Abstract
Mammalian spermatogenesis is maintained by spermatogonial stem cells (SSCs). However, since evidentiary assays and unequivocal markers are still missing in non-human primates (NHPs) and man, the identity of primate SSCs is unknown. In contrast, in mice, germ cell transplantation studies have functionally demonstrated the presence of SSCs. LIN28 is an RNA-binding pluripotent stem cell factor, which is also strongly expressed in undifferentiated mouse spermatogonia. By contrast, two recent reports indicated that LIN28 is completely absent from adult human testes. Here, we analyzed LIN28 expression in marmoset monkey (Callithrix jacchus) and human testes during development and adulthood and compared it with that in mice. In the marmoset, LIN28 was strongly expressed in migratory primordial germ cells and gonocytes. Strikingly, we found a rare LIN28-positive subpopulation of spermatogonia also in adult marmoset testis. This was corroborated by western blotting and quantitative RT–PCR. Importantly, in contrast to previous publications, we found LIN28-positive spermatogonia also in normal adult human and additional adult NHP testes. Some seasonal breeders exhibit a degenerated (involuted) germinal epithelium consisting only of Sertoli cells and SSCs during their non-breeding season. The latter re-initiate spermatogenesis prior to the next breeding-season. Fully involuted testes from a seasonal hamster and NHP (Lemur catta) exhibited numerous LIN28-positive spermatogonia, indicating an SSC identity of the labeled cells. We conclude that LIN28 is differentially expressed in mouse and NHP spermatogonia and might be a marker for a rare SSC population in NHPs and man. Further characterization of the LIN28-positive population is required.
Keywords: LIN28, pluripotency, primate, spermatogonial stem cell, testis
Introduction
Mammalian spermatogonial stem cells are unipotent. They exclusively produce sperm cells over several decades in human and non-human primates (NHPs) and over years in rodents. However, mouse spermatogonial stem cells have been shown to spontaneously dedifferentiate after removal from the testicular stem cell niche (Kanatsu-Shinohara et al., 2004; Guan et al., 2006; Seandel et al., 2007; Ko et al., 2009), eventually leading to a pluripotent embryonic stem (ES) cell-like state. Although several groups aimed at translating these findings from the mouse model to the NHP model or directly to the human model (Conrad et al., 2008; Golestaneh et al., 2009; Kossack et al., 2009; Mizrak et al., 2010), it is still an open issue whether spermatogonia-derived pluripotent cell lines can be derived from the primate testis (Ko et al., 2010, 2011; Tapia et al., 2011). Since we failed in two independent laboratories (J.G. S.S.; Center for Reproductive Medicine and Andrology and K.E. and R.B.; German Primate Center) to establish pluripotent cell lines from marmoset monkey testes in numerous attempts over the last years, we started searching for possible differences in the expression of pluripotency factors between rodent and primate spermatogonial stem cells that may contribute to the difficulties during spontaneous human and monkey spermatogonial stem cell dedifferentiation to a pluripotent ES cell-like state.
Besides the ‘classical’ pluripotency transcription factors such as OCT4 and NANOG, several other factors have been shown to have essential roles for the maintenance of the undifferentiated state of ES cell and/or during induced pluripotent stem (iPS) cell generation. Among them are the transcription factors SALL4 (Elling et al., 2006; Tsubooka et al., 2009), KLF4 (Jiang et al., 2008) and the RNA-binding protein LIN28. In Caenorhabditis elegans, where lin-28 was first investigated, it has been shown that the expression of this protein is strictly regulated during development and timely presence of the protein is essential for normal development of the larvae (Moss et al., 1997). Also, the mouse orthologue of C. elegans lin-28 exhibits strict temporal and cell-specific expression during development (Moss and Tang, 2003). Recently, it has been demonstrated that LIN28 acts at the top of the cascade of factors that orchestrate primordial germ cell (PGC) specification (West et al., 2009), and in adult mouse testes, LIN28 is expressed in undifferentiated spermatogonia (Zheng et al., 2009). In contrast, a recent paper reported complete absence of LIN28 in more than 50 post-pubertal healthy human control testes, while robust LIN28 expression was seen in human testicular germ cell tumors (Cao et al., 2011). The absence of LIN28 in the adult testes was confirmed by Gillis and colleagues, while gonocyte-specific expression of LIN28 was detected in the fetal human testes (Gillis et al., 2011). LIN28 has also been shown to significantly enhance human iPS cell generation (Yu et al., 2007; Warren et al., 2010) and in the common marmoset monkey (Callithrix jacchus), this factor was even essential for complete reprogramming of somatic cells to an iPS cell state (Tomioka et al., 2010). Here, we confirmed that LIN28 is strongly expressed in adult mouse spermatogonia. Interestingly, we detected LIN28 also in a very small subpopulation of marmoset monkey spermatogonia. Moreover, in contrast to published data, LIN28 is also expressed in adult human testes and additional old-world NHP testes, where it is restricted to a very small subpopulation of spermatogonial cells. Numerous LIN28-positive germ cells in fully seasonally involuted hamster and monkey testes suggest that the LIN28-positive germ cells are spermatogonial stem cells.
Materials and Methods
Sample collection and processing were described recently (Eildermann et al., 2012a).
Human material
Fetal human gonads and pediatric testis
Parental approval to perform autopsy at the Department of Pathology, University of Göttingen, Germany, was obtained for each fetus. This included the use of gonadal tissue for histopathological investigations. The fetal gonads of males were retrieved from the archive of the Department of Pathology. Human gonads of six males from second and third trimesters were obtained after spontaneous miscarriages (gestational ages ranging from 18 to 35 weeks). Fetal gonads were collected within 24 h of death and post-mortem examinations were carried out in the Department of Pathology, University of Göttingen. Testes were dissected, fixed in 10% formalin and embedded in paraffin, and processed routinely for histological examination. Cases with obvious conditions potentially interfering with gonadal development such as chromosomal aberrations, malformations or growth retardation were excluded from the study. Gestational ages were calculated clinically in relation to the mother's last menstrual cycle and correlated with the foot length and the crown-heel length at autopsy. The biopsy of the pediatric testis from the 1-year-old boy exhibited an age-appropriate developmental stage and was obtained for diagnostic purposes.
Human adult testes samples
All patients had given written informed consent (Az. 2006-588-fs of Ethikkommission the Medical Faculty of the University of Münster) to these investigations. Ten tumor-free histological control samples from patients with contralateral germ cell tumors were analyzed. Five additional tumor-free archival testes with normal spermatogenesis from prostate carcinoma patients undergoing orchiectomy for therapeutic purposes were also used. Testicular tissue was fixed by immersion in Bouin's fixative and embedded in paraffin using standard techniques. For histological evaluation, 5 µm paraffin sections were stained with hematoxylin. Histological evaluation revealed normal spermatogenesis in all the testes.
Animal material
Marmoset monkey testis tissue samples
Testes of 15 adult, 14 pubertal (between post-natal weeks 20 and 42) and 13 newborn animals were analyzed. Five embryonal/fetal testes (between gestational days 68 and 117) were obtained after spontaneous miscarriage, or after timed surgical retrieval of the embryos (License # 33.9.42502-04/066/06). All studies were done in accordance with the German law on the protection of animals and were approved by the legal authorities.
Additional NHP species
The additional NHP testes were obtained from the DPZ tissue bank, in which many control tissue samples from different studies are archived. Furthermore, this tissue bank also includes material from Zoo animals that were autopsied at the DPZ Pathology Unit. All the tissues were obtained in accordance with the respective legal requirements.
Mouse tissues
Mouse (strain CD1) tissues were retrieved immediately after killing by cervical dislocation (adult animals) or decapitation (post-natal animals). The tissues were fixed in Bouin's solution and paraffin-embedded according routine procedures. Pregnancies were timed by checking the vaginal plugs. At least two embryos/animals were analyzed per developmental stage. All the embryos and tissues were obtained in accordance with the respective legal requirements.
Djungarian hamster tissue
Djungarian hamster (Phodopus sungorus) testes were obtained from an animal facility of the School of Engineering & Science, Jacobs University, Bremen. Testes from three animals with full spermatogenesis and three involuted testes with inactive spermatogenesis from short-day conditions (8 h light, 16 h dark) were used. The animals ranged in age from 6 to 13 months and were kept under the respective conditions for 3–7 months. The tissues were fixed in Bouin's solution and paraffin-embedded according to routine procedures. All tissues were obtained in accordance with the respective legal requirements.
