Abstract
Early studies reported that the size of adipose cells correlates with insulin resistance. However, a recent study comparing moderately obese, sensitive and resistant subjects, with comparable body mass index (BMI, ~ 30), did not detect any significant difference in the size of the large cells, but rather a smaller proportion of large cells in the resistant subjects, suggesting impaired adipogenesis. We hypothesize that a decreased proportion, rather than the size, of large adipose cells is also associated with insulin resistance in first-degree relatives of type 2 diabetics. Thirty-five leaner (BMI 18 – 34) subjects who were relatively healthy were recruited. Insulin sensitivity was measured by euglycemic, hyperinsulinemic clamp. Needle biopsies of abdominal subcutaneous fat were assayed for adipose cell size by fitting the cell size distribution with two exponentials and a Gaussian function. The fraction of large cells was defined as the area of the Gaussian peak and the size of the large cells was defined as its center (cp). Glucose infusion rate and cp were negatively correlated, but insulin sensitivity and the proportion of large cells were not correlated. BMI and cp were also strongly correlated, but a relationship of modest correlation between cell size and insulin resistance was still significant after correcting for BMI. In contrast to moderately obese subjects, in the first-degree relatives of type 2 diabetics both BMI and the size of the large adipose cells predict the degree of insulin resistance; no correlation is found between the proportion of large adipose cells and insulin resistance.
INTRODUCTION
Insulin resistance is the precursor of Type 2 diabetes, but its molecular and cellular mechanisms remain poorly understood. Obesity leads to insulin resistance, which is manifested by decreased insulin-stimulated glucose uptake in muscle and adipose tissue, and by impaired insulin-suppressed glucose production in liver (1, 2). Obesity is a major risk factor for Type 2 diabetes (3).
Two types of obesity have been described, hypertrophic due to increased size of adipose cells and hyperplastic due to increased number of adipose cells. Numerous early studies of human obesity led to the notion that hypertrophic obesity (or enlarged adipose cell size) is closely correlated with many metabolic abnormalities associated with insulin resistance (4, 5). This hypothesis was further supported by cross-sectional studies in Pima Indians (6, 7) and other populations (8, 9) showing that enlarged mean subcutaneous abdominal adipose cell size is associated with insulin resistance, and predicts Type 2 diabetes, independent of body fat content or body mass index (BMI). However, in a recent study of weight-matched, moderately obese subjects, it was found, using a newer and more in-depth method of studying the distribution of cell size, that the adipose cells exhibited a non-unimodal distribution with a prominent tail of small adipose cells that can be fit by two exponential functions and a Gaussian peak of large adipose cells, whose mean diameter is given by cp, center of the peak (10). Similar results have been obtained by others (11, 12). In this study the size of large adipose cells, as assessed by cp, did not correlate with insulin resistance. Instead, insulin resistance was associated with a surplus of small adipose cells (cells under the exponential tail) and, correspondingly, a deficit of large adipose cells (cells under the Gaussian peak, or cells to the right of the nadir). We interpret this result to indicate that insulin resistance is a condition in which new cells are recruited in response to increased need to store fat, but accumulate because the small adipose cells are not capable of achieving full size. When the ability to store additional fat is impaired, we suggest, the excess calories are stored as fat in other insulin target tissues such as liver and muscle, resulting in insulin resistance, consistent with the lipotoxicity hypothesis (13, 14).
We now hypothesize that a decreased proportion, rather than the size, of large adipose cells is associated with insulin resistance in the first-degree relatives of type 2 diabetics. Thus we recruited thirty-five leaner resistant and sensitive subjects who were the first-degree relatives of type 2 diabetics. To our surprise, we find an inverse correlation between insulin sensitivity and the size of the large adipose cells, but no correlation between insulin sensitivity and the proportion of large cells. A strong correlation is also found between the size of large adipose cells and BMI, but the relationship between cell size and insulin resistance is still significant, albeit of modest size, after correcting for BMI.
METHODS AND PROCEDURES
Subjects
In a daily newspaper advertisement, a group of 35 subjects with BMI 18–34 were recruited. Informed consent was obtained from all subjects. These subjects had a known family history of diabetes with at least two first-degree relatives with Type 2 diabetes but did not themselves have Type 2 diabetes, as assessed by fasting plasma glucose (< 7 mM). The study was approved by the Ethics Review Board of the University of Gothenburg.
Clinical Procedures
After admission, all subjects underwent the following investigations. Body weight and height and waist and hip circumferences were recorded with standard techniques. The proportions of body fat and lean body mass (LBM) were determined using bioelectrical impedance (single frequency, 50 kHz; Animeter, HTS, Odense, Denmark). Fasting levels of plasma glucose, insulin, and triglycerides were measured using standard laboratory methods. HbA1c was determined using high-performance liquid chromatography (Mono-S method). In this study, all HbA1c values were converted to NGSP standard levels using the formula: .
