Abstract
Early childhood caries (ECC) prevalence has increased significantly in children ages 2–5 years.1 ECC disproportionately affects lower socioeconomic and minority groups, is a predictor for future decay, but is preventable and manageable2. Caries risk assessment systematically derives a patient’s caries risk and is important during an infant oral health visit beginning at age one. Information obtained through a risk assessment can guide a disease management care path tailored to an individual’s age and risk to effectively treat and manage one’s caries disease process.3
A recent national health survey from 2007 found California and Texas, the two most-populated states in the U.S., to rank among the lowest in children’s oral health.6 In addition, Hispanic children are the most at risk for poor oral health, since 28.5 % of Hispanic children compared to 19.1 % of white children, have not seen a dentist by the age of 17. Increased awareness of the causes and consequences of ECC could help families, especially those who suffer from disparities in access to care, obtain dental care and institute preventive measures within their family practices.
Many parents and caretakers are unaware of the role they play in bacterial transmission to their child. Caregivers pass organisms and bacteria to infants orally through close contact (vertical transmission).7 Furthermore, women from vulnerable underserved communities, and some of their providers, fail to recognize the value of good oral health and relevant importance of regular dental visits and care during pregnancy.8
It is critical that oral health providers, whether at academic centers, in private practice, or at safety net sites (such as health centers and hospital clinics) embrace risk assessment and disease management in addressing ECC. In its Life Course Model 2010 concept paper, "a conceptual framework that helps explain health and disease patterns particularly health disparities — across populations and over time," the U.S. Department of Health and Human Services Maternal and Child Health Bureau (MCHB) posits, "that interventions that reduce risks and increase protective factors can change the health trajectory of individuals and populations". It further suggested “the need to: refocus resources and strategies for a greater emphasis on early (“upstream”) determinants of health; incorporate earlier detection of risks coupled with earlier intervention; and promote protective factors while reducing risk factors at the individual, family and community levels."5,9
Caries Management by Risk Assessment (CAMBRA) is designed for use with newborns to five-year-old children. It is easy to use and offers an approach to disease prevention management that integrates risk assessment of childhood caries as an integral component of a comprehensive oral health visit.
Caries Management by Risk Assessment (CAMBRA)
CAMBRA assists providers to systematically:
-
*
Assess each child and his caregiver’s caries risk in an individualized manner;
-
*
Tailor a specific preventive therapeutic management plan or “care path;”
-
*
Customize a restorative plan in conjunction with preventive care;
-
*
Plan a timely, specific, and appropriate periodicity schedule based on the child’s caries risk
To effectively prevent and management the disease of caries, care should begin early, ideally during an Age One comprehensive oral exam visit. There are six basic steps in the infant oral care visit. A caries risk assessment is the first of these six critical steps, giving the provider more information to help them consider the risk and health status of each patient before beginning the exam.5
Caries risk assessment provides information pertaining to three specific overarching domains:
Risk and/or biological factors such as continual bottle use, sleeping with a bottle, frequency and types of snacks, child taking any medications as well as other risk factors;
Protective factors such as the use of fluoridated tap water, use of fluoridated toothpaste or the use of xylitol on a continuous basis; and
Clinical findings such as presence of early demineralized enamel surface, cavitated lesions, plaque, lack of salivary flow, etc. (information to be obtained from Step 4)
Through a short and brief interview with the caregiver, information is gathered to assess the child’s risk of caries development and disease progression as low, moderate, or high. For example, a child may be at high risk if the child goes to bed or has a constant exposure with a bottle containing liquids with natural or artificial sugar, or snacks throughout the day, etc. Protective factors include brushing with a smear of fluoridated toothpaste at least once daily, especially before bed at night or drinking tap fluoridated water regularly.