Immunohistochemistry on sectioned tissues
Immunohistochemical staining for LIN28 was basically performed as described previously (Behr et al., 2007). Tissues were fixed over night in Bouin's solution immediately after recovery. After several washes in 70% EtOH for at least 2 days, the tissues were embedded in paraffin and sectioned at 5 µm. Tissue sections were deparaffinized and rehydrated and an antigen retrieval step was performed by microwaving the sections in 10 mM citrate buffer for 10 min. Endogenous peroxidase was inhibited by an incubation with peroxidase blocking reagent (DakoCytomation Carpinteria, CA, USA, LSAB+ system-HRP, K0679). The LIN28 antibody (Cell Signaling, Cat. # 3978S) was produced by immunizing rabbits with a synthetic peptide corresponding to amino acid sequence surrounding Ala177 of human LIN28A. It was used at a 1:70 dilution (in provided antibody diluent from Cell Signaling) for immunohistochemistry (IHC). Other antibodies were used in the following dilutions in Tris-buffered saline plus 3% bovine serum albumin (BSA): VASA (DDX4 = DEAD box Protein 4) [R&D Systems, Cat.#AF2030, (0.2 mg/ml)] 1:100, Undifferentiated embryonic cell transcription factor 1 (UTF1) (Millipore, Cat. #MAB4337) 1:25, SALL4 (abcam, Cat. #ab57577) 1:200, Caspase-3 (Cell Signaling, Cat. #9661) 1:200 and PLAP (DakoCytomation, Cat. #M7191) 1:25. All incubation steps were done in a humid chamber and incubations with the primary antibody were performed overnight at 4°C. DakoCytomation Universal LSAB Plus-kit including biotinylated second antibody polymer and horse-radish peroxidase (HRP)-conjugated streptavidin was employed for the detection of bound primary antibody. 3,3′-diaminobenzidine chromogen was used as substrate for the HRP and Mayer's hematoxylin as counterstain. Control stainings were carried out using non-specific rabbit immunoglobulin G (IgG) instead of the LIN28 antibody at the same protein concentration. Double staining was performed using the Envision Doublestain System, Rabbit/Mouse (DakoCytomation, Cat. #K5361) according to the manufacturer's instructions. Pictures were taken using the Nuance CRi multispectral camera (distributed by INTAS, Göttingen, Germany) which allows the detection of selected wavelengths also in the visible, i.e. immunhistochemical staining, spectrum.
Quantification of LIN28-positive cells in marmoset testes
The stained slides were scanned using the Pannoramic Midi and analyzed using the Pannoramic Viewer (3D Histech). The gonocytes were recognized by their uniform round nuclear shape, and the spermatogonia were recognized according the description given previously (Clermont, 1963; Weinbauer et al., 2001). For each age (newborn, pubertal and adult), four animals were evaluated. For newborn and juvenile animals, the total number and the number of LIN28-positive germ cells per tubule were counted in 20 tubules per section. For adult animals, all tubules per section were counted and the percentage of tubules containing at least one LIN28-positive spermatogonium was evaluated. The numbers of counted tubules are given in Supplementary data, Table S1. As an internal control, a second section from an independent area of each testis was assessed for one animal of each age.
Quantification of LIN28-positive cells in human testes
The stained slides were scanned using the Pannoramic Midi and analyzed using the Pannoramic Viewer (3D Histech). For fetal and prepubertal testis, one section per testis was used, and for adult testis, three sections per patient. For the 6 fetal testis samples 100 tubules per sections were evaluated in each case, for 1 prepubertal and 15 adult samples, all tubules per section were evaluated. The numbers of counted tubules are given in Supplementary data, Table S2. The percentage of tubules containing at least one LIN28-positive spermatogonium was evaluated.
ES cell culture
Common marmoset monkey ES cells (line cjes001) were cultured as described previously by Muller et al. (2009).
Immunofluorescence staining
ES cells were grown on x-irradiated mouse embryonic fibroblasts [mouse embryonic feeders (MEFs)] in foil-bottom 24-well plates (LumoxTM, Greiner Bio-One, Stuttgart, Germany) for 4 days, fixed for 30 min in 2% paraformaldehyde (PFA), 0.02% Triton X-100 and then washed twice in phosphate-buffered saline (PBS). The staining with primary antibodies was done according to the manufacturer's recommendations. Antibodies were diluted in PBS supplemented with 5% BSA. After 16 h incubation with primary antibody (LIN28, Cell Signaling #3978S) dilution (1:100) at 4°C (or alternatively 1 h at 37°C), cells were washed twice in PBS and incubated for another 60 min with the anti-rabbit secondary antibody covalently linked to Alexa dye A488. Images were taken on a Zeiss Axio Observer Z1 microscope. Counterstaining reagent was 4(6-diamidino-2-phenylindole).
Western blot analysis
Protein from ∼50 mg tissue or cell culture material from up to one 9 cm dish was isolated using the RNeasy mini Kit from Qiagen (Appendix F in the handbook describes the Protein precipitation from buffer RLT lysates). Protein precipitate was dissolved in 200 µl resuspension buffer (0.15 M NaCl, 15 NP-40, 1% lithium dodecyl sulfate, 2% SARKOSYL (N-Lauroylsacosin natrium salt)). For western blot analysis, 15–20 µl of the protein-lysate (including 10 × DTT and 4 × loading buffer) and 5 µl Novex sharp prestained protein standard from invitrogen was loaded onto a NuPAGE Novex 4–12% Bis–Tris gel to separate proteins. Alternatively, mouse proteins were directly lysed in SDS and sample buffer and run on the gel. Proteins were then transferred to a nitrocellulose membrane. The membrane was washed in PBS-T (1 × PBS with 0.1% Tween-20) and blocked for 30 min in 5% skim milk/0.1% normal goat serum/PBS-T. Primary-antibody incubation was performed for 1 h at room temperature or overnight at 4°C. The LIN28 antibody (Cell Signaling #3978S 1:1000) was diluted in 5% skim milk/PBS-T. After washing in PBS-T, membranes were incubated with a secondary HRP conjugated antibody (goat-anti- rabbit-HRP from RandD #HAF008). Signal-detection was carried out using the ECL-Kit from Amersham (RPN2209) and an Intas Chemo Cam.
Quantitative real-time PCR
Relative quantitative real-time (qRT)-PCR for LIN28 was performed on testicular RNA from four newborn, four 8-week-old and four adult monkeys and on testicular RNA from three newborn mice, three 9-day-old mice and three adult mice. For marmoset, the primers were designed on the basis of the whole marmoset genome which is available in the trace archive (http://blast.ncbi.nlm.nih.gov/Blast.cgi?PROGRAM=blastn&BLAST_SPEC=TraceArchive&BLAST_PROGRAMS=megaBlast&PAGE_TYPE=BlastSearch). The gene sequences were annotated by aligning them to the corresponding human and mouse genes; they displayed a nucleotide homology of ∼85% between marmoset and human/mouse. Primers were designed with Primer Express®, crossing exon boundaries, to yield RNA-specific and marmoset-specific detection. Primers were tested to yield a distinct, single amplicon by 2% agarose gel-electrophoresis. Primer sequences for marmoset were forward—5′-GACGTCTT TGTGCACCAGAGTAA-3′ and reverse—5′-CGGCCTCACCTTC CTTCAA-3′. For mice the following primers were used: forward—5′-GGTGGTGTGTTCTGTATTGGGA-3′ and reverse—5′-AGTTG TAGCACCTGTCTCCTTTG-3′. Identity of the amplicon was confirmed by DNA sequencing. For qPCR a primer concentration optimizing run according to the Power SYBR® Green PCR Master Mix and RT–PCR Protocol from Applied Biosystems, including a dissociation curve, was performed for each gene. Briefly, 2 µg testicular RNA was reverse transcribed, using random hexamers, by Superscript II (Invitrogen, Karlsruhe) to obtain cDNA; 2 µl of 1:2 diluted cDNA was used for each 20 µl PCR reaction with Power SYBR Green Mastermix (Applied Biosystems). The suitable primer concentration was determined experimentally. Six hundred nano molecules were found to be optimal for mouse forward and reverse as well as marmoset forward primers, while 900 nM were suitable for the marmoset reverse primer. The PCR program consisted of initial steps of activation and denaturation, which were run once for 10 min at 50°C and 5 min at 95°C, respectively, followed by 40 cycles of annealing (15 s at 95°C) and elongation (1 min at 60°C) steps. The extent of fluorescence of the SYBR green dye was detected and analyzed using the ABI Prism® 7000 SDS software. Each sample was assayed in triplicate und normalized to glyceraldehyde-3-phosphate dehydrogenase expression. Relative quantification was based on the 2[–ΔΔC(T)] method according to (Livak and Schmittgen, 2001) using adult testicular RNA as calibrator.
