Abstract
Background
This study investigates the possible benefits of radiofrequency ablation (RFA) in patients with non-resectable colorectal liver metastases.
Methods
This phase II study, originally started as a phase III design, randomly assigned 119 patients with non-resectable colorectal liver metastases between systemic treatment (n = 59) or systemic treatment plus RFA ( ± resection) (n = 60). Primary objective was a 30-month overall survival (OS) rate >38% for the combined treatment group.
Results
The primary end point was met, 30-month OS rate was 61.7% [95% confidence interval (CI) 48.2–73.9] for combined treatment. However, 30-month OS for systemic treatment was 57.6% (95% CI 44.1–70.4), higher than anticipated. Median OS was 45.3 for combined treatment and 40.5 months for systemic treatment (P = 0.22). PFS rate at 3 years for combined treatment was 27.6% compared with 10.6% for systemic treatment only (hazard ratio = 0.63, 95% CI 0.42–0.95, P = 0.025). Median progression-free survival (PFS) was 16.8 months (95% CI 11.7–22.1) and 9.9 months (95% CI 9.3–13.7), respectively.
Conclusions
This is the first randomized study on the efficacy of RFA. The study met the primary end point on 30-month OS; however, the results in the control arm were in the same range. RFA plus systemic treatment resulted in significant longer PFS. At present, the ultimate effect of RFA on OS remains uncertain.
Keywords: colorectal cancer, liver metastases, multimodality treatment, radiofrequency ablation, unresectable
introduction
Every year 1 million people worldwide are diagnosed with colorectal cancer. Liver metastases will develop at some point during the course of the disease in up to 50% of these patients. When resection of liver metastases is possible, 5-year overall survival (OS) approaches 50% [1–3]. For non-resectable disease, systemic therapy is the standard of care and has been shown to prolong median survival to ∼2 years [2, 4–7]. Over the past decade, several techniques for local tumor destruction have emerged as alternative treatments for patients with non-resectable colorectal liver metastases [8–10]. Among these treatments, radiofrequency ablation (RFA) has been most frequently used to obtain complete tumor clearance from the liver, either alone or in combination with resection. Conclusive data on the beneficial survival effect of this approach are, however, lacking. There are no published randomized controlled trials (RCTs) investigating the use of RFA in these patients and data on efficacy have to be extracted from single-arm retrospective or prospective studies [11–15]. These studies show a wide variability in reported results, mainly because of differences in patient selection, concomitant treatments and varying end points.
Given the lack of strong evidence on the efficacy of RFA, European as well as American professional associations have issued expert statements on the possible best use of RFA [16–18]. However, these guidelines are hampered by the lack of evidence from RCTs and emphasize the compelling need for such studies.
Therefore, in an attempt to determine the additional value of RFA in patients with non-resectable colorectal metastases confined to the liver, a randomized phase III study was designed by the European Intergroup to compare the efficacy of combination treatment of RFA plus systemic treatment versus systemic treatment alone. Because accrual became slow, the study was amended and downsized to a randomized phase II trial.
methods
patients
Eligible patients had to have non-resectable liver metastases from colorectal adenocarcinoma without detectable extrahepatic disease, as judged on abdomino-pelvic computed tomography (CT) or magnetic resonance imaging (MRI) and chest CT by the consulting hepatobiliary surgeon and radiologist. Non-resectable was defined as no possibility to completely resect all tumor lesions. Prior chemotherapy for liver metastases only was allowed provided that at least disease stabilization was achieved. Previous adjuvant chemotherapy was allowed when terminated at least 12 months before detection of metastatic disease.