All subjects were nondiabetic and underwent a 75-g oral glucose tolerance test (OGTT), with measurements of blood levels of glucose and insulin at times 0 and 120 minutes. The degree of insulin sensitivity was measured by the euglycemic, hyperinsulinemic clamp over 120 minutes and expressed as glucose infusion rate (GIR) normalized by total body weight or lean body mass (15). Briefly, insulin was infused into an intravenous cannula placed in the antecubital vein at a rate of 60 mU/m2/min. Blood glucose levels were maintained at 5 mM by infusing glucose at a variable rate. After reaching a plateau (~ 60–90 min), the average rate of glucose infusion required to maintain euglycemia was calculated over 60 min (60–120 min) and normalized by the kilograms of whole body weight or LBM. Glucose was analyzed in arterialized venous blood using an automatic glucose analyzer (YSI 2700 SELECT, Yellow spring instruments, Ohio, USA). Insulin was analyzed with a standard radioimmunoassay (Pharmacia, Uppsala, Sweden). The clinical and laboratory characteristics are shown in Table 1.
Table 1.
Clinical and laboratory characteristics of the subjects (N=35)a
| Variable | Mean ± SD | Range |
|---|---|---|
| Age (year) | 41 ± 7 | 28–49 |
| Height (m) | 1.75 ± 0.09 | 1.59–1.96 |
| Weight (kg) | 78.9 ± 13.7 | 60.5–108.7 |
| BMI (kg/m2) | 25.7 ± 3.9 | 18–34 |
| Waist/hip circumference ratio | 0.88 ± 0.08 | 0.74–1.09 |
| Fasting insulin (mU/L)b | 8.4 ± 5.5 | 3.1–24 |
| Fasting glucose (mM) | 4.9 ± 0.5 | 4.2–6.2 |
| 2h-plasma glucose OGTT (mM) | 6.5 ± 2.3 | 2.9–13 |
| Fasting triglyceride (mM) | 1.06 ± 0.47 | 0.43–1.8 |
| HbA1c (%) | 4.2 ± 0.3 | 3.7–5 |
| M (GIR/LBM 60 min) | 10.8 ± 3.9 | 4.2–17.9 |
| M (GIR/TBM 60 min) | 8.75 ± 3.87 | 2.2–17.3 |
Abbreviation: BMI, body mass index, kg/m2; LBM or TBM, lean or total body mass, kg; OGTT, oral glucose tolerance test; GIR, glucose infusion rate, mg/min; M, insulin sensitivity, mg/kg/min.
weight history. Thirty subjects had weight changes less than 5 kg within 6 months and were considered stable; the other 5 subjects had weight changes as follows: EBOG and JMOL lost 5 and 15 kg within 6 months, respectively; SRHO lost 17 kg within 18 months; and MAND and STHO gained 10 and 11 kg during 24 months, respectively.
two subjects were not assessed (N=33).
Measurement of adipose cell size distribution
Abdominal subcutaneous fat biopsies (20–30 mg) were obtained with a needle aspiration technique from the paraumbilical region after local infiltrative anesthesia with lidocaine (10 mL, 0.5%), fixed with osmium tetroxide in a water bath at 37°C for 48 h, and assayed for adipose cell size with a Beckman Coulter Multisizer III (Miami, Florida, USA) with a 400-μm aperture, which effectively counts particles with sizes of 20–400 μm. The instrument was set to count 6,000 particles and the fixed cell suspension was diluted so that coincident counting was <10%. Each subject had two fat samples, each of which was counted twice. The cell size distribution data used for analysis were the averages of the averaged values of each sample with duplicate counts. After collection of pulse sizes, the data were expressed using particle diameters and displayed as histograms of counts against diameter using linear bins and a linear scale for the x-axis.
Adipose cell size was analyzed by fitting a formula with seven parameters to the cell size distribution: , where x=cell diameter, and x0= the smallest diameter; h1 and w1 = height and width of the first exponential; h2 and w2 = height and width of the second exponential; and hp, cp, and wp = height, center, and width squared of the Gaussian curve (10). The small cells correspond to the double exponential, and the large cells to the Gaussian curve. The exponential curve on the left occurs because adipogenesis represents a process in which newly recruited adipose cells are injected into the population at a small size and fill up with stored triglyceride. This will in general produce a monotonically decreasing region from the lower threshold of detection (limited by the aperture size) to the nadir, which is the trail of cells of small size leading up to the mature group. Our previous adipogenesis reports invoke a growth law that involves an acceleration of growth rate as size increases (although it eventually declines at very large size). This will always produce a pronounced left tail and a nadir to the left of the right peak (16, 17).