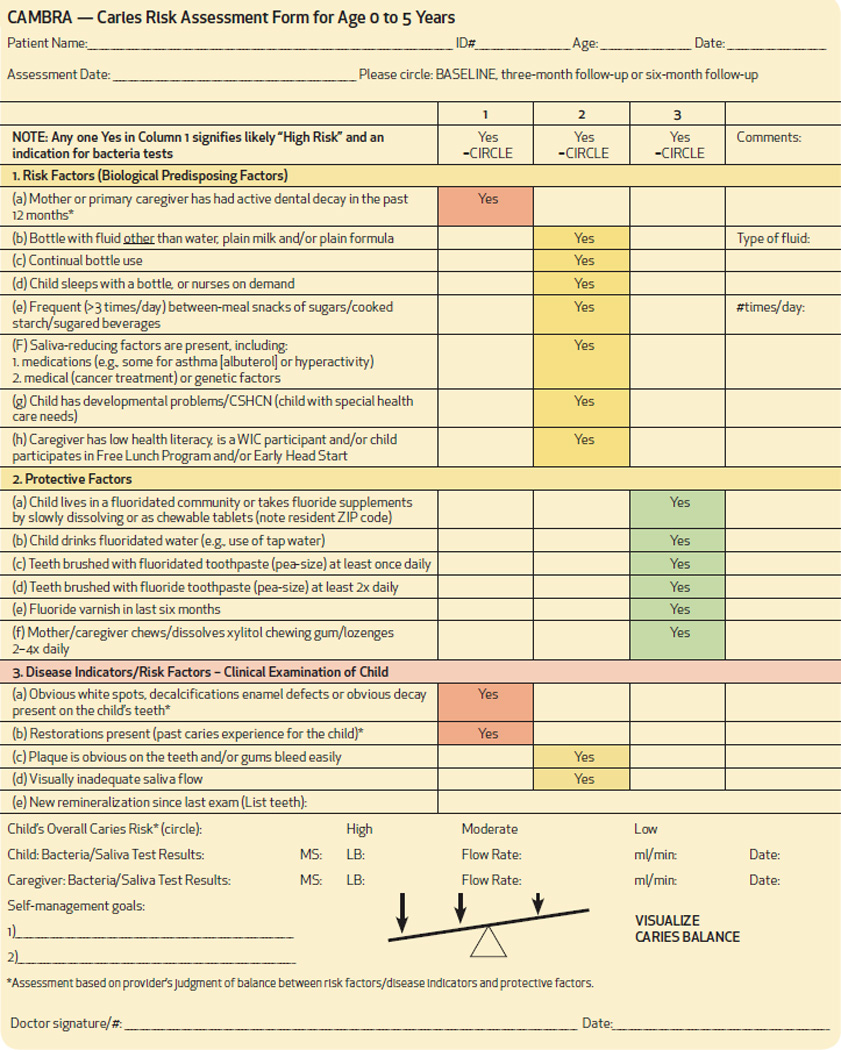
Three findings are always associated with a high caries-risk. These are: 1) new carious lesions in the primary caregiver within the past 12 months; 2) prior caries and/or restorations in the child; and 3) white spot lesions, decalcification enamel defects or other obvious decay in the child (Table 1). The information obtained from a caries risk assessment allows the care provider to formulate a caries risk profile for the child, an essential first step to determining the prevention and treatment plan, as well as the periodicity of patient follow-up/recall (one month, three months, six months or one year).5 Caries risk assessment can be easily and efficiently, performed by dental and medical providers.
Table 1.
CAMBRA Form
 |
Step Two consists of proper positioning of the infant. The knee-to-knee position allows an effective and efficient visualization of the child’s oral cavity and dentition. The child is lying supine, with his head resting in the care provider’s lap. This position allows the child to see his parent and the parent to see what the care provider sees.
Step Three involves a toothbrush prophylaxis, which is effective in removal of plaque on most teeth. Using the tell-show-do technique, the care provider can demonstrate the proper technique for brushing the child’s teeth. Step Four is the clinical examination, and Step Five is the application of fluoride varnish, which is to prevent tooth decay. Fluoride varnish is to be applied every three to six months depending on the caries risk of the child.5,11,12
Step Six involves the care provider working with the parent to determine a mutually agreed upon set of self-management goals appropriate for the family (Table 2) The care provider is to first transmit a summary of the findings obtained from the caries risk assessment and clinical examination, and explain to the parent the causes of the caries process. Together with the parents, one or two self-management goals are to be identified for the family to work on to reduce the child’s risk factors and enhance his protective factors. 5 Parents should be encouraged to adopt a healthy oral lifestyle for their child assist their childr, en from an early age to maintain a proper diet and oral hygiene and establish and maintain a dental home for their child.5 When self-management goals are revisited during recall visits, caregivers and their children can receive positive reinforcement as they see how meeting their goals can improve their child’s caries-risk and result in better oral health outcomes.