There had to be <10 liver metastases, with a maximum diameter of 4 cm for those lesions to be treated by RFA. Patients were eligible when metastatic involvement of the liver was ≤50% and complete treatment of all liver lesions was judged possible, either by RFA alone or by combination with resection of resectable lesions and RFA of the remaining non-resectable liver deposits. Patients had to be aged between 18 and 80 years, World Health Organization performance status of one or less, adequate bone marrow, liver and renal function. Exclusion criteria included presence of the primary tumor, any other malignancy in the past 10 years (except carcinoma of the cervix in situ or nonmelanoma skin cancer), higher than grade 1 sensory neuropathy, clinical significant cardiovascular disease, uncontrolled hypertension, bleeding disorders or coagulopathy, active infection, any contra-indication to the use of 5-FU/LV/oxaliplatin or bevacizumab and major surgical procedures within 28 days before the start of bevacizumab. Clinical investigations were carried out within 21 days before randomization. The study was approved by the medical ethics committees of all participating centers. Written informed consent was obtained from all patients.
procedures
Randomization was done at the EORTC headquarters with the minimization technique and was stratified for center, previous chemotherapy for liver metastases, previous adjuvant chemotherapy and route of randomization (before or during surgery). Eligible patients were randomly assigned at a 1:1 ratio to receive RFA plus systemic treatment or systemic treatment alone. In both arms, treatment had to start within 4 weeks of randomization. In the RFA group, systemic treatment had to begin between 4 and 8 weeks after the RFA procedure.
The strategy to obtain complete tumor clearance, either by RFA alone or in combination with resection, as well as the way RFA was carried out (during open surgery, laparoscopically or percutaneously) was decided by the hepatobiliary surgeon and the multidisciplinary team. All RFA procedures were carried out by experienced surgeons or radiologists, according to the guidelines of the manufacturer (Radionics, RadioTherapeutics, Rita).
From April 2002 to October 2005, systemic treatment in both arms consisted of 5-FU/LV/oxaliplatin. After October 2005, bevacizumab was added as it had become accepted as the standard of care in most participating centers for the population under study. Systemic therapy was given for 6 months in the absence of disease progression or unacceptable toxicity. In the systemic treatment alone arm, resection was allowed if during systemic treatment non-resectable disease was converted to resectable disease. RFA was not allowed at any time in this study arm. After protocol treatment, further systemic treatment was at the discretion of the multidisciplinary team. Second-line chemotherapy based on irinotecan was strongly recommended in cases of disease progression during protocol treatment.
Treatment of 5-FU/LV/oxaliplatin consisted of the FOLFOX 4 regimen (oxaliplatin 85 mg/m2, LV 200 mg/m2, 5-FU bolus 400 mg/m2 followed by 600 mg/m2 22-h infusion, every 14 days [19], or oxaliplatin 85 mg/m2, l-folinic acid 175 mg, 5-FU bolus 400 mg/m2 followed by 2400 mg/m2 46-h infusion every 14 days or oxaliplatin 85 mg/m2 every 14 days and weekly LV 200 mg/m2 and 5-FU 2600 mg/m2 24-h infusion, for 6 weeks followed by 1 week of rest). Bevacizumab was administered at 5 mg/kg body weight, once every 2 weeks. The relative dose intensity (RDI) was calculated as the ratio of the received DI [total dose (mg/m2)/total duration in weeks] to the DI indicated in the protocol.
Adverse effects were graded according to the National Cancer Institute—Common Toxicity Criteria scale version 2.0. Patients were followed up for safety until day 30 after the last administration of study treatment.
Tumor response was assessed every 6 weeks during protocol treatment and thereafter 3 monthly for 2 years and afterward 6 monthly. Follow-up investigations consisted of abdomino-pelvic CT, chest X-ray and measurement of serum carcinoembryonic antigen concentration.
Tumor response was assessed according to RECIST by the local radiologist [20]. Tumor recurrence at the RFA site was judged by the appearance on CT imaging of one or more new lesions at the edge of the RFA lesion or a 20% increase in the largest diameter of the RFA-treated lesion.
Health-related quality of life (HRQoL) was assessed by the EORTC QLQ-C30 questionnaire at randomization (baseline) and every 6 weeks after the start of systemic therapy until end of protocol treatment, thereafter during the standard follow-up assessments.