From the fitted curves, the fraction of large cells was then calculated. The size of the large cells was defined as the center of the Gaussian peak (cp). Some subjects who displayed two peaks were fitted with one exponential and two Gaussian functions. We previously demonstrated that the adipose cell size distributions of fat biopsies as measured by the Multisizer 3 were similar to those of isolated fat cells from the same biopsies (10); the advantages of this method over the traditional microscopic techniques for measurement of fat cell size (18, 19) were also discussed previously (10).
Statistics
Statistical analysis was performed with Systat Software (SPW11.2, Systat Software, San Jose, CA, USA) by linear regression. P<0.05 was considered statistically significant.
RESULTS
Clinical characteristics of subjects
Table 1 shows the clinical and laboratory characteristics of the subjects. They had a broad range of age (28–49 years old) and BMI (18–34). All the subjects were relatively healthy, and had normal levels of fasting plasma glucose (4.2–6.2 mM) and normal levels of blood HbA1c (3.7–5.0). The degree of insulin sensitivity was assessed with the euglycemic, hyperinsulinemic clamp and normalized by total body weight or lean body mass. Glucose infusion rates (GIR) had a range of 2.2 to 17.3 mg/kg/min, if normalized by total body weight, and a range of 4.2–17.9 mg/kg/min, if normalized by LBM. These data indicate that the study group was a mix of insulin sensitive and resistant subjects.
Adipose cell size distribution
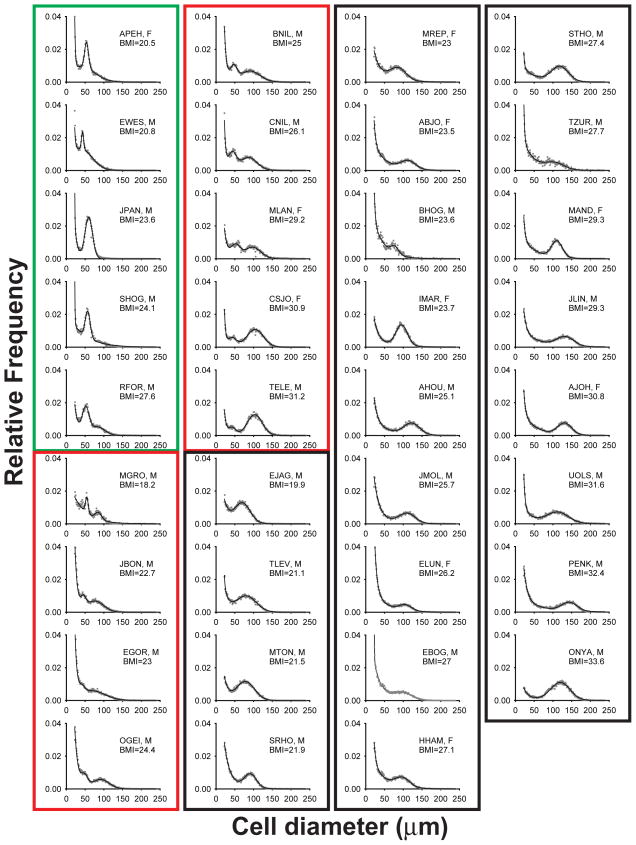
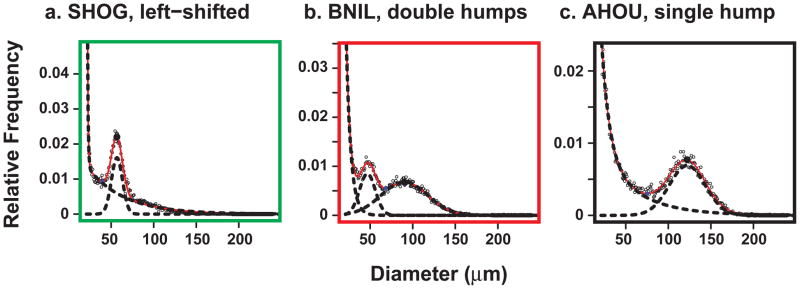
Figure 1 shows the adipose cell size distribution data of all 35 subjects as measured by the Multisizer technology using cells from osmium tetroxide-fixed abdominal subcutaneous adipose tissue biopsies. In agreement with previously published data (10), the adipose cells from most of the subjects exhibit a bimodal distribution with the small adipose cells corresponding to the double exponential and the large adipose cells to the Gaussian curve. Interestingly a subset of the subjects displays two Gaussian peaks. Figure 2 shows three examples of typical curve fits from three representative subjects. Based on the shape of the cell size distribution, the subjects were categorized into three distinctive groups: 1) subjects (N=5) with a left-shifted Gaussian peak, 2) subjects (N=9) with double Gaussian peaks, and 3) subjects (N=21) with a normal single hump. In this study the size of the large adipose cells was defined as the center of the right Gaussian peak (cp) in the case of subjects with double peaks.
Figure 1.