Table 2.
Self-Management Goals
 |
Risk-Based Care Paths
Once a child’s caries risk has been determined, the care provider, in partnership with the child’s caregiver, can determine a multifaceted care path appropriate for the family, based on the child’s age and individualized needs. Research supports the use of fluoride varnish in combination with improved diet and oral hygiene counseling, and families should be encouraged to drink fluoridated tap water and/or use fluoridated toothpaste, which are very important aspects and measures of preventive care.11,12 However, a Care Path or decision tree can aid the provider in determining a specific combination of diagnostic, preventive and restorative procedures, and the periodicity of these recommended measures that are appropriate for the child and his family to improve and/or stabilize a child’s caries risk profile.
Care Paths are expected to be dynamic and change over time as the effectiveness of new as well as current protocols are validated by scientific evidence. Tables 3 and 4 are examples of the care paths at this time recommended for children ages 0–2 and ages 3–5, respectively. Newer products, such as remineralizing gels, or new uses for existing products such as glass ionomer to be used as sealants, are now available and can be considered viable treatment options. As evidence continues to evolve, and new studies advocate for the use of agents, such as probiotics and topical applications of providone-iodine to reduce high oral bacteria levels, these modalities will be added to the CarePath. While the evidence for the effectiveness of preventive and treatment modalities continues to grow, standardized and widely accepted protocols are limited. Therefore, each practitioner should use careful consideration based on emerging available evidence and one’s own experience when considering when and how to introduce use of newer modalities into their patients’ care paths.
Table 3.
Example of Recommended Care Path for Children Ages 0–2
| Example of a Caries Management Protocol for 0–2 Year Olds | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Risk Category Ages 0 to 2 |
Diagnostic | Preventive Intervention | Restoration** | ||||||||
| Periodic Oral Exams |
Radiographs* | Saliva Test | Fluoride | Xylitol | Sealants | Antibacterials/ Probiotics |
Anticipatory Guidance/ Counseling |
Self- Management Goals |
White Spot/ Precavitated Lesions |
Existing Lesions | |
| Low | Annual | Posterior bitewings at 12–24 month intervals If proximal surfaces cannot be examined visually or with a probe | Optional baseline |
In office: No Home: Brush 2x day w/ smear of F toothpaste |
Not Required | No | No | Yes | No | n/a | n/a |
| Moderate | Ever/ 6 months | Posterior bitewings at 6–12 month intervals if proximal surfaces cannot be examined visually or with a probe | Suggested |
In office: F varnish initial visit & recalls Home: Brush 2x day w/smear of F toothpaste Caregiver: OTC sodium fluoride treatment rinses |
Child: Xylitol wipes 3–4x daily Caregiver: 2 sticks of gum or 2 mints 4x day, total 6–10 grams a day |
Glass ionomer-based materials recommended on deep pits and fissures | No | Yes | No | Treat w/ fluoride products as indicated to promote remineralization | n/a |
| Moderate; noncompliant | Every 3–6 months | Posterior bitewings at 6–12 month intervals if proximal surfaces cannot be examined visually or with a probe | Recommended |
In office: F vanish initial visit & recalls Home: Brush 2x day w/smear of F toothpaste then apply a smear of calcium-phosphate paste left on at bedtime Caregiver: OTC sodium fluoride treatment rinses |
Child: Xylitol wipes 3–4x daily Caregiver: 2 sticks of gum or 2 mints 4x day, total 6–10 grams a day |
Glass ionomer-based materials recommended on deep pits and fissures | Recommend CHX for caregiver/ Recommend probiotics | Yes | Yes | Treat w/fluoride products as indicated to promote remineralization | n/a |
| High | Every 3 months | Anterior (#2 occlusal film) and posterior bitewings at 6–12 month intervals if proximal surfaces cannot be examined visually or with a probe | Recommended |
In office: F varnish initial visit & recalls Home: Brush 2x day w/smear of F toothpaste then apply a smear of calcium-phosphate paste left on at bedtime Caregiver: OTC sodium fluoride treatment rinses |