The primary end point of the phase II trial was the 30-month survival rate. Survival rates at 30 months of 38% and 53% were expected in the systemic treatment group and in the RFA plus systemic treatment group, respectively. The primary objective of the phase II trial was to demonstrate that the 30-month OS rate in the combined treatment group is >38%. Using a Fleming one-stage design with type I error of 10%, 76 patients were to be randomized to the combination treatment group in order to achieve a power of 90% under the alternative of a true 30-month survival rate of 53% in the combined treatment group. Secondary end points were OS, progression-free survival (PFS) and HRQoL.
statistical analysis
The analyses of 30-month survival rate, OS and PFS were intent-to-treat analyses. To be more conservative in the analysis of the primary end point, patients lost to follow-up before 30 months after randomization were counted as failures. Confidence intervals (CIs) for 30-month survival rates were computed using Fisher's exact methods. A sensitivity analysis for the primary end point was done on all eligible patients. Overall PFS and OS were estimated by the Kaplan–Meier method and compared by the log-rank test. Patients who were still event free when last seen were censored at the date of last follow-up.
results
We recruited 119 patients from 22 hospitals between 16 April 2002 and 20 June 2007. In June 2007, the study was closed early because of slow accrual. At that time, 119 out of 152 (78.3%) patients had been randomly assigned. The baseline characteristic of the 119 patients was balanced between the two treatment groups (Table 1).
Table 1.
Baseline characteristics
| RFA plus systemic treatment (N = 60), N (%) | Systemic treatment (N = 59), N (%) | Total (N = 119), N (%) | |
|---|---|---|---|
| Age (years) | |||
| Median (range) | 64 (31–79) | 61 (38–79) | 63 (31–79) |
| Sex | |||
| Male | 37 (61.7) | 42 (71.2) | 79 (66.4) |
| Female | 23 (38.3) | 17 (28.8) | 40 (33.6) |
| WHO performance status | |||
| 0 | 47 (78.3) | 47 (79.7) | 94 (79.0) |
| 1 | 13 (21.7) | 12 (20.3) | 25 (21.0) |
| No. of liver metastases | |||
| 1 | 15 (25.0) | 7 (11.9) | 22 (18.5) |
| 2 | 6 (10.0) | 4 (6.8) | 10 (8.4) |
| 3 | 8 (13.3) | 7 (11.9) | 15 (12.6) |
| 4 | 9 (15.0) | 8 (13.6) | 17 (14.3) |
| 5 | 6 (10.0) | 10 (16.9) | 16 (13.4) |
| 6 | 3 (5.0) | 9 (15.3) | 12 (10.1) |
| 7 | 6 (10.0) | 8 (13.6) | 14 (11.8) |
| 8 | 3 (5.0) | 2 (3.4) | 5 (4.2) |
| 9 | 4 (6.7) | 4 (6.8) | 8 (6.7) |
| Median | 4 | 5 | 4 |
| Synchronicity of liver metastases | |||
| Metachronous metastases | 37 (61.7) | 31 (52.5) | 68 (57.1) |
| Synchronous metastasesa | 23 (38.3) | 28 (47.5) | 51 (42.9) |
| Time from surgery for primary cancer to randomization (days) | |||
| Median | 290 | 308 | 295 |
| T stage of primary cancer | |||
| pT2 | 9 (15.0) | 4 (6.8) | 13 (10.9) |
| pT3 | 42 (70.0) | 48 (81.4) | 90 (75.6) |
| pT4 | 9 (15.0) | 6 (10.2) | 15 (12.6) |
| Unknown | 0 (0.0) | 1 (1.7) | 1 (0.8) |
| N stage of primary cancer | |||
| pN0 | 17 (28.3) | 21 (35.6) | 38 (31.9) |
| pN1 | 22 (36.7) | 24 (40.7) | 46 (38.7) |
| pN2 | 20 (33.3) | 12 (20.3) | 32 (26.9) |
| Unknown | 1 (1.7) | 2 (3.4) | 3 (2.5) |
| Adjuvant chemotherapy for primary cancerb | |||
| No | 50 (83.3) | 49 (83.1) | 99 (83.2) |
| Yes | 10 (16.7) | 10 (16.9) | 20 (16.8) |
| Prior chemotherapy for metastatic diseaseb | |||
| No | 51 (85.0) | 51 (86.4) | 102 (85.7) |
| Yes | 9 (15.0) | 8 (13.6) | 17 (14.3) |
| Previous liver surgery for CRC metastases | |||
| No | 51 (85.0) | 49 (83.1) | 100 (84.0) |
| Yes | 9 (15.0) | 10 (16.9) | 19 (16.0) |
| Route of randomizationb | |||
| Before surgery | 46 (76.7) | 44 (74.6) | 90 (75.6) |
| During surgery | 14 (23.3) | 15 (25.4) | 29 (24.4) |
| CEA (ng/ml) | |||
| Median | 7.0 | 8.0 | 8.0 |
| Range | 1.0–1887.0 | 1.0–174.0 | 1.0–1887.0 |
| N | 51 | 53 | 104 |
Comparison for all different baseline characteristics is not statistically significant.