Multisizer profile of cell size distribution of all subjects (n=35) with cell diameter (μm) using linear bins against relative frequency in percent. Dotted lines represent the experimental data and the solid lines the fitted data. For clarity, all values of relative frequencies were trancated to 0.04. Based on the shape of the cell size distribution, the subjects were categorized into three distinctive groups: 1) subjects (n=5, green box) with a left-shifted Gaussian peak, 2) subjects (n=9, red box) with double Gaussian peaks, and 3) subjects (n=21, black box) with a normal single hump. In the inset of each cell size distribution, the four-letter code represents the name of the patient followed by its gender (M, male and F, female); BMI, body mass index.
Figure 2.
Three examples of fitted curves from three different subjects. a, SHOG, left-shifted. b, BNIL, double hump. c, AHOU, single hump. SHOG, BNIL, and AHOU are code names of the patients and represent the three different groups marked with three different colors (green, red, and black) as explained in the main text and in Figure1.
Correlation of the size of large adipose cell with insulin resistance
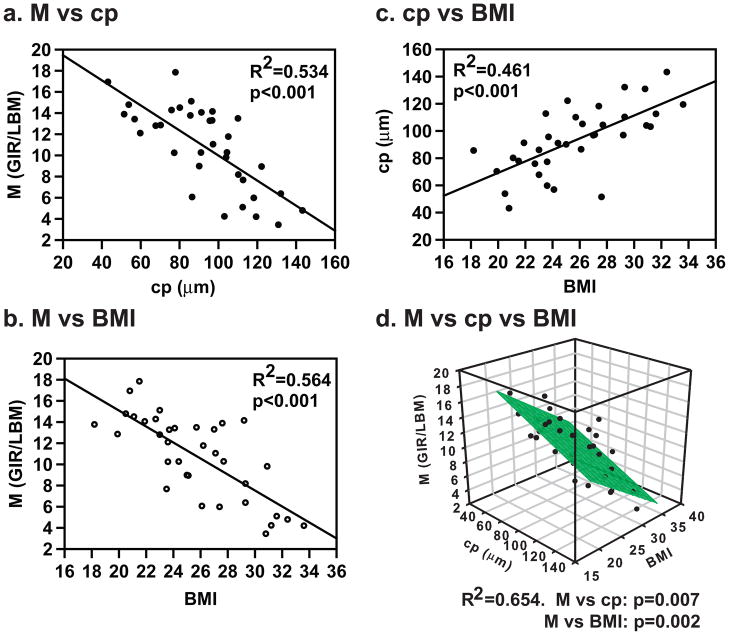
To identify the parameters that predict the degree of insulin resistance, we analyzed the association of insulin sensitivity with the size of the large adipose cells (cp) and BMI by simple linear regression. Figure 3a, b shows that the insulin sensitivity (M) normalized by lean body mass has an inverse correlation with cp (R2=0.534, p<0.001) and BMI (R2=0.564, p<0.001). The size of the large adipose cells also correlated with BMI (R2=0.461, p<0.001, Figure 3c). Figure 3d shows that insulin sensitivity still correlates, inversely, with the size of the large adipose cells after correction for BMI as assessed by multiple linear regression using BMI as the first independent variable and cp as the second independent variable, or vice versa. The R2 increases modestly to 0.654 with both BMI and cp included in the model. These results were obtained after removing one evident outlier. If that subject is included, the results are the same, except that cp no longer makes a significant contribution to M once BMI is included in the model. See Figure S1 in the Supplemental material for details. When only the weight-stable subjects (Table 1) were included in the analysis, the results were the same (M vs cp, R2=0.578, p<0.001; and BMI vs cp, R2=0.469, p<0.001). Similar correlation results were obtained between cp and insulin sensitivity (M) normalized by total body weight (not shown). Furthermore, we found that the average volume per cell also correlated negatively with insulin sensitivity (data not shown), in agreement with the classical studies. We also found that the inverse correlation of the size of large adipose cells with insulin sensitivity is independent of sex (data not shown). However, we found no correlation between insulin sensitivity and the fraction of large adipose cells in this group (not shown), in contrast to that of the study done at Stanford by McLaughlin et al. (2007).
Figure 3.
Inverse correlation of insulin sensitivity with the size of large adipose cells (cp) (a) and BMI (b) analyzed by simple linear regression. c, Correlation of cp with BMI. d, Inverse correlation of insulin sensitivity with BMI and cp analyzed by multiple linear regression. BMI, body mass index; cp, the center of the Gaussian peak; GIR, glucose infusion rate; LBM, lean body mass; M, insulin sensitivity.