Child: Xylitol wipes 3–4x daily Caregiver: 2 sticks of gum or 2 mints 4x day, total 6–10 grams a day |
Glass ionomer-based materials recommended on deep pits and fissures | Recommend CHX for caregiver/ Recommend probiotics | Yes | Yes | Treat w/ fluoride products as Indicated to promote remineralization | ITR [interim therapeutic restorations) with glass ionomer-based materials or conventional restorative treatment as patient cooperation and family circumstances allow |
| High; noncompliant | Every 1–3. months | Anterior (#2 occlusal film) and posterior bitewings at 6–12 month intervals if proximal surfaces cannot be examined visually or with a probe | Recommended |
In office: F varnish initial visit & recalls Home: Brush 2x day w/smear of F toothpaste then apply a smear of calcium-phosphate paste left on at bedtime Caregiver: OTC sodium fluoride treatment rinses |
Child: Xylitol wipes 3–4x daily Caregiver: 2 sticks of gum or 2 mints 4x day, total 6–10 grams a day |
Glass ionomer-based materials recommended on deep pits and fissures | Recommend CHX for caregiver/ Recommend probiotics | Yes | Yes | Treat w/ fluoride products as indicated to promote remineralization | ITR [interim therapeutic restorations) with glass ionomer-based materials or conventional restorative treatment as patient cooperation and family circumstances allow |
| Extreme | Every 1–3 months | Anterior (#2 occlusal film) and posterior bitewings at 6–12 month intervals if proximal surfaces cannot be examined visually or with a probe | Recommended |
In office: F varnish initial visit & recalls Home: Brush 2x day w/smear of F toothpaste then apply a smear of calcium-phosphate paste left on at bedtime Caregiver: OTC sodium fluoride treatment rinses |
Child: Xylitol wipes 3–4x daily Caregiver: 2 sticks of gum or 2 mints 4x day, total 6–10 grams a day |
Glass ionomer-based materials recommended on deep pits and fissures | Recommend CHX for caregiver/ Recommend probiotics | Yes | Yes | Treat w/fluoride products as indicated to promote remineralization | ITR [interim therapeutic restorations) with glass ionomer-based materials or conventional restorative treatment as patient cooperation and family circumstances allow |
Table 4.
Example of Recommended Care Path for Children Ages 3–5
| Example of a Caries Management Protocol for 3–5-Year-Olds | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Risk Category – Ages 3 to 5 |
Diagnostic | Preventive Intervention | Restoration** Existing Lesions |
||||||||
| Periodic Oral Exams |
Radiographs* | Saliva Test | Fluoride | Xylitol &/or Baking Soda |
Sealants | Antibacterials/ Probiotics |
Anticipatory Guidance/ Counseling |
Self- Management Goals |
White Spot/ Precavitrated Lesions |
||
| Low | Annual | Posterior bitewings at 12–24 month intervals If proximal surfaces cannot be examined visually or with a probe | Optional baseline |
In office: No Home: Brush 2x day w/pea-size amount of F toothpaste |
Not Required | No | No | Yes | No | n/a | n/a |
| Moderate | Every 6 months | Posterior bitewings at 6–12 month intervals if proximal surfaces cannot be examined visually or with a probe | Suggested |
In office: F Varnish initial visit & recalls Home: Brush 2x day w/pea-size amount of F toothpaste Caregiver: OTC sodium fluoride treatment rinses |
Child: Xylitol wipes 3–4x dally-products to substitute for sweet treats or when unable to brush Caregiver: 2 sticks of gum or 2 mints 4x day, total 6–10 grams a day |
Glass ionomer-based materials recommended on deep pits and fissures | No | Yes | No | Treat w/fluoride products as indicated to promote remineralization | n/a |
| Moderate; noncompliant | Every 3–5 months | Posterior bitewings at 6–12 month Intervals if proximal surfaces cannot be examined visually or with a probe | Recommended |
In office: F varnish initial visit & recalls Home: Brush 2x day w/pea-size amount of F toothpaste, then apply pea-size amount of calcium-phosphate paste left on at bedtime Caregiver: OTC sodium fluoride treatment rinses |
Child: Xylltol wipes 3–4x daily; products to substitute for sweet treats or when unable to brush Caregiver: 2 sticks of gum or 2 mints 4x day, total 6–10 grams a day |