Results
Validation of the LIN28 antibody for marmoset monkey LIN28
The specificity of the LIN28 antibody for the respective marmoset protein was tested by western blot analysis. The sample containing a mixture of marmoset ES cell and MEF protein showed one very predominant band at the expected size of ∼28 kDa. The control sample lacking ES cell protein and consisting only of MEF protein showed no signal (Fig. 1A). Omission of the primary antibody resulted in no signal. Moreover, heterologously expressed marmoset LIN28 revealed the same western blot signal as shown in Fig. 1A (data not shown). Immunofluorescence showed the expected cytoplasmic LIN28 distribution in marmoset ES cells (Fig. 1B). In summary, these data indicate that the antibody used in this study specifically detects marmoset monkey LIN28 protein also.
Figure 1.
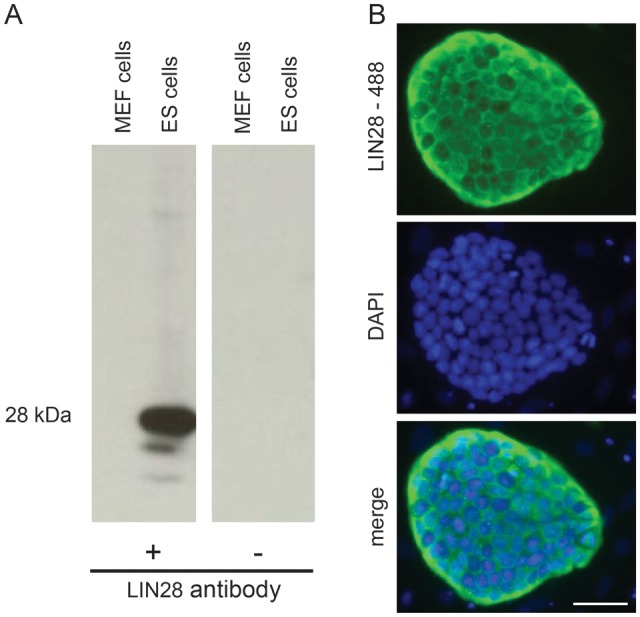
The LIN28 antibody detects marmoset monkey LIN28 protein in pluripotent ES cells. (A) Western blot analysis. Marmoset ES cells cultured on mouse embryonic feeder (MEF) cells showed a strong signal of the expected size of ∼28 kDa. Protein from MEF cells alone contains no LIN28. Omission of the primary antibody also resulted in no signal verifying the specificity of the LIN28 antibody. (B) Immunofluorescent staining of LIN28 in an undifferentiated colony of marmoset ES cells cultured on MEF cells. LIN28 is specifically detected in the cytoplasm of ES cells. The scale bar represents 50 µm.
LIN28 expression in marmoset monkey testicular germ cells during development
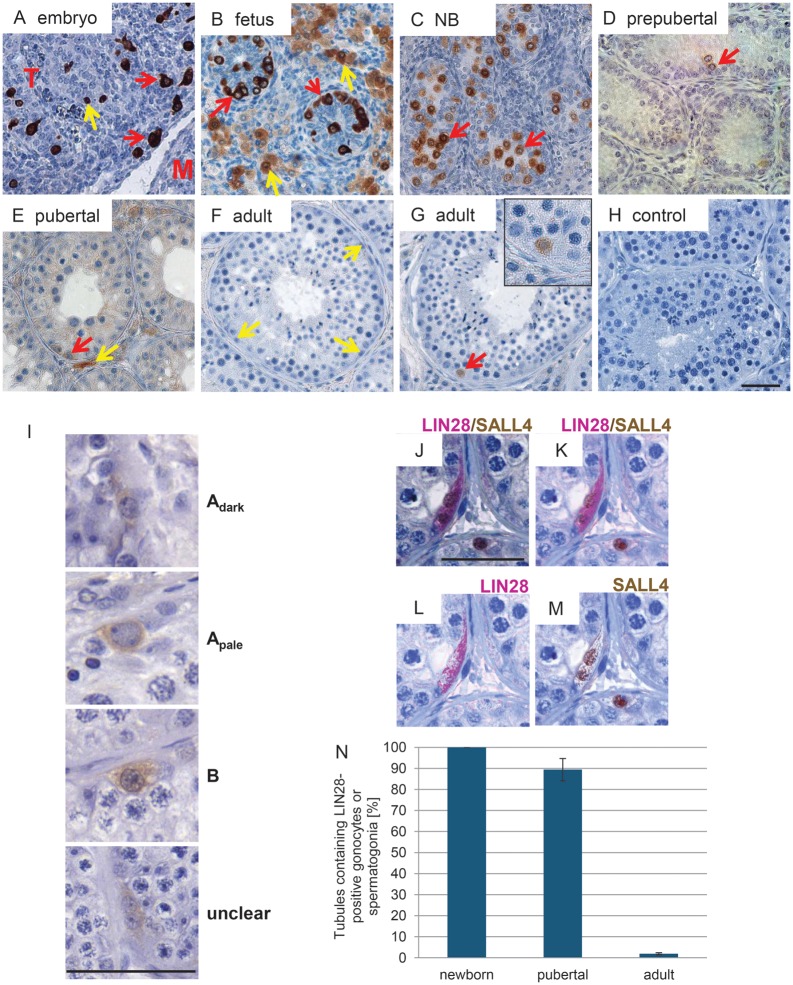
Prenatal testicular development is severely delayed in the marmoset monkey compared with human (Li et al., 2005). During the early stage of testicular organogenesis [embryonic day (E) 75], LIN28 was strongly detectable in PGCs during their late phase of migration (Fig. 2A, red arrows) and in germ cells at the transition from PGCs to gonocytes during the formation of the testicular cords (Fig. 2A, yellow arrow). Staining was seen in the cytoplasmic and in the nuclear compartment with greater intensity in the cytoplasm. At estimated fetal stage E90 [based on crown-rump length according to (Phillips, 1976) and head diameter], when the testicular cords were fully established (Fig. 2B), the gonocytes were enclosed in the cords. LIN28 was strongly expressed and localized predominantly to the cytoplasm of the germ cells (Fig. 2B, red arrows). Unexpectedly, at this stage, the majority of the gonocytes were descended to the basement membrane of the cord and only a few germ cells had a more central position. An interstitial cell population, most likely fetal Leydig cells, was also LIN28-positive, yet exhibiting lower staining intensity (Fig. 2B, yellow arrows). Basically, the same pattern was seen in the neonatal testis (Fig. 2C). However, the staining intensity between different individual germ cells varied. Furthermore, most germ cells that were localized at the basement membrane at E90 (Fig. 2B) had moved again to the central compartment of the forming seminiferous tuble (Fig. 2C, red arrows). In the pubertal testes, the differentiating germ cells are devoid of LIN28 (Fig. 2D and E) and only few spermatogonia remained LIN28-positive (red arrows). Additionally, there are some interstitial LIN28-positive cells (Fig. 2E, yellow arrows). The adult testis is almost negative for LIN28 and most of the tubule cross sections show no stained spermatogonia (Fig. 2F, yellow arrows). However, importantly, in very few tubular cross sections of the adult marmoset testis, there were specifically labeled LIN28-positive cells (Fig. 2G, red arrow, and inset). Weakly LIN28-positive cells could also be seen within the interstitial compartment. Those cells are most likely Leydig cells.
Figure 2.