Comparison of the current study (Gothenburg subjects) with that of moderately obese subjects (Stanford subjects)
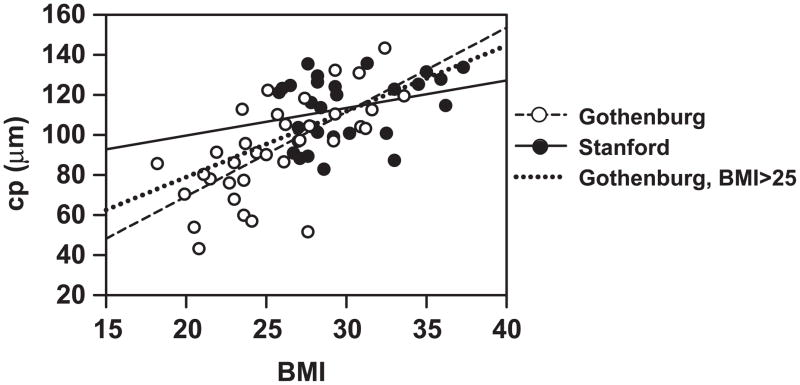
Figure 4 compares the Gothenburg and Stanford studies. In the moderately obese subjects of the Stanford study, no correlation is observed between cp and BMI. However, cp correlates well with BMI in the Gothenburg study, which included leaner individuals. On the other hand, Figure 4 does show that the regression slope for the Gothenburg subjects with BMI > 25 is shallower than for the group as a whole and closer to that of the Stanford subjects. Further, neither the slope nor intercept of the former is significantly different than those of the latter (p values 0.38 and 0.35, respectively).
Figure 4.
Comparison of the Gothenburg study in this paper and the Stanford study (10). The size of large adipose cells (cp) correlates well with BMI in the leaner Gothenburg subjects (R2=0.461, p<0.001) but not in the moderately-obese subjects used in the Stanford study (R2=0.081, p<0.141). The dotted line shows the regression slope for the Gothenburg subjects with BMI > 25. For this group, neither the slope nor intercept is significantly different between the Stanford subjects and the Gothenburg subjects (p values 0.38 and 0.35, respectively). Abbreviations are the same as in Figure 3.
In the Stanford study, no differences were found in either the mean cell size (insulin-resistant subjects, 65 ± 15 μm vs insulin-sensitive subjects, 72 ± 8 μm, p=0.34) or the peak diameter of large cells (insulin-resistant subjects, 116 ± 16 μm vs insulin-sensitive subjects, 111 ± 17 μm, p=0.69). In the present study, the mean adipose cell size is 69 ± 14 μm (n=35), and the average diameter of the large adipose cells is 93 ± 24 μm (n=35). While the mean adipose cell sizes are comparable, the average diameter of the large adipose cells of the subjects in this study, which we consider the more meaningful measure, was noticeably smaller (~17%) than that in the previous study (93 ± 24 μm for Gothenburg (n=35) vs 113 ± 16 μm for Stanford (n=28); p = 0.0002).
DISCUSSION
This study demonstrates that insulin sensitivity is inversely correlated with the size of the large adipose cells (cp; Figure 3a) or the average cell volume (not shown) in first-degree relatives of type 2 diabetics who did not themselves have frank diabetes as assessed by fasting plasma glucose (< 7 mM). The size of the large adipose cells is largely a surrogate for BMI (Figure 3c), but a small effect of cp on glucose disposal remains after accounting for BMI (Figure 3d). These data confirm the classical view that the size of large adipose cells is an independent predictor of the degree of insulin resistance. However, taken together with other recent studies, the relationship between adipose cell size and insulin resistance is better viewed as context-dependent. For example, McLaughlin et al. (the Stanford study) (10) did not find a statistically significant correlation between cp and insulin resistance in a group of moderately obese subjects; that study found instead a correlation between the fraction of large adipose cells and insulin sensitivity. We do not find such a correlation in the leaner Gothenburg subjects.
Evidence that large adipose cells in adipose tissue are associated with insulin resistance has accumulated for many years. With isolated adipose cells from obese human subjects, Salans et al. (5) showed that the larger the adipose cells were, the less responsive they were to insulin in glucose utilization; Franck et al. (18) demonstrated that large adipose cells have decreased capacity of insulin to stimulate GLUT4 translocation to the plasma membrane. Many other investigators extended this observation to the whole body in vivo (4–9). While the fat distribution in different depots has been suggested to also play an important role in metabolic aberrations, a significant correlation was found between the mean size of the subcutaneous adipose cells and many abnormalities (e.g., hyperinsulinaemia and glucose intolerance) associated with insulin resistance (4). Due to the confounding effect of obesity, however, it had not been very clear which of the cellularity characteristics in the adipose tissue is the most important factor for predicting insulin resistance in vivo.