Glass ionomer-based materials recommended on deep pits and fissures | Recommend CHX for caregiver/ Recommend probiotics | Yes | Yes | Treat w/fluoride products as Indicated to promote remineralization | n/a |
| High | Every 3 months | Anterior (#2 occlusal film) and posterior bitewings at 6–12 month intervals If proximal surfaces cannot be examined visually or with a probe | Recommended |
In office: F varnish initial visit & recalls Home: Brush 2x day w/pea-size amount of F toothpaste, then apply pea-size amount of calcium-phosphate paste left on at bedtime Caregiver: OTC sodium fluoride treatment rinses |
Child: XylitoI wipes 3–4x daily: products to substitute for sweet treat 5 or when unable to brush Caregiver: 2 sticks of gum or 2 mints 4x day |
Glass ionomer-based materials recommended on deep pits and fissures | Recommend CHX for caregiver/Recommend probiotics | Yes | Yes | Treat w/fluoride products as indicated to promote remineralization | ITR [interim therapeutic restorations) with glass ionomer-based materials or conventional restorative treatment as patient cooperation and family circumstances allow |
| High; noncompliant | Every 1–3 months | Anterior (#2 occlusal film) and posterior bitewings at 6-T2 month Intervals If proximal surfaces cannot be examined visually or with a probe | Recommended |
In office: F varnish initial visit & recalls Home: Brush 2x day w/pea-size amount of F toothpaste, then apply pea-size a mount of calcium-phosphate paste left on at bedtime Caregiver: OTC sodium fluoride treatment rinses |
Child: Xylitol wipes/products to substitute for sweet treats or when unable to brush Caregiver: 2 sticks of gum or2 mints 4x day. total 6–10 grams a day |
Glass ionomer-based materials recommended on deep pits and fissures | Recommend CHX for caregiver/Recommend probiotics | Yes | Yes | Treat w/fluoride products as Indicated to promote remineralization | ITR (interim therapeutic restorations] with glass ionomer-based materials or conventional restorative treatment as patient cooperation and family circumstances allow |
| Extreme | Every 1–3 months | Anterior [#2 occlusal film) and posterior bitewings at 6–12 month Intervals if proximal surfaces cannot be examined visually or with a probe | Recommended |
In office: F varnish initial visit & recalls Home: Brush 2x day w/pea-size amount of F toothpaste, then apply pea-size amount of calcium-phosphate paste left on at bedtime Caregiver: OTC sodium fluoride treatment rinses |
Child Xylital wipes 3–4x daily; products to substitute for sweet treats or when unable to brush Caregiver: 2 sticks of gum or 2 mints 4x day. total 6–10 grams a day |
Glass ionomer-based materials recommended on deep pits and fissures | Recommend CHX for caregiver/Recommend probiotics | Yes | Yes | Treat w/fluoride products as indicated to promote remineralization | TTR (Interim therapeutic restorations] with glass ionomer-based materials or conventional restorative treatment as patient cooperation and family circumstances allow |
Quality Improvement—Potential for Transforming Oral Health Care Delivery and Improving Outcomes
Evidence supports the effectiveness of dietary control, fluoride use, and other modalities such as xylitol, in preventing and controlling dental caries, a chronic infectious disease caused by acids produced by oral bacteria metabolizing fermentable carbohydrates.13 Since the risk for caries development and caries activity differs among individuals and may change in each individual over time, caries risk assessment performed initially, and periodically thereafter, allows for a determination of a patient’s relative risk, from which an adoption of an evidence-based prevention plan that can be customized.
Disease management of caries (e.g., CAMBRA) is modeled after the medical management of chronic conditions in which the patient (or child’s family) is engaged in day-to-day health behavior modifications that address disease etiology. It requires parent/family engagement in dietary control and applications of fluorides and other preventive modalities. The progress in stabilizing and reducing the risk for the caries process is monitored using caries risk assessment tools.