LIN28 expression in prenatal, post-natal and adult marmoset monkey testes. (A) LIN28-positive PGCs enter the developing testis at gestational day 75. Some stained cells are inside the forming testicular cords (yellow arrows), while other PGCs are still in the stromal compartment (red arrows). Many cells have extensions suggesting migratory activity. T, testis; M, mesonephros (B) Fetal testis at gestational day 90. Testicular cords clearly display an epithelium and the germ cells within the cords are LIN28-positive. Most of the germ cells have contact with the basal membrane of the developing germinal epithelium (red arrows). LIN28 signals in the cells of the interstitium (yellow arrows) become weaker compared with the germ cells’ signal. (C) Newborn testis after ∼145 days of gestation. Most germ cells detached again from the basal membrane (red arrow). LIN28 signal intensity became heterogenous with some germ cells showing strong signals and others exhibiting only very faint staining. (D) Prepubertal testis at post-natal week 20. LIN28 staining is restricted to few germ cells (red arrows). (E) Pubertal testis showing first meiotic cells. Few spermatogonia (red arrow) and some interstitial cells (yellow arrow) express LIN28. (F) Typical adult seminiferous tubule lacking LIN28-positive cells. Faint staining can be seen in some interstitial cells. (G) One of the rare tubular cross sections containing a LIN28-positive spermatogonium (for quantification see Fig. 2N and Supplementary data). The inset highlights the morphology of the stained cell. (H) Negative control. LIN28 antibody was replaced by non-specific rabbit IgG at the same protein concentration as the LIN28 antibody was used. (I) Representative examples of different spermatogonial subtypes in the marmoset monkey testis expressing LIN28. Shown are an Adark spermatogonium, an Apale spermatogonium, a B spermatogonium as well as LIN28-positive spermatogonia that cannot be assigned to one specific spermatogonial subtype. (J–M) LIN28-positive spermatogonia co-express SALL4. The brightfield-picture J) shows two LIN28+/SALL4+ spermatogonia and one LIN28-negative spermatogonium expressing SALL4 (K-M) show the same picture false-colored as an overlay (K) or highlighting only LIN28 (L) or SALL4 (M) staining. All scale bars represent 50 µm.
In the adult marmoset testes, we detected 39 clearly stained spermatogonia (see Supplementary data, Table S1). Noteworthy, staining was seen in all the three morphological spermatogonial subtypes (Fig. 2I): Two cells were clearly assigned to Adark spermatogonia, 11 to Apale spermatogonia and 8 to type B spermatogonia. Eighteen LIN28-positive spermatogonia could not be clearly assigned to a specific spermatogonial subtype. They were classified as ‘unclear’.
A double staining of LIN28 and SALL4 revealed that all observed LIN28-positive spermatogonia co-expressed SALL4, whereas not all SALL4-positive cells expressed LIN28. Figure 2J shows the original bright-field picture of two spermatogonia co-expressing LIN28 and SALL4, while another SALL4-positive spermatogonium lacks LIN28 signals. Figure 2K–M shows the same picture false-colored to better distinguish between the LIN28 signals and the SALL4 signals.
Quantitative analysis of the LIN28-positive germ cells revealed that 100% of the tubules in the newborn testis contained LIN28-positive gonocytes (Fig. 2N). At this developmental stage, 30–80% of all germ cells were LIN28-positive. In pubertal testes, the percentage of tubules containing LIN28-positive (pre-) spermatogonia decreased to ∼90%. In the adult marmoset testis only 0.5–3.3% of all evaluated tubules displayed any LIN28-positive spermatogonia demonstrating that in the adult testis the LIN28-positive population of spermatogonia is very rare. The absolute numbers of evaluated tubules and counted LIN28-positive cells are given in Supplementary data, Table S1.
LIN28 expression in mouse testicular germ cells during development
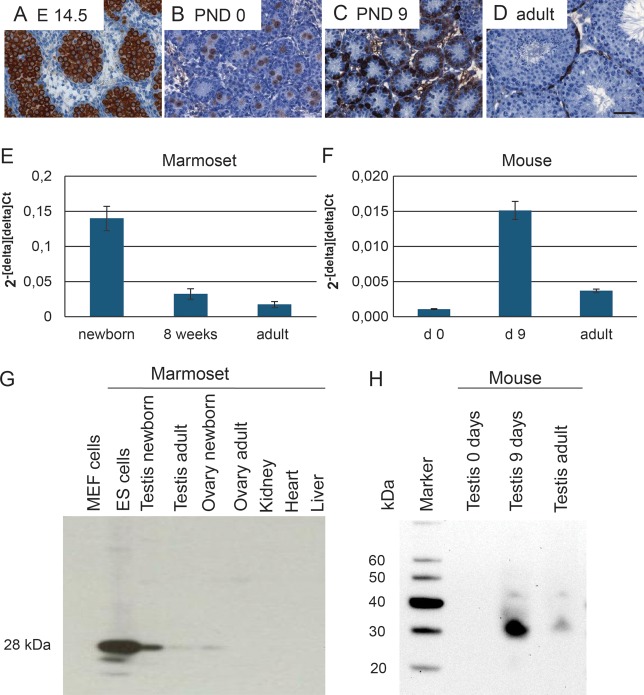
As a reference, we included fetal, neonatal, postnatal day 9 and adult mouse testes (Fig. 3A–D). As in the primate testis, mouse gonocytes and immature spermatogonia were LIN28-positive (Fig. 3A–C). However, in contrast to the adult monkey and human testes, the adult mouse testis still shows strong LIN28 expression in several spermatogonia per tubulular cross section (Fig. 3D).
Figure 3.
LIN28 protein and mRNA in marmoset and mouse testes. (A) LIN28 is present in the cytoplasm of mouse gonocytes and (B and C) immature spermatogonia, as well as in adult spermatogonia (D). The scale bar represents 50 µm. Quantitative RT–PCR with marmoset (E) and mouse (F) whole testis RNA. (E) Newborn, 8 weeks-old, and adult marmoset testes. There is a strong and significant decrease in the relative mRNA abundance during testicular development. (F) The expression profile of the mouse testis shows a strong increase from birth to PND9 and subsequent decrease to adulthood. The adult relative expression levels were still above the newborn levels. (G) Western blot analysis of LIN28 in newborn and adult marmoset monkey testis and control tissues. Omission of the primary antibody resulted in no signal (data not shown). (H) Western blot analysis of LIN28 in neonatal, post-natal day 9 and adult mouse testes.
LIN28 RNA and protein profiles in post-natal marmoset and mouse testes are divergent
To determine the relative abundance of LIN28 mRNA during post-natal testis development in the marmoset and the mouse, we performed qRT-PCR. In the marmoset testis, we detected a constant decrease in relative LIN28 mRNA abundance, which reflects the immunohistochemical findings: strong expression in the newborn, intermediate expression at 8-week-old testes and low expression in adult testes. In the mouse, the expression profile was different with a strong increase from birth to PND9 and subsequent decrease to adulthood. However, adult relative expression levels were still above the newborn levels (Fig. 3F). The mRNA data were corroborated by western blot analyses (Fig. 3G and H). In the newborn marmoset, we detected a clear band, while only an extremely faint band was visible in adult testis. Control organs such as newborn ovary or adult tissues including ovary, heart, liver and kidney showed only weak (newborn ovary) or no LIN28 signal (Fig. 3G). In the mouse, LIN28 was almost undetectable at birth. At PND9, there was a very intense signal and in adult testis, a rather weak one (Fig. 3H). Thus, mRNA and protein data correlate very well and corroborate the immunohistochemical data.