In this study we used the newer technology of cell counting and sizing by the Coulter Counter Multisizer III (10). With this method, adipose cell size can be analyzed by fitting the cell size distribution to a formula, and thus the size and the number of cells can be more accurately measured. Notably, the cell size distribution as measured with the Multisizer III is either bimodal or trimodal (Figures 1, 2) instead of unimodal as many previous investigators have reported, using either microscopic techniques to measure adipose cell diameters or the older version of Coulter Counter to estimate the size of fat cells by measuring the cellular lipid weight (4–6, 8, 9, 20). By using this newer approach, we find that the classical view that large adipose cell size correlates with insulin resistance holds for our current study group.
However, the present study and the Stanford study (10) were conducted using the same methodology, but came up with superficially opposite results. Nevertheless, despite the apparent discrepancy, we see the two studies as complementary rather than contradictory, and we believe that both fit within the paradigm of insulin resistance as a disorder of impaired adipogenesis. A key difference between the two populations is shown in Figure 4: the Gothenburg subjects are leaner and for them cp increases with BMI, whereas for the more obese Stanford subjects, cp does not increase with BMI. This is consistent with the observation of Arner et al. (21) that average adipose cell volume could be fit by a saturating, hyperbolic function of total fat mass. This is further supported by the fact that the regression slope and intercept for the Gothenburg subjects with BMI > 25 are not significantly different from those of the Stanford subjects (Figure 4). Our interpretation of the common finding of a saturating relationship between fat mass and fat cell size is that it becomes harder to increase fat cell size once the diameter exceeds a certain level.
The results of this study and those of the Stanford study (10) put the historical discussion of hypertrophy vs. hyperplasia in a new light. We suggest that the first response to an increase in body fat mass is hypertrophy, the expansion of existing adipose cells. This would appear to be more efficient and may be the preferred route for leaner subjects. For more obese subjects, the possibilities for expansion are limited and hyperplasia becomes necessary. This hypothesis is supported by a recent study from the Jensen laboratory (22) reporting that short-term weight gain led to increased abdominal subcutaneous adipose cell size without change in cell number. Moreover, among the female subjects, the subjects who had smaller adipose cells at baseline had the greatest degree of hyperplasia and those with larger cells experienced a decrease in the average size of the large cells (diameter > 35μm). This indicates recruitment and/or expansion of previously undetected small cells.
Note that it is necessary both to recruit new cells and to expand them, as larger adipose cells cannot expand further to store more fat. Those subjects who are unable to expand the small adipose cells, plausibly those with the smaller proportion of large adipose cells in the cross-sectional measurement of McLaughlin et al. (10), could be more susceptible to a spillover of fat to other peripheral tissues where it promotes insulin resistance (13). In fact, rosiglitazone treatment led to transient recruitment and expansion of small adipose cells and a sustained increase in fat cell size, thus improving insulin sensitivity both in Zucker fa/fa rats (23) and type 2 diabetic human subjects (A. Sherman, B. Eliasson, S. Mullen, U. Smith, and SW Cushman, manuscript in preparation). On the other hand, however, non-diabetic, insulin-resistant human subjects treated with pioglitazone for 90 days showed an increase in the proportion of small adipose cells and a broadening of the distribution of large cells but no increase in large cell size (24). Thus, improved insulin sensitivity appears to be associated with increased adipose cell size dynamism, and both recruitment and expansion of adipose cells, but which aspect predominates may depend on the metabolic status of the subjects and, perhaps, species differences. It is not so clear why having larger adipose cells is associated with insulin resistance for the subjects in the present study. We do not have enough subjects in the higher BMI/larger cp range to assess whether the capacity for recruitment and expansion of small cells correlates with insulin resistance.
Other differences are observed between the two groups: the present group is younger as well as leaner, has a higher proportion of males, and is more ethnically homogeneous. Genetic factors may also account for the discrepancy. In the study of McLaughlin et al. (10) the subjects had no known family history of type 2 diabetes, whereas the subjects in this study were first-degree relatives of type 2 diabetics (25, 26). Although the genetic factors that control adipose cell size have not yet been identified, they may be involved in the association of the size of the large adipose cells with insulin resistance.
Our data do not settle whether large adipose cell size is a cause of insulin resistance or a consequence of it or both. In this study, cp is highly correlated with BMI, so it is mostly a surrogate for adiposity, but we do see a correlation between cp and glucose disposal even after taking BMI into account. Indeed, from a cell biological standpoint, cp would seem to be a more fundamental quantity than BMI. Large adipose cells are reported to have increased expression of pro-inflammatory adipokines (27) and increased lipolysis (28, 29), both of which may cause insulin resistance (30–32). Note, however that a recent study of healthy, moderately obese individuals did not find an association between the size of the large adipose cells and expression of inflammatory genes (33); rather, inflammation was associated with an increased fraction of small cells, parallel to the findings on insulin resistance in a similar population (10).