aLiver metastases detected within 3 months after primary cancer diagnosis.
bStratification factors.
CEA, carcinoembryonic antigen; CRC, colorectal cancer; RFA, radiofrequency ablation; WHO, World Health Organization.
Supplemental Figure S1 (available at Annals of Oncology online) shows the trial profile. In the combined treatment group, three patients were found to be ineligible. Reasons for ineligibility were diagnosis of bone metastases on MRI [1], lesion to be treated with RFA larger than 40 mm on baseline CT scan [1], liver metastases considered to be resectable on baseline CT scan [1]. Of the 60 patients randomized to the combined treatment group, 50 (83.3%) patients underwent RFA and started post-ablation systemic therapy; among these, 30 were treated with RFA only and 20 with RFA and additional resection. Of the 10 patients not receiving planned combined treatment, 3 did not receive RFA, 6 did not receive systemic treatment and of 1 patient no treatment data were available. Of all patients treated by RFA, 51 (91.1%) underwent RFA during open surgery.
Of the 59 patients randomized to systemic treatment alone, all patients started systemic treatment. One patient was found to be ineligible, the patient was resectable on initial CT imaging and underwent liver resection after the start of chemotherapy. Five additional patients (8.5%) underwent liver resection per protocol because their non-resectable disease was converted to resectable disease during or after chemotherapy. Two patients received surgery plus RFA after progression on chemotherapy, both judged as major protocol violations.
In the combined treatment group, the median number of cycles was 8.5 (0–12), in the systemic treatment group, it was 10 [1–12] (Table 2). Table 3 shows the postoperative complications in the combined treatment arm and the toxic effects of systemic treatment of both study arms. There was one postoperative death due to sepsis in the combined treatment arm. Toxicity from systemic treatment was comparable in both arms.
Table 2.
Treatment compliance by treatment group
| RFA plus systemic treatment (N = 60) | Systemic treatment (N = 59) | |
|---|---|---|
| Treatment received | N (%) | N (%) |
| Folfox | 43 (71.7) | 46 (78.0) |
| Folfox + bevacizumab | 8 (13.3) | 13 (22.0) |
| RFA only | 6 (10.0) | |
| No treatment | 3 (5.0) | |
| Administration of chemotherapy | ||
| No. of cycles | N (%) | N (%) |
| 0 | 9 (15.0) | 0 (0.0) |
| 1–4 | 14 (23.3) | 15 (25.4) |
| 5–8 | 7 (11.7) | 4 (6.8) |
| 9–11 | 8 (13.3) | 13 (22.0) |
| 12 | 22 (36.7) | 27 (45.8) |
| Median | 8.5 | 10.0 |
| RDI (%) | Median (range) | Median (range) |
| RDI 5-FU (N = 110) | 83 (49–105) | 91 (50–104) |
| RDI folinic acid (N = 109) | 92 (46–200) | 95 (51–200) |
| RDI oxaliplatin (N = 110) | 80 (18–101) | 83 (37–103) |
| RDI bevacizumab (N = 21) | 94 (34–102) | 97 (13–101) |
RDI, relative dose intensity; RFA, radiofrequency ablation.
Table 3.