LIN28 is expressed in a rare population of adult human spermatogonia
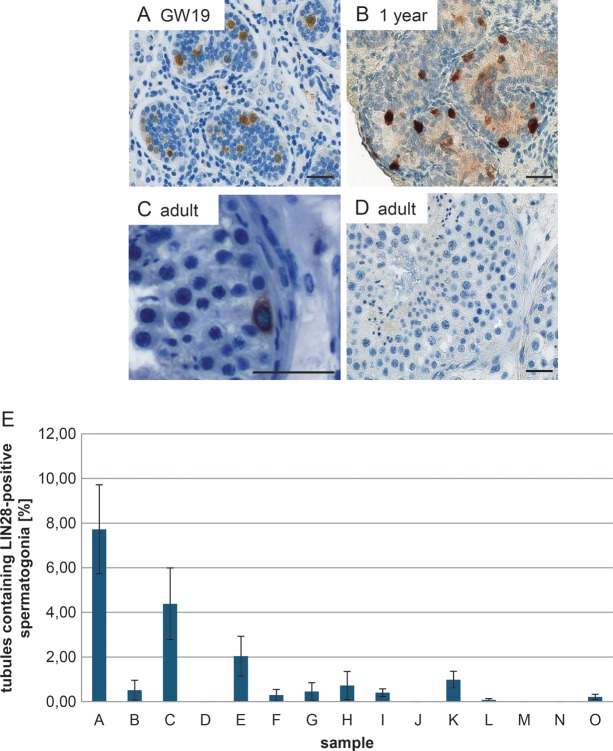
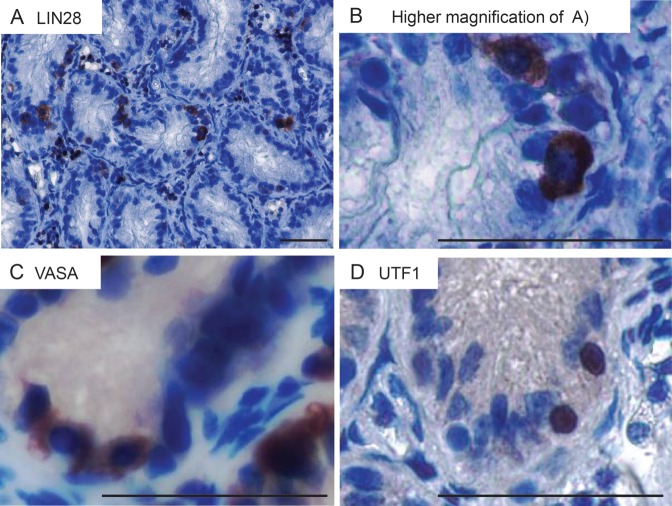
On the basis of the findings in the adult marmoset monkey testis, we carefully analyzed a panel of adult human testis samples with qualitatively and quantitatively normal spermatogenesis (n = 15). We included fetal testes and a prepubertal sample as controls and to confirm the developmental expression of LIN28 in the human testis. The fetal samples were fixed in 10% PFA and not in Bouin's solution like the other samples used in this study. To exclude an influence of the different fixatives on the staining, testis samples from a marmoset monkey were fixed in Bouin's solution and 10% PFA, respectively. The LIN28 staining showed only a slight difference in the staining intensity between both fixatives (Supplementary data, Fig. S1). However, the basic staining patterns were fully comparable between both fixatives thereby excluding a significant effect of the fixation on our data. As shown in Fig. 4A and B, LIN28 is present in fetal gonocytes and spermatogonia of the postnatal testis. In contrast to previous papers (Cao et al., 2011; Gillis et al., 2011), a careful analysis revealed the presence of a very few clearly LIN28-positive spermatogonia in the adult testis also (Fig. 4C). However, the vast majority of all tubules showed no LIN28-positive spermatogonia (Fig. 4D). In the evaluated samples, the percentage of tubules showing at least one LIN28-positive spermatogonia ranged from 0 to 7.7% (Fig. 4E). Four out of 15 samples showed no LIN28-positive spermatogonia (Fig. 4E, details for the histological evaluation see Supplementary data, Table S2). The number of detected LIN28-positive cells showed no correlation with the age of the patient (data not shown).
Figure 4.
LIN28 expression in human germ cells during testis development. LIN28 is present in human gonocytes (A) and immature spermatogonia (B). Very few spermatogonia in the adult testis are LIN28-positive (C); for quantification see (E), however, the vast majority of all tubules showed no LIN28-positive spermatogonia. (D) Negative control. The scale bar represents 50 µm. (E) Percentage of tubular cross sections containing at least one LIN28-positive cell in adult testis. Bars A–J represent histologically normal samples from patients with germ cell tumors in the contra-lateral testis, K–O represent normal testis samples from prostate carcinoma patients without any evidence of germ cell tumors undergoing orchiectomy for therapeutic purposes.
LIN28 in additional NHP species
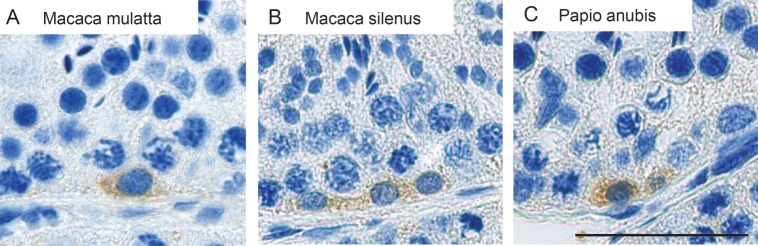
We analyzed testes of additional adult old world monkey species, which represent the closest phylogenetic relatives to man available to us. In the rhesus monkey (Macaca mulatta) and the lion-tailed macaque (Macaca silenus) as well as in the baboon (Papio anubis), we also found single or paired LIN28-positive spermatogonia (Fig. 5A–C).
Figure 5.
LIN28 in additional NHP species. LIN28 is present in single or paired spermatogonia in the rhesus monkey (Macacca mulatta) (A) and the lion-tailed macaque (Macacca silenus) (B) as well as in the baboon (Papio anubis) (C). The scale bar represents 50 µm.
LIN28 expression in hamster and monkey testes during seasonal involution
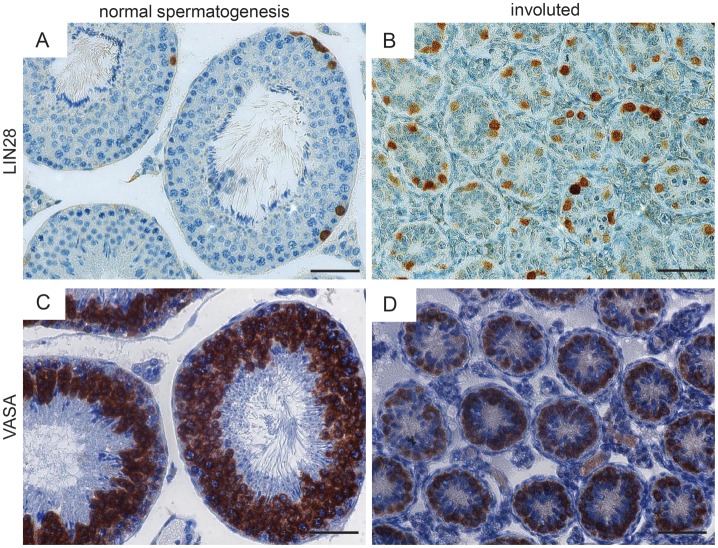
There are several mammalian (including primate) species that show severe testicular involution during the annual reproductive cycle. In these species, spermatogenesis is active only before/during the mating period to supply fertile ejaculates. After the mating period, spermatogenesis is down-regulated and the testis regresses to a rather fetal state, i.e. in extreme cases, the germ cell population consists only/mostly of the most primitive spermatogonial stem cells, while differentiating germ cells are mostly lacking. Starting from these spermatogonial stem cells, spermatogenesis is re-initiated prior to the next mating period. We analyzed LIN28 expression in involuted Djungarian hamster (P. sungorus) (Fig. 6) and NHP (Ring-tailed lemur; Lemur catta) testicular samples (Fig. 7). The testes showed strong testicular involution and had only very few germ cells in the seminiferous tubules. Very impressively, under these special circumstances, a significant fraction of germ cells in the hamster as well as in the NHP testis were LIN28-positive as evidenced by staining of the same samples for the general germ cell marker VASA. UTF-1 is an established spermatogonial marker in human and rat testis (van Bragt et al., 2008; von Kopylow et al., 2010). UTF1-expression by the germ cells of the involuted monkey tubules indicated their stem cell identity (Fig. 7). Importantly, in both species, we detected neither Caspase3 (apoptosis marker) nor placenta-like alkaline phosphates (PLAP; germ cell tumor marker) expression in the germ cells (data not shown), while the controls available to us (human testicular tumors and marmoset monkey placenta for PLAP and marmoset testis for Caspase3) showed the expected staining pattern. Altogether, these data strongly indicate that LIN28 is expressed in vital and functional stem cells, from which spermatogenesis is re-initiated after a physiological phase of spermatogenic quiescence.
Figure 6.
Spermatogonia in involuted hamster testes are LIN28-positive. (A) Djungarian hamster testis with normal full spermatogenesis. Few spermatogonia are intensely LIN28-positve. (B) Fully involuted hamster testis showing LIN28-positive spermatogonia, which are present at very high density in this condition. (C) Germ cells including most spermatogonia in the normal testis are also positive for VASA. (D) Also in the involuted testis the germ cells are VASA-positive. There are more VASA- than LIN28-positive cells. The scale bar represents 50 µm.
Figure 7.
Spermatogonia in involuted primate testes are LIN28-positive. (A) Fully involuted monkey (Lemur catta) testis showing only very few germ cells in the inactive germinal epithelium consisting mainly of somatic Sertoli cells. However, many of the germ cells present are clearly LIN28-positive. (B) Higher magnification of (A). The germ cells also express VASA (C) and UTF1 (D). The scale bar represents 50 µm.