Another important finding of this study is that some subjects had two peaks, which can be roughly fitted with two Gaussian functions (Figure 1). It is currently not clear why some subjects have two peaks, but others only one. We speculate that the left peak represents a population of intermediate size adipose cells resulting from either a recent wave of recruitment or enlargement of existing small adipose cells. Because of their relatively small size, these adipose cells have the potential to take up and store more fat; as a result the left peak would move to the right. Such shifts were seen in the adipose tissue of mice fed either a regular diet or a high-fat diet (16). Similar double-peaked adipose cell size distributions and movement of the left peak to the right were also observed in the adipose tissue of Zucker fa/fa rats treated with the anti-diabetic drug rosiglitazone (23, 34).
Further studies are needed with lean, healthy individuals without known family histories of type 2 diabetes to assess the generality of the results. Longitudinal studies with human subjects may be useful for determining whether and under what conditions the left-shifted peaks of adipose cells size that we observed are capable of shifting to the right as seen in rodents. The main limitation of this study, however, is that the results are only associative and cannot distinguish whether the larger diameter of the large adipose cells is the cause or the consequence of insulin resistance or vice versa. It will be of great interest and importance to identify the physiological and/or genetic factors that regulate the size of adipose cells.
Supplementary Material
Acknowledgments
B. E. and U. S. were supported by the Swedish Research Council, the Novo Nordisk Foundation, and the Swedish Diabetes Association. J. Y., S. C., and A. S. were supported by the Intramural Research Program of NIDDK, NIH. We thank Junghyo Jo and Vipul Periwal (LBM, NIDDK) for insightful comments. We thank Tracey McLaughlin for permission to reanalyze the data from a previous study.
Footnotes
DISCLOSURE
The authors declare no conflict of interest.
References
- 1.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37(12):1595–607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 2.Reaven GM. Pathophysiology of insulin resistance in human disease. Physiol Rev. 1995;75(3):473–86. doi: 10.1152/physrev.1995.75.3.473. [DOI] [PubMed] [Google Scholar]
- 3.Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest. 2000;106(4):473–81. doi: 10.1172/JCI10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krotkiewski M, Bjorntorp P, Sjostrom L, Smith U. Impact of obesity on metabolism in men and women. Importance of regional adipose tissue distribution. J Clin Invest. 1983;72(3):1150–62. doi: 10.1172/JCI111040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salans LB, Knittle JL, Hirsch J. The role of adipose cell size and adipose tissue insulin sensitivity in the carbohydrate intolerance of human obesity. J Clin Invest. 1968;47(1):153–65. doi: 10.1172/JCI105705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weyer C, Foley JE, Bogardus C, Tataranni PA, Pratley RE. Enlarged subcutaneous abdominal adipocyte size, but not obesity itself, predicts type II diabetes independent of insulin resistance. Diabetologia. 2000;43(12):1498–506. doi: 10.1007/s001250051560. [DOI] [PubMed] [Google Scholar]
- 7.Weyer C, Wolford JK, Hanson RL, et al. Subcutaneous abdominal adipocyte size, a predictor of type 2 diabetes, is linked to chromosome 1q21--q23 and is associated with a common polymorphism in LMNA in Pima Indians. Mol Genet Metab. 2001;72(3):231–8. doi: 10.1006/mgme.2001.3147. [DOI] [PubMed] [Google Scholar]
- 8.Lundgren M, Svensson M, Lindmark S, Renstrom F, Ruge T, Eriksson JW. Fat cell enlargement is an independent marker of insulin resistance and ‘hyperleptinaemia’. Diabetologia. 2007;50(3):625–33. doi: 10.1007/s00125-006-0572-1. [DOI] [PubMed] [Google Scholar]
- 9.Lonn M, Mehlig K, Bengtsson C, Lissner L. Adipocyte size predicts incidence of type 2 diabetes in women. FASEB J. 2010;24(1):326–31. doi: 10.1096/fj.09-133058. [DOI] [PubMed] [Google Scholar]
- 10.McLaughlin T, Sherman A, Tsao P, et al. Enhanced proportion of small adipose cells in insulin-resistant vs insulin-sensitive obese individuals implicates impaired adipogenesis. Diabetologia. 2007;50(8):1707–15. doi: 10.1007/s00125-007-0708-y. [DOI] [PubMed] [Google Scholar]
- 11.Smith SR, Xie H, Baghian S, et al. Pioglitazone changes the distribution of adipocyte size in type 2 diabetes. Adipocytes. 2006;2(1):11–22. [Google Scholar]