Postoperative complications and tolerance to systemic treatment
| RFA plus systemic treatment (N = 57) |
Systemic treatment (N = 59) | |||
|---|---|---|---|---|
| RFA (N = 30), N (%) | RFA plus resection (N = 27)a, N (%) | Total (N = 57), N (%) | ||
| Postoperative complications | ||||
| Respiratory failure | 0 (0.0) | 1 (3.7) | 1 (1.8) | |
| Cardiac failure or infarction | 1 (3.3) | 2 (7.4) | 3 (5.3) | |
| Hepatic dysfunction bilirubin >10 mg/dl for 3 days | 1 (3.3) | 2 (7.4) | 3 (5.3) | |
| Wound infection | 2 (6.7) | 1 (3.7) | 3 (5.3) | |
| Intra-abdominal infection (abscess) | 1 (3.3) | 1 (3.7) | 2 (3.5) | |
| Other infection | 1 (3.3) | 0 (0.0) | 1 (1.8) | |
| Fever | 5 (16.7) | 7 (25.9) | 12 (21.1) | |
| Malaise | 1 (3.3) | 1 (3.7) | 2 (3.5) | |
| Fatigue | 4 (13.3) | 2 (7.4) | 6 (10.5) | |
| Hemorrhage | 0 (0.0) | 2 (7.4) | 2 (3.5) | |
| Need for reoperation | 0 (0.0) | 3 (11.1) | 3 (5.3) | |
| Renal failure | 0 (0.0) | 1 (3.7) | 1 (1.8) | |
| Other | 2 (6.7) | 3 (11.1) | 5 (8.8) | |
| Hospitalization >24 h: due to complication | 4 (13.3) | 6 (22.2) | 10 (17.5) | |
| Postoperative death | 0 (0.0) | 1 (3.7) | 1 (1.8) | |
| Tolerance to systemic treatment | (N = 51) N (%) | (N = 59) N (%) | ||
| Grade 3–4 neutropenia | 14 (27.5) | 12 (20.3) | ||
| Grade 3–4 cardiotoxicity | 5 (9.8) | 1 (1.7) | ||
| Grade 3–4 diarrhea | 10 (19.6) | 10 (16.9) | ||
| Grade 3–4 vomiting | 5 (9.8) | 4 (6.8) | ||
| Grade 3 nausea (no grade 4) | 7 (13.7) | 6 (10.2) | ||
| Grade 3–4 gastrointestinal others | 4 (7.8) | 4 (6.8) | ||
| Grade 3–4 pulmonary | 3 (5.9) | 1 (1.7) | ||
| Grade 3–4 renal | 1 (2.0) | 1 (1.7) | ||
| Grade 3 neuropathy (no grade 4) | 9 (17.6) | 8 (13.6) | ||
| Grade 3 fatigue (no grade 4) | 7 (13.7) | 4 (6.8) | ||
| Grade 3 hypertension (no grade 4) | 2 (3.9) | 2 (3.4) | ||
Patients may have several complications; therefore, number of complications does not add up to the total number of patients.
aOne patient treated by resection only.
RFA, radiofrequency ablation.
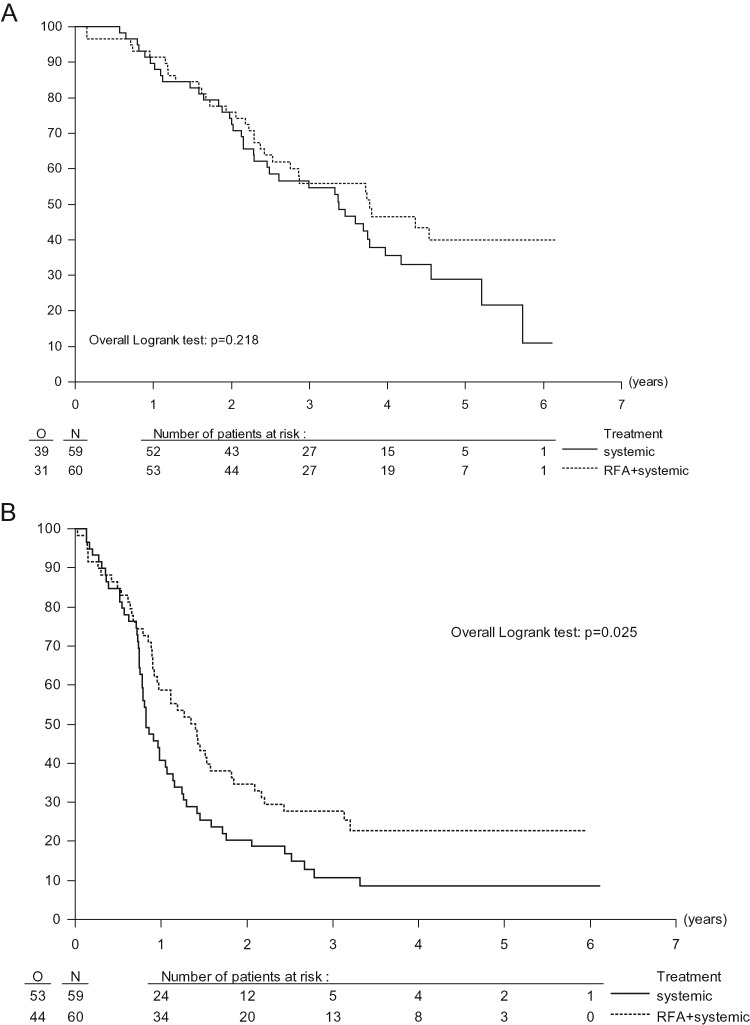