Despite the evidence supporting the effectiveness of bio-behavioral approaches and interventions in preventing and controlling caries, including caries risk management, to improve patient outcomes, disease management of caries has not been widely implemented in clinical dental practice. It is known that barriers exist in our current oral care delivery systems, which do not easily permit effective implementation of disease management of caries in clinical practice. These barriers include providers’ lack of knowledge, skills and comfort; parents’ knowledge, preferences and expectations; reimbursement favoring surgical management of caries; and coordination, and follow-up. While reimbursement methodologies need to be altered from the current “one size fits all” to include coverage of caries risk assessment and more frequent and intensive preventive services for higher-risk individuals, quality improvement (QI) can foster and accelerate the adoption of changes needed to redesign current care delivery systems. QI methods also have allowed for testing of system changes in order to produce better system performance and improved outcomes for patients and populations.
QI is defined by Batalden as the combined and unceasing efforts of everyone—healthcare professionals, patients and their families, researchers, payers, planners and educators—to make changes that will lead to better patient outcomes (health), better system performance (care) and better professional development (learning).14 Quality improvement necessarily involves making changes that systematically incorporate evidence-based knowledge. It functions at the system level by which care delivery takes place, the physical and information level along with the complex social structures—i.e., health care professionals. In recent years, hospitals and medical health care systems have increasingly been using QI to effectively enhance patient safety, improve quality of care, and management of chronic disease and preventive care.
Although QI is not yet familiar territory to dentistry, it offers the potential to transform oral health care delivery in order to provide better oral healthcare, improve oral health outcomes, and to reduce costs of treatment of caries. A demonstration project funded by the DentaQuest institute was implemented at two hospital-based dental practices that care for a disproportionate number of young children with ECC to test the feasibility of applying a disease management approach for ECC. Applying the principles of QI, it focused on making changes within the practices’ oral health care delivery systems needed to implement an evidence-based disease management approach for ECC. Thirty months of results from the demonstration project have shown that a disease management approach to address ECC can be implemented into practice and has the potential to deliver better care, improve clinical outcomes and reduce the overall cost of care.15
Ultimately, in order for caries risk assessment to be successfully implemented as a universal model for quality improvement, the public and private systems of care must enact an equitable financial reimbursement model for these preventive treatments and its intervention care paths based on a medical model based on age and risk that is comparable and or equitable to current surgical care compensation. Furthermore, providers and insurers alike must embrace the dental ethics perspective of early disease prevention and early intervention that can benefit their future patients’ short- and long-term oral health outcomes.
Summary
While caries risk assessment was first endorsed by the American Dental Association , the American Academy of Pediatric Dentistry, and the American Association of Public Health Dentistry, its use by non-dental professionals has become more widespread.16–18 The American Academy of Pediatrics now recommends the use of a risk assessment protocol during well child visits to all its providers.19 Community-based organizations, principally those working with underserved and vulnerable populations such as Early Head Start and Women, Infants and Children, have found CAMBRA an essential tool as part of their comprehensive infant oral care programs.
CAMBRA’s easy to use organized format of disease indicators, risk and protective factors, clinical findings, and self-management goals helps to facilitate oral health education, deepens the appreciation of oral health information, and increases the understanding of how individual behaviors can affect caries development and progression. By embracing the concepts of caries risk assessment, early intervention and the establishment of a dental home, care providers could reduce their patients’ risk of early childhood caries and improve children’s oral and overall health.
The adoption of CAMBRA has not been universal in the dental community, which remains primarily focused on restorative treatment rather than on prevention and management of the disease. Since restorative and surgical care by itself does not address disease etiology, patients and caregivers must have the opportunity to increase their own oral health literacy, understand the causes and consequences of poor oral health and the value of “maintaining healthy teeth, healthier.” As families gain an understanding of the value of preventive care and managing their oral disease risk, they will expect to partner with their oral health provider on plans for preventive care, monitoring, early intervention and treatments, as they have come to demand with their physicians. As QI becomes more familiar to dentistry, dental practices will be able to use QI methods to redesign their systems of care and train themselves and their staff to deliver customized risk-based prevention and disease management to patients. In doing so, the dental profession will have the potential to improve patient oral health outcomes and result in a reduction in cost of care.