Discussion
Primate SSCs are still enigmatic. Their exact identity and the functional roles of different morphologically characterized types of undifferentiated spermatogonia are even nowadays unclear (for review see, Hermann et al., 2010). This is mainly due to the fact that the tools to study and identify spermatogonial stem cells are still very limited in primates, while in the mouse model transgenic and transplantation approaches and the combination of both were very instructive during the past two decades (Brinster and Zimmermann, 1994; Guan et al., 2006; Nakagawa et al., 2007; Yoshida, 2008; Barroca et al., 2009; Ko et al., 2009; Suzuki et al., 2009; Nakagawa et al., 2010); for review see (Phillips et al., 2010). Strikingly, in NHPs and human, where the SSCs are thought to be a subpopulation of the morphological populations of Adark and/or Apale spermatogonia, emerging data indicate that these spermatogonial populations exhibit a very heterogenous molecular phenotype (Hermann et al., 2009) and even to date it is still a matter of debate whether Adark spermatogonia contribute to sperm production under normal physiological circumstances or whether they only represent reserve stem cells which repopulate a germinal epithelium after a gonadotoxic insult (Ehmcke and Schlatt, 2006; Ehmcke et al., 2006; Hermann et al., 2010). Moreover, it is still not fully understood how and which type of spermatogonia replenishes the SSC pool. These fundamental gaps in our knowledge about SSCs in primates are very difficult or even impossible to close since the important tools which advanced mouse SSC research during the recent years, e.g. SSC transplantation and in vivo-imaging using transgenic mouse strains, will not be available in primates as routine within the foreseeable future. Therefore, analysis of SSCs in primates relies mainly on whole mount analysis of cultured tissue fragments and on in situ characterization of SSCs in fixed tissue sections using IHC.
For that purpose, it is important to establish a specific, meaningful and thus unequivocal panel of markers for the characterization and probably selection of specific types of spermatogonia in general and SSCs in particular. Some useful markers for different purposes have been described during recent years (Aponte et al., 2005; Hermann et al., 2010; Plant, 2010). However, additional markers would be of great advantage to better describe and characterize spermatogonial subpopulations.
To our knowledge so far, the LIN28-positive cells are a much rarer cell type than any other primate spermatogonial cell population described. Therefore, additional studies have to be performed using double (LIN28 together with e.g. PLZF, NGN3, c-KIT, respectively) and whole mount labeling, since the length of the LIN28-positive spermatogonial clones is of interest. However, the fact that those spermatogonia remaining in the testis during severe involution are clearly LIN28-positve in the NHP and rodent testis strongly argues for a spermatogonial stem cell identity of the LIN28-positive cells.
LIN28 is a pluripotency factor that is highly expressed in pluripotent mouse ES cells, mouse embryonal carcinoma and human teratocarcinoma cells (Moss and Tang, 2003). LIN28 was also very supportive or even necessary during iPS cell generation in human and NHP cells (Yu et al., 2007; Tomioka et al., 2010; Warren et al., 2010). However, in contrast to most other reprogramming proteins, which are DNA-binding transcription factors, LIN28 is a well-conserved RNA-binding protein exhibiting unique molecular features as it is the only known mammalian protein that combines two specific domains: the cold shock domain, which binds to single-stranded nucleic acids, and the aminosäuren cystein/histidin zinc finger motif. The latter motif is known from nucleocapsid proteins of retro-viruses. It is involved in packaging the viral RNA into the virus particles [(Moss and Tang, 2003) and references therein]. Thus, LIN28 is an RNA-binding protein and as such, it predominantly localizes to the cytoplasm of the spermatogonia as shown in this study. A recent paper showed that LIN28 binds to and enhances the translation of mRNAs, including OCT4, that are involved in the growth and survival of human ES cells (Qiu et al., 2010; Peng et al., 2011). Since there are additional reports on LIN28 expression in different types of (cancer) stem cells, it is very likely that LIN28 has a general important function in stem cells.
During recent years, several publications reported the culture of spermatogonial stem cells from mouse testis and the subsequent derivation of multipotent/pluripotent ES cell-like cells (Guan et al., 2006; Seandel et al., 2007; Kanatsu-Shinohara et al., 2008; Ko et al., 2009). However, the attempts to derive the corresponding human cells were not as convincing as in the mouse (Conrad et al., 2008; Golestaneh et al., 2009; Kossack et al., 2009; Mizrak et al., 2010) and the origin and developmental potential of cultured cells from human testis is still under debate (Ko et al., 2010; 2011; Tapia et al., 2011; Chikhovskaya et al., 2012; Eildermann et al., 2012b). One major problem in the human system is that the final proof of pluripotency cannot be achieved, since chimera formation between preimplantation embryo and pluripotent stem cells appears impossible (Tachibana et al., 2012). In addition to the debated data published for human testis cells, we failed to isolate and culture ES cell-like cells from the NHP testis (Eildermann et al., 2012b). Taking this into consideration, we searched for biological differences between the mouse and the primate testes and analyzed the expression of several pluripotency factors in fetal and postnatal testes of man, macaques, marmosets and mice. While some pluripotency factors such as SALL4 showed expression in gonocytes and in adult spermatogonia in all the species analyzed (Eildermann et al., 2012a), others were present in gonocytes (like OCT4), but were absent from spermatogonia of all the species. In general, the expression patterns of the factors analyzed in primates and mice were comparable. A very exciting exception of these conserved expression patterns of the pluripotency factors in the primate and mouse germ line was LIN28, as reported in this study and in parts also shown previously by others (Zheng et al., 2009; Cao et al., 2011; Gillis et al., 2011). The data published by Zheng et al. (2009) were fully confirmed in our study. We also confirmed a huge discrepancy between LIN28 expression in the mouse and in the primate testes including human testes. However, while Cao et al. (2011) and Gillis et al. (2011) reported a complete absence of LIN28 from adult spermatogonia, we identified a very small subpopulation of spermatogonia clearly expressing LIN28 in the monkey and human testes. This discrepancy to previous reports could be due to the very low frequency of the LIN28-positive cells in primate testis. Indeed, there are human testis sections even in our own study that do not contain any LIN28-positive cells. If we had not detected LIN28-positive spermatogonia in the monkey testis, which we initially analyzed in more detail, it is possible that we (also) would have missed the stained spermatogonia in the human testis. Importantly, our data do not support the idea that the stained spermatogonia are undergoing apoptosis as it was suggested for LIN28-positive human gonocytes (Gillis et al., 2011). They rather support the idea that the LIN28-positive cells may represent a spermatogonial stem cell population. The enormous difference between primate and mouse testis in the number of spermatogonia expressing LIN28 may contribute to the discrepancy regarding derivation of pluripotent ES cell-like cells from mouse and primate testis.
This discrepant LIN28 expression in mouse and marmoset testis also explains the differential expression profile during development (see Fig. 3E and F). In the marmoset testis, the percentage (and probably also the absolute number) of LIN28-positive cell is continuously decreasing during development. In contrast, in the post-natal mouse testis, the population of LIN28 expressing cells, i.e. gonocytes and immature spermatogonia, is quickly expanding during the first days of post-natal testis development and only subsequently diluted by the more differentiated germ cells such as spermatocytes and spermatids.
In summary, LIN28 is expressed only in a very small subpopulation of spermatogonia in the adult monkey and human testis, which is in striking contrast to the mouse testis. Taking the functional role of LIN28 in pluripotent stem cells into account, it is likely that LIN28 is expressed in SSCs and may thus serve as a novel SSCs marker in NHPs and in man. Furthermore, since LIN28 is essential for germ cell specification in mice (West et al., 2009), it is tempting to speculate that this RNA-binding protein is also involved in maintaining the identity of adult SSC. This assumption is supported by the fact that LIN28 is expressed in (i) spermatogonia of involuted rodent and primate testes (as shown in this study) and (ii) neoplastic germ cells (Cao et al., 2011; Gillis et al., 2011), which are known to express many pluripotent stem cell markers (Rajpert-De Meyts, 2006; Emerson and Ulbright, 2010).
Supplementary data
Supplementary data are available at http://molehr.oxfordjournals.org/.