- 12.Pasarica M, Xie H, Hymel D, et al. Lower total adipocyte number but no evidence for small adipocyte depletion in patients with type 2 diabetes. Diabetes Care. 2009;32(5):900–2. doi: 10.2337/dc08-2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Unger RH. Lipotoxic diseases. Annu Rev Med. 2002;53:319–36. doi: 10.1146/annurev.med.53.082901.104057. [DOI] [PubMed] [Google Scholar]
- 14.Samuel VT, Petersen KF, Shulman GI. Lipid-induced insulin resistance: unravelling the mechanism. Lancet. 2010;375(9733):2267–77. doi: 10.1016/S0140-6736(10)60408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237(3):E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 16.Jo J, Gavrilova O, Pack S, et al. Hypertrophy and/or Hyperplasia: Dynamics of Adipose Tissue Growth. PLoS Comput Biol. 2009;5(3):e1000324. doi: 10.1371/journal.pcbi.1000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jo J, Guo J, Liu T, et al. Hypertrophy-driven adipocyte death overwhelms recruitment under prolonged weight gain. Biophys J. 2010;99(11):3535–44. doi: 10.1016/j.bpj.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Franck N, Stenkula KG, Ost A, Lindstrom T, Stralfors P, Nystrom FH. Insulin-induced GLUT4 translocation to the plasma membrane is blunted in large compared with small primary fat cells isolated from the same individual. Diabetologia. 2007;50(8):1716–22. doi: 10.1007/s00125-007-0713-1. [DOI] [PubMed] [Google Scholar]
- 19.Jernas M, Palming J, Sjoholm K, et al. Separation of human adipocytes by size: hypertrophic fat cells display distinct gene expression. FASEB J. 2006;20(9):1540–2. doi: 10.1096/fj.05-5678fje. [DOI] [PubMed] [Google Scholar]
- 20.Salans LB, Cushman SW, Weismann RE. Studies of human adipose tissue. Adipose cell size and number in nonobese and obese patients. J Clin Invest. 1973;52(4):929–41. doi: 10.1172/JCI107258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arner E, Westermark PO, Spalding KL, et al. Adipocyte turnover: relevance to human adipose tissue morphology. Diabetes. 2010;59(1):105–9. doi: 10.2337/db09-0942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tchoukalova YD, Votruba SB, Tchkonia T, Giorgadze N, Kirkland JL, Jensen MD. Regional differences in cellular mechanisms of adipose tissue gain with overfeeding. Proc Natl Acad Sci U S A. 2010;107(42):18226–31. doi: 10.1073/pnas.1005259107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.MacKellar J, Cushman SW, Periwal V. Differential effects of thiazolidinediones on adipocyte growth and recruitment in Zucker fatty rats. PLoS One. 2009;4(12):e8196. doi: 10.1371/journal.pone.0008196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McLaughlin TM, Liu T, Yee G, et al. Pioglitazone Increases the Proportion of Small Cells in Human Abdominal Subcutaneous Adipose Tissue. Obesity (Silver Spring) 2009;18(5):926–31. doi: 10.1038/oby.2009.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carvalho E, Jansson PA, Nagaev I, Wenthzel AM, Smith U. Insulin resistance with low cellular IRS-1 expression is also associated with low GLUT4 expression and impaired insulin-stimulated glucose transport. FASEB J. 2001;15(6):1101–3. [PubMed] [Google Scholar]
- 26.Carvalho E, Jansson PA, Axelsen M, et al. Low cellular IRS 1 gene and protein expression predict insulin resistance and NIDDM. FASEB J. 1999;13(15):2173–8. doi: 10.1096/fasebj.13.15.2173. [DOI] [PubMed] [Google Scholar]
- 27.Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr. 2004;92(3):347–55. doi: 10.1079/bjn20041213. [DOI] [PubMed] [Google Scholar]
- 28.Ostman J, Backman L, Hallberg D. Cell size and lipolysis by human subcutaneous adipose tissue. Acta Med Scand. 1973;193(5):469–75. doi: 10.1111/j.0954-6820.1973.tb10610.x. [DOI] [PubMed] [Google Scholar]
- 29.Large V, Reynisdottir S, Langin D, et al. Decreased expression and function of adipocyte hormone-sensitive lipase in subcutaneous fat cells of obese subjects. J Lipid Res. 1999;40(11):2059–66. [PubMed] [Google Scholar]
- 30.Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112(12):1821–30. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McGarry JD. What if Minkowski had been ageusic? An alternative angle on diabetes. Science. 1992;258(5083):766–70. doi: 10.1126/science.1439783. [DOI] [PubMed] [Google Scholar]
- 32.McGarry JD, Dobbins RL. Fatty acids, lipotoxicity and insulin secretion. Diabetologia. 1999;42(2):128–38. doi: 10.1007/s001250051130. [DOI] [PubMed] [Google Scholar]
- 33.McLaughlin T, Deng A, Yee G, et al. Inflammation in subcutaneous adipose tissue: relationship to adipose cell size. Diabetologia. 2010;53(2):369–77. doi: 10.1007/s00125-009-1496-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.MacKellar J, Cushman SW, Periwal V. Waves of adipose tissue growth in the genetically obese Zucker fatty rat. PLoS One. 2010;5(1):e8197. doi: 10.1371/journal.pone.0008197. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.