It is important to recognize that, in order for a successful paradigm shift to disease prevention to occur full scale, dental insurance benefits must support risk-based disease management by reimbursing for risk assessments, monitoring and regularly assessing, and more frequent preventive care treatments for high caries risk patients. Despite these challenges, many practices and clinics have successfully implemented CAMBRA. Two other articles are presented in this journal as a helpful guideline to CAMBRA adoption and incorporation into practice, "Successful Business Models for Implementation of Caries Risk Assessment in Private Practice Settings" and '"CAMBRA: Changing the Face of Prevention at Federally Qualified Health Centers."
Contributor Information
Francisco Ramos-Gomez, University of California, Los Angeles School of Dentistry, Section of Pediatrics and Researcher for UCSF’s Center to Address Disparities in Children’s Oral Health - CANDO.
Man-Wai Ng, Children’s Hospital Boston, and Assistant Professor, Oral and Developmental Biology, Harvard School of Dental Medicine.
References
- 1.Dye BA, Arevalo O, Vargas CM. Trends in pediatric dental caries by poverty status in the United States: 1988–1997 and 1994–2004. Int J Paediatric Dent. 2010;20(2):132–143. doi: 10.1111/j.1365-263X.2009.01029.x. [DOI] [PubMed] [Google Scholar]
- 2. [Accessed Aug. 8, 2011];Dental Health Foundation, the 2006 oral health needs assessment of children. dentalhealthfoundation.org/images/lib_PDF/dhf_2006_report.pdf.
- 3.Ramos-Gomez FJ, Crystal YO, et al. Pediatric dental care: prevention and management protocols based on caries risk assessment. J Calif Dent Assoc. 2010 Nov;38(11):790. [PMC free article] [PubMed] [Google Scholar]
- 4.Child and Adolescent Health Measurement Initiative. [Accessed Aug. 8, 2011];National Survey of Children’s Health, Data Resource Center for Child and Adolescent Health. nschdata.org. 2007
- 5.American Academy of Pediatric Dentistry. Policy on the dental home. AAPD Reference Manual. 31(6):22–23. [Google Scholar]
- 6.Gajendra S, Kumar JV. Oral health and pregnancy: a review. NY State Dent J. 2004;70(1):40–44. [PubMed] [Google Scholar]
- 7.Fine A, Kotelchuck M. Rethink MCH: the life course model as an organizing framework concept paper. U.S. Department of Health and Human Services Health Resources and Services; [Google Scholar]
- 8.Twetman S. Prevention of early childhood caries (ECC)–review of literature published 1998–2007. Eur Arch Paedtr Dent. 2008 Mar;9(1):12–18. doi: 10.1007/BF03321590. [DOI] [PubMed] [Google Scholar]
- 9.Altarum Institute, Topical fluoride for high-risk children, development of decision support matrix, recommendations from MCHB expert panel. Washington, DC: 2007. [Google Scholar]
- 10.Tinanoff N, Resine S. Update on early childhood caries since the surgeon general’s report. Acad Pediatr. 2009 Nov-Dec;9(6):396–403. doi: 10.1016/j.acap.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Batalden PB, Davidoff F. What is “quality improvement” and how can it transform healthcare? [Accesed Aug. 8, 2011];Qual Saf Health Care 2007. 2007 16:2–3. doi: 10.1136/qshc.2006.022046. clinicalmicrosystem.org/toolkits/getting_started/EditorialQSHC.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ng M. [Accessed Aug. 8, 2011]; unpublished data, http://www.nnoha.org/goopages/pages_downloadgallery/download.php?filename=13501.ppt&orig_name=2010_man_wai_ng.ppt&cdpath=/npohcpresentations/2010_man_wai_ng.ppt. [Google Scholar]
- 13.American Dental Association. [Accessed August 8, 2011];ADA statement on early childhood caries. 2004 http://www.ada.org/2057.aspx.
- 14.American Academy of Pediatric Dentistry. Guideline on caries risk assessment and management for infants, children, and adolescents. Pediatr Dent. 2010;32(special issue):101–108. [PubMed] [Google Scholar]
- 15. [Accessed August 8, 2011];American Association of Public Health Dentistry, First oral health assessment policy. 2004 http://aaphd.org/default.asp?page=FirstHealthPolicy.htm.
- 16. [Accessed Aug. 8, 2011];American Academy of Pediatrics, Bright Futures. brightfutures.aap.org/practice_guides_and_other_resources.html.


