA. 93:3684–3688 10.1073/pnas.93.8.3684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springauf A., Gründer S. 2010. An acid-sensing ion channel from shark (Squalus acanthias) mediates transient and sustained responses to protons. J. Physiol. 588:809–820 10.1113/jphysiol.2009.182931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernino S., Amador M., Luetje C.W., Patrick J., Dani J.A. 1992. Calcium modulation and high calcium permeability of neuronal nicotinic acetylcholine receptors. Neuron. 8:127–134 10.1016/0896-6273(92)90114-S [DOI] [PubMed] [Google Scholar]
- Waldmann R., Champigny G., Bassilana F., Heurteaux C., Lazdunski M. 1997. A proton-gated cation channel involved in acid-sensing. Nature. 386:173–177 10.1038/386173a0 [DOI] [PubMed] [Google Scholar]
- Watanabe J., Beck C., Kuner T., Premkumar L.S., Wollmuth L.P. 2002. DRPEER: a motif in the extracellular vestibule conferring high Ca2+ flux rates in NMDA receptor channels. J. Neurosci. 22:10209–10216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White M.M., Aylwin M. 1990. Niflumic and flufenamic acids are potent reversible blockers of Ca2(+)-activated Cl- channels in Xenopus oocytes. Mol. Pharmacol. 37:720–724 [PubMed] [Google Scholar]
- Wiemuth D., Gründer S. 2010. A single amino acid tunes Ca2+ inhibition of brain liver intestine Na+ channel (BLINaC). J. Biol. Chem. 285:30404–30410 10.1074/jbc.M110.153064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiemuth D., Sahin H., Falkenburger B.H., Lefèvre C.M., Wasmuth H.E., Gründer S. 2012. BASIC—a bile acid-sensitive ion channel highly expressed in bile ducts. FASEB J. In press [DOI] [PubMed] [Google Scholar]
- Wu G., Hamill O.P. 1992. NPPB block of Ca(++)-activated Cl- currents in Xenopus oocytes. Pflugers Arch. 420:227–229 10.1007/BF00374996 [DOI] [PubMed] [Google Scholar]