At the time of analysis, 49 (41.2%) of 119 patients were still alive, 29 in the combined treatment group and 20 in the systemic treatment group. The median follow-up time was 4.4 years in both treatment groups. At 30 months, only three patients (two in the combined treatment group and one in the systemic treatment group) have been lost to follow-up. Counting patients lost to follow-up before 30 months as failures (conservative approach), OS rate at 30 months was 61.7% (95% CI 48.2–73.9) for the combined treatment group and 57.6% (95% CI 44.1–70.4) for the systemic treatment group. The median OS was 45.3 (95% CI 33.1–NA) months for combination treatment and 40.5 (95% CI 29.5–50.1) months for systemic treatment only [hazard ratio (HR) = 0.74, 95% CI 0.46–1.19, P = 0.22, Figure 1A]. Median PFS was 16.8 months (95% CI 11.7–22.1) in the group assigned to combination treatment and 9.9 months (95% CI 9.3–13.7) in the group assigned to systemic treatment only. The HR for combined treatment versus systemic treatment only was 0.63 (95% CI 0.42–0.95, P = 0.025), corresponding to an absolute of 17% increase in the rate of PFS at 3 years from 10.6% (95% CI 4.2–20.5) to 27.6% (95% CI 16.9–39.5) (Figure 1B).
Figure 1.
Overall survival and progression-free survival. (A) Overall survival by treatment group. (B) Progression-free survival by treatment group. RFA, radiofrequency ablation.
The liver, either alone or in combination with extrahepatic disease, was the first site of progressive disease in 27 patients in the combined treatment group (45%) compared with 45 patients in the systemic treatment alone group (76.3%) (P < 0.0001; supplemental Figure S1, available at Annals of Oncology online). In total, on 56 patients treated with RFA, 9 patients (16.1%) had local recurrence at the RFA site at first progression. On a per-lesion basis, in total 11 lesions (in 9 patients) out of 170 RFA-treated lesions recurred locally at first progression (6.5%). In addition, three lesions (in two patients) recurred locally after initial disease progression at another site.