Authors’ roles
N.A. and K.E.: study design, collection and organization of data, data analysis and interpretation and final approval of the manuscript. C.D. and S. Schweyer: contribution of study material and final approval of the manuscript. J.E.: data analysis and interpretation and final approval of the manuscript. A.L., M.B. and S.K.: contribution of study material and final approval of the manuscript. J.G.: collection and assembly of data and final approval of the manuscript. S. Schlatt: contribution of study material, data analysis and interpretation and final approval of the manuscript. R.B.: study design, assembly of data, data analysis and interpretation, manuscript writing and final approval of the manuscript.
Funding
This study was supported in part by BMBF grant 01GN0809/10 entitled ‘Pluripotent cells in primate testes’ to R.B. and S. Schlatt and by the German Research Foundation by unrestricted grants within the Research Unit ‘Germ Cell Potential’ (FOR-BE 2296/6-2) to R.B., J.G. and S. Schlatt. Funding to pay the Open Access publication charges for this article was provided by the German Primate Center, which is a Leibniz Institute financed by the Bundesrepublik Deutschland and the Bundesländer.
Conflict of interest
None declared.
Supplementary Material
Acknowledgements
We thank Nicole Umland, Angelina Berenson, Marion Seidel, Nicole Terwort and Karen Grote for excellent technical assistance.
References
- Aponte PM, van Bragt MP, de Rooij DG, van Pelt AM. Spermatogonial stem cells: characteristics and experimental possibilities. APMIS. 2005;113:727–742. doi: 10.1111/j.1600-0463.2005.apm_302.x. [DOI] [PubMed] [Google Scholar]
- Barroca V, Lassalle B, Coureuil M, Louis JP, Le Page F, Testart J, Allemand I, Riou L, Fouchet P. Mouse differentiating spermatogonia can generate germinal stem cells in vivo. Nat Cell Biol. 2009;11:190–196. doi: 10.1038/ncb1826. [DOI] [PubMed] [Google Scholar]
- Behr R, Deller C, Godmann M, Muller T, Bergmann M, Ivell R, Steger K. Kruppel-like factor 4 expression in normal and pathological human testes. Mol Hum Reprod. 2007;13:815–820. doi: 10.1093/molehr/gam064. [DOI] [PubMed] [Google Scholar]
- Brinster RL, Zimmermann JW. Spermatogenesis following male germ-cell transplantation. Proc Natl Acad Sci USA. 1994;91:11298–11302. doi: 10.1073/pnas.91.24.11298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao D, Allan RW, Cheng L, Peng Y, Guo CC, Dahiya N, Akhi S, Li J. RNA-binding protein LIN28 is a marker for testicular germ cell tumors. Hum Pathol. 2011;42:710–718. doi: 10.1016/j.humpath.2010.09.007. [DOI] [PubMed] [Google Scholar]
- Chikhovskaya JV, Jonker MJ, Meissner A, Breit TM, Repping S, van Pelt AM. Human testis-derived embryonic stem cell-like cells are not pluripotent, but possess potential of mesenchymal progenitors. Hum Reprod (Oxford, England) 2012;27:210–221. doi: 10.1093/humrep/der383. [DOI] [PubMed] [Google Scholar]
- Clermont Y. The cycle of the seminiferous epithelium in man. Am J Anat. 1963;112:35–51. doi: 10.1002/aja.1001120103. [DOI] [PubMed] [Google Scholar]
- Conrad S, Renninger M, Hennenlotter J, Wiesner T, Just L, Bonin M, Aicher W, Buhring HJ, Mattheus U, Mack A, et al. Generation of pluripotent stem cells from adult human testis. Nature. 2008;456:344–349. doi: 10.1038/nature07404. [DOI] [PubMed] [Google Scholar]
- Ehmcke J, Schlatt S. A revised model for spermatogonial expansion in man: lessons from non-human primates. Reproduction (Cambridge, England) 2006;132:673–680. doi: 10.1530/rep.1.01081. [DOI] [PubMed] [Google Scholar]
- Ehmcke J, Wistuba J, Schlatt S. Spermatogonial stem cells: questions, models and perspectives. Hum Reprod Update. 2006;12:275–282. doi: 10.1093/humupd/dmk001. [DOI] [PubMed] [Google Scholar]
- Eildermann K, Aeckerle N, Debowski K, Godmann M, Christiansen H, Heistermann M, Schweyer S, Bergmann M, Kliesch S, Gromoll J, et al. Developmental expression of the pluripotency factor Sal-like protein 4 in the monkey, human and mouse testis: restriction to premeiotic germ cells. Cells Tissues Organs. 2012a doi: 10.1159/000335031. 10.1159/000335031. [DOI] [PubMed] [Google Scholar]
- Eildermann K, Gromoll J, Behr R. Misleading and reliable markers to differentiate between primate testis-derived multipotent stromal cells and spermatogonia in culture. Hum Reprod (Oxford, England) 2012b doi: 10.1093/humrep/des091. 10.1093/humrep/des091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elling U, Klasen C, Eisenberger T, Anlag K, Treier M. Murine inner cell mass-derived lineages depend on Sall4 function. Proc Natl Acad Sci USA. 2006;103:16319–16324. doi: 10.1073/pnas.0607884103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson RE, Ulbright TM. Intratubular germ cell neoplasia of the testis and its associated cancers: the use of novel biomarkers. Pathology. 2010;42:344–355. doi: 10.3109/00313021003767355. [DOI] [PubMed] [Google Scholar]
- Gillis AJ, Stoop H, Biermann K, van Gurp RJ, Swartzman E, Cribbes S, Ferlinz A, Shannon M, Oosterhuis JW, Looijenga LH. Expression and interdependencies of pluripotency factors LIN28, OCT3/4, NANOG and SOX2 in human testicular germ cells and tumours of the testis. Int J Andrology. 2011;34:e160–e174. doi: 10.1111/j.1365-2605.2011.01148.x. [DOI] [PubMed] [Google Scholar]
- Golestaneh N, Kokkinaki M, Pant D, Jiang J, DeStefano D, Fernandez-Bueno C, Rone JD, Haddad BR, Gallicano GI, Dym M. Pluripotent stem cells derived from adult human testes. Stem Cells Dev. 2009;18:1115–1126. doi: 10.1089/scd.2008.0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan K, Nayernia K, Maier LS, Wagner S, Dressel R, Lee JH, Nolte J, Wolf F, Li M, Engel W, et al. Pluripotency of spermatogonial stem cells from adult mouse testis. Nature. 2006;440:1199–1203. doi: 10.1038/nature04697. [DOI] [PubMed] [Google Scholar]
- Hermann BP, Sukhwani M, Simorangkir DR, Chu T, Plant TM, Orwig KE. Molecular dissection of the male germ cell lineage identifies putative spermatogonial stem cells in rhesus macaques. Hum Reprod (Oxford, England) 2009;24:1704–1716. doi: 10.1093/humrep/dep073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann BP, Sukhwani M, Hansel MC, Orwig KE. Spermatogonial stem cells in higher primates: are there differences from those in rodents? Reproduction. 2010;139:479–493. doi: 10.1530/REP-09-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Chan YS, Loh YH, Cai J, Tong GQ, Lim CA, Robson P, Zhong S, Ng HH. A core Klf circuitry regulates self-renewal of embryonic stem cells. Nat Cell Biol. 2008;10:353–360. doi: 10.1038/ncb1698. [DOI] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M, Inoue K, Lee J, Yoshimoto M, Ogonuki N, Miki H, Baba S, Kato T, Kazuki Y, Toyokuni S, et al. Generation of pluripotent stem cells from neonatal mouse testis. Cell. 2004;119:1001–1012. doi: 10.1016/j.cell.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M, Lee J, Inoue K, Ogonuki N, Miki H, Toyokuni S, Ikawa M, Nakamura T, Ogura A, Shinohara T. Pluripotency of a single spermatogonial stem cell in mice. Biol Reprod. 2008;78:681–687. doi: 10.1095/biolreprod.107.066068. [DOI] [PubMed] [Google Scholar]
- Ko K, Tapia N, Wu G, Kim JB, Bravo MJ, Sasse P, Glaser T, Ruau D, Han DW, Greber B, et al. Induction of pluripotency in adult unipotent germline stem cells. Cell Stem Cell. 2009;5:87–96. doi: 10.1016/j.stem.2009.05.025. [DOI] [PubMed] [Google Scholar]
- Ko K, Arauzo-Bravo MJ, Tapia N, Kim J, Lin Q, Bernemann C, Han DW, Gentile L, Reinhardt P, Greber B, et al. Human adult germline stem cells in question. Nature. 2010;465:E1. doi: 10.1038/nature09089. discussion E3. [DOI] [PubMed] [Google Scholar]
- Ko K, Reinhardt P, Tapia N, Schneider RK, Arauzo-Bravo MJ, Han DW, Greber B, Kim J, Kliesch S, Zenke M, et al. Brief report: evaluating the potential of putative pluripotent cells derived from human testis. Stem Cells. 2011;29:1304–1309. doi: 10.1002/stem.671. [DOI] [PubMed] [Google Scholar]
- Kossack N, Meneses J, Shefi S, Nguyen HN, Chavez S, Nicholas C, Gromoll J, Turek PJ, Reijo-Pera RA. Isolation and characterization of pluripotent human spermatogonial stem cell-derived cells. Stem Cells. 2009;27:138–149. doi: 10.1634/stemcells.2008-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li LH, Donald JM, Golub MS. Review on testicular development, structure, function, and regulation in common marmoset. Birth Defects Res. 2005;74:450–469. doi: 10.1002/bdrb.20057. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Mizrak SC, Chikhovskaya JV, Sadri-Ardekani H, van Daalen S, Korver CM, Hovingh SE, Roepers-Gajadien HL, Raya A, Fluiter K, de Reijke TM, et al. Embryonic stem cell-like cells derived from adult human testis. Hum Reprod (Oxford, England) 2010;25:158–167. doi: 10.1093/humrep/dep354. [DOI] [PubMed] [Google Scholar]
- Moss EG, Tang L. Conservation of the heterochronic regulator Lin-28, its developmental expression and microRNA complementary sites. Dev Biol. 2003;258:432–442. doi: 10.1016/s0012-1606(03)00126-x. [DOI] [PubMed] [Google Scholar]
- Moss EG, Lee RC, Ambros V. The cold shock domain protein LIN-28 controls developmental timing in C. elegans and is regulated by the lin-4 RNA. Cell. 1997;88:637–646. doi: 10.1016/s0092-8674(00)81906-6. [DOI] [PubMed] [Google Scholar]
- Muller T, Fleischmann G, Eildermann K, Matz-Rensing K, Horn PA, Sasaki E, Behr R. A novel embryonic stem cell line derived from the common marmoset monkey (Callithrix jacchus) exhibiting germ cell-like characteristics. Hum Reprod (Oxford, England) 2009;24:1359–1372. doi: 10.1093/humrep/dep012. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Nabeshima Y, Yoshida S. Functional identification of the actual and potential stem cell compartments in mouse spermatogenesis. Dev Cell. 2007;12:195–206. doi: 10.1016/j.devcel.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Sharma M, Nabeshima Y, Braun RE, Yoshida S. Functional hierarchy and reversibility within the murine spermatogenic stem cell compartment. Science. 2010;328:62–67. doi: 10.1126/science.1182868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng S, Chen LL, Lei XX, Yang L, Lin H, Carmichael GG, Huang Y. Genome-wide studies reveal that Lin28 enhances the translation of genes important for growth and survival of human embryonic stem cells. Stem Cells (Dayton, Ohio) 2011;29:496–504. doi: 10.1002/stem.591. [DOI] [PubMed] [Google Scholar]
- Phillips IR. The Embryology of the Common Marmoset (Callithrix jacchus) Berlin, Heidelberg, New York: Springer-Verlag; 1976. [PubMed] [Google Scholar]
- Phillips BT, Gassei K, Orwig KE. Spermatogonial stem cell regulation and spermatogenesis. Philos Trans R Soc Lond. 2010;365:1663–1678. doi: 10.1098/rstb.2010.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant TM. Undifferentiated primate spermatogonia and their endocrine control. Trends Endocrinol Metab. 2010;21:488–495. doi: 10.1016/j.tem.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu C, Ma Y, Wang J, Peng S, Huang Y. Lin28-mediated post-transcriptional regulation of Oct4 expression in human embryonic stem cells. Nucleic Acids Res. 2010;38:1240–1248. doi: 10.1093/nar/gkp1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajpert-De Meyts E. Developmental model for the pathogenesis of testicular carcinoma in situ: genetic and environmental aspects. Hum Reprod Update. 2006;12:303–323. doi: 10.1093/humupd/dmk006. [DOI] [PubMed] [Google Scholar]
- Seandel M, James D, Shmelkov SV, Falciatori I, Kim J, Chavala S, Scherr DS, Zhang F, Torres R, Gale NW, et al. Generation of functional multipotent adult stem cells from GPR125+ germline progenitors. Nature. 2007;449:346–350. doi: 10.1038/nature06129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Sada A, Yoshida S, Saga Y. The heterogeneity of spermatogonia is revealed by their topology and expression of marker proteins including the germ cell-specific proteins Nanos2 and Nanos3. Dev Biol. 2009;336:222–231. doi: 10.1016/j.ydbio.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Tachibana M, Sparman M, Ramsey C, Ma H, Lee H-S, Penedo MCT, Mitalipov S. Generation of Chimeric Rhesus Monkeys. Cell. 2012 doi: 10.1016/j.cell.2011.12.007. doi:org/10.1016/jcell201112007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapia N, Arauzo-Bravo MJ, Ko K, Scholer HR. Concise review: challenging the pluripotency of human testis-derived ESC-like cells. Stem Cells. 2011;29:1165–1169. doi: 10.1002/stem.669. [DOI] [PubMed] [Google Scholar]
- Tomioka I, Maeda T, Shimada H, Kawai K, Okada Y, Igarashi H, Oiwa R, Iwasaki T, Aoki M, Kimura T, et al. Generating induced pluripotent stem cells from common marmoset (Callithrix jacchus) fetal liver cells using defined factors, including Lin28. Genes Cells. 2010;15:959–969. doi: 10.1111/j.1365-2443.2010.01437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsubooka N, Ichisaka T, Okita K, Takahashi K, Nakagawa M, Yamanaka S. Roles of Sall4 in the generation of pluripotent stem cells from blastocysts and fibroblasts. Genes Cells. 2009;14:683–694. doi: 10.1111/j.1365-2443.2009.01301.x. [DOI] [PubMed] [Google Scholar]
- van Bragt MP, Roepers-Gajadien HL, Korver CM, Bogerd J, Okuda A, Eggen BJ, de Rooij DG, van Pelt AM. Expression of the pluripotency marker UTF1 is restricted to a subpopulation of early A spermatogonia in rat testis. Reproduction (Cambridge, England) 2008;136:33–40. doi: 10.1530/REP-07-0536. [DOI] [PubMed] [Google Scholar]
- von Kopylow K, Kirchhoff C, Jezek D, Schulze W, Feig C, Primig M, Steinkraus V, Spiess AN. Screening for biomarkers of spermatogonia within the human testis: a whole genome approach. Hum Reprod (Oxford, England) 2010;25:1104–1112. doi: 10.1093/humrep/deq053. [DOI] [PubMed] [Google Scholar]
- Warren L, Manos PD, Ahfeldt T, Loh YH, Li H, Lau F, Ebina W, Mandal PK, Smith ZD, Meissner A, et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell. 2010;7:618–630. doi: 10.1016/j.stem.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinbauer GF, Aslam H, Krishnamurthy H, Brinkworth MH, Einspanier A, Hodges JK. Quantitative analysis of spermatogenesis and apoptosis in the common marmoset (Callithrix jacchus) reveals high rates of spermatogonial turnover and high spermatogenic efficiency. Biol Reprod. 2001;64:120–126. doi: 10.1095/biolreprod64.1.120. [DOI] [PubMed] [Google Scholar]
- West JA, Viswanathan SR, Yabuuchi A, Cunniff K, Takeuchi A, Park IH, Sero JE, Zhu H, Perez-Atayde A, Frazier AL, et al. A role for Lin28 in primordial germ-cell development and germ-cell malignancy. Nature. 2009;460:909–913. doi: 10.1038/nature08210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S. Spermatogenic stem cell system in the mouse testis. Cold Spring Harb Symp Quant Biol. 2008;73:25–32. doi: 10.1101/sqb.2008.73.046. [DOI] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- Zheng K, Wu X, Kaestner KH, Wang PJ. The pluripotency factor LIN28 marks undifferentiated spermatogonia in mouse. BMC Dev Biol. 2009;9:38. doi: 10.1186/1471-213X-9-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.