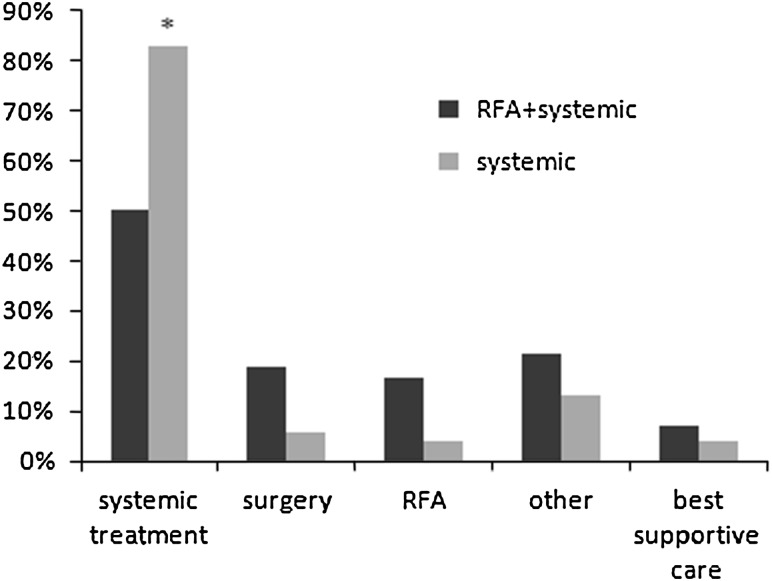
The percentage of patients treated for first progression was comparable between both arms, 37 out of 42 patients (88.1%) in the combination treatment group and 46 out of 53 patients (86.8%) in the systemic treatment group. Salvage treatment consisted of systemic treatment in 21 patients (50%) in the combined treatment group compared with 44 patients (83%) in the systemic treatment group (P < 0.001; Figure 2).
Figure 2.
Salvage treatment of first progression by treatment group. Some patients were treated by a combination of treatment modalities. *P < 0.001. RFA, radiofrequency ablation.
A total of 110 patients had at least one valid HRQoL assessment and were available for analysis. Compliance was good at baseline and after RFA in the combined treatment group but low for the other follow-up measurements. Based on observed data in the combined treatment arm, HRQoL scales were impaired after RFA. While a 20-point difference is considered a significant effect, mean global QoL dropped by 27 points. However, recovery to a level at ∼10 points below baseline was achieved before the start of systemic treatment (4–8 weeks after RFA). Thereafter, HRQoL scores were similar in both treatment groups, although the limited sample size limits definite conclusions on HRQoL.
discussion
Within the European Intergroup frame work, the present study was originally designed and started as a randomized phase III study. However, strong preferences for either of both treatment arms limited the referral for trial participation, and the study had to be downscaled to the present randomized phase II design in 2003. The phase II design of the present study clearly limits definite conclusions on the benefit of combined treatment on OS. Although the primary end point was met, median OS was not statistically different between both arms.
In addition, OS was higher than expected in the systemic treatment alone arm. At the time the study was designed, survival data for systemic therapy in patients with metastatic disease confined to the liver were still limited. A median OS time of 22 months (corresponding to a 30-month survival rate of ∼38%) was anticipated based on overall analysis of patients with metastatic colorectal cancer [21, 22]. At the time of study design, few patients went on to second-line treatment at the time of disease progression (since in the late 1990s little or nothing was available), and it was never anticipated that four out of five patients in both arms of the study would be offered significant further (and in some cases multiple) multidisciplinary interventions at the point of first-line treatment failure. The observed 30-month OS in the systemic treatment group comes closer to more recent studies in patients with liver only disease [23–25].
The present study was originally designed as a randomized phase III study to detect a difference in survival between both arms. Due to slow accrual, the study was downsized to a randomized phase II trial which does not allow any direct comparison in OS. However, we observed a significant improvement in PFS for patients treated by combination treatment of RFA plus systemic treatment. Median PFS was prolonged by nearly 7 months in the combined treatment group. Given the limited power of the present study design to detect a difference in OS, it remains to be awaited whether this effect on PFS translates into a difference in OS survival after longer follow-up. The translation of improved PFS into prolonged OS may be biased by imbalances in salvage treatments as well as by potential inaccurate determination of PFS. Although the percentage of patients undergoing salvage treatment was comparable between both study groups, the choice of salvage treatment was not balanced. In the systemic treatment group, a significant higher percentage of patients received systemic treatment as salvage treatment, while in the combined treatment group, salvage treatment more often consisted of local treatment by surgery or RFA. Apparently, it was felt by the treatment team that RFA resulted in adequate local control of the liver lesions allowing a wider indication for further local treatment of recurrent disease. This imbalance in the salvage treatments compromises the interpretation of OS data.
With regard to reliable assessment of PFS, the compliance to the tight follow-up protocol was high. Despite, it may be argued that local recurrence after RFA is not always easy to detect, which could result in an underestimation of PFS in the RFA group. However, the imaging protocol to detect local recurrence turned out to be quite accurate since during a median follow-up of 4.4 years, the number of patients that developed local recurrence, not detected as first site of recurrence, was low (two cases only).
Furthermore, local control of liver metastases by RFA caused a significant difference in the site of first progression between both treatment groups. It may be hypothesized that effective local control of the liver metastases by RFA postponed disease progression until the presentation of extrahepatic disease, which generally occurred at a later time point. Although a longer PFS in the combined treatment group can be expected because of the high local control rate of the liver lesions, the gain of almost 7 months in PFS is clinically significant and may indicate a beneficial effect of this approach.
In addition, with a small sample size there is always the risk of imbalance in known or unknown patient characteristics which can make one of the groups more ‘favourable’ than the other. Although we did not observe any significant imbalances, we cannot be completely affirmative that the observed difference in PFS is not influenced by any patient imbalance between the arms.
RFA was safe and well tolerated. Tolerance to chemotherapy was similar between both treatment arms and comparable to other studies in which identical systemic treatment was given after liver surgery [26]. HRQoL after RFA showed only a short decline with recovery to baseline values within 8 weeks after the procedure.
With the availability of an increasing number of local tumor ablative techniques, the treatment options for patients with non-resectable colorectal liver metastases increase considerably. This is especially so at the present time when new medical devices can be rapidly introduced without the need to subject them for official approval of efficacy.
This present phase II study is the first randomized study on the use of RFA for unresectable colorectal liver metastases. The study shows that local tumor ablation by RFA in combination with systemic therapy results in an excellent survival, which however was also achieved in the control arm. Long-term follow-up data of the present trial are awaited, but as the trial is not powered to detect a difference in OS, definitive proof of the benefit of RFA on OS may be difficult. The median PFS was significantly prolonged by RFA plus systemic therapy. Imbalances in salvage treatments between both study arms may have impeded the translation of PFS into OS. Since the study was not powered to detect differences in OS, the ultimate effect of RFA on OS is still uncertain. Despite these limitations, it is highly unlikely that ultimate efficacy on OS will ever be tested again, given the difficult accrual of the present study. Whether PFS, increasingly used in assessing the efficacy of treatments in metastatic cancer, could be an acceptable surrogate end point in such setting remains debatable.
funding
The sponsor of this trial was EORTC. The trial was supported by grants from Cancer Research UK (C837/A3488) and ALM-CAO (Arbeitsgruppe Lebermetastasen und—tumoren in der Chirurgischen Arbeitsgemeinschaft Onkologie), Dutch Cancer Foundation (Commissie Klinisch Toegepast Onderzoek), (5U10 CA11488-32–5U10 CA011488-40) from the National Cancer Institute (Bethesda, MD), by a donation from Kankerbestrijding/KWF from The Netherlands through the EORTC Charitable Trust and by an unrestricted scientific grant from Sanofi-Aventis. Radionics, Radiotherapeutics and Rita provided free RFA needles. The content of this report is solely the responsibility of the authors. Trial management and data analysis were undertaken at the EORTC Data Center (Brussels, Belgium) independently of any commercial interest. The trial is registered with ClinicalTrials.gov, number NCT00043004.
disclosure
The authors have declared no conflicts of interest.
Supplementary Material
acknowledgements
Investigators who participated through the collaborative groups: V J Verwaal, The Netherlands Cancer Institute—Antoni van Leeuwenhoekziekenhuis, Amsterdam, The Netherlands; T Gruenberger, Medical University Vienna—General Hospital, Vienna, Austria; J Klaase, Medisch Spectrum Twente, Enschede, The Netherlands; S Falk, University Hospitals Bristol NHS Foundation Trust—Bristol Haematology and Oncology Centre, Bristol Avon, UK; J Wals, Atrium Medisch Centrum, Heerlen, The Netherlands; R L Jansen, Akademisch Ziekenhuis Maastricht, The Netherlands; P Lindnér, Sahlgrenska Sjukhuset, Goteborg, Sweden; S Mulier, Cliniques Universitaires de Mont Godinne, Yvoir, Belgium; K Bosscha, Jeroen Bosch Ziekenhuis, 's Hertogenbosch, The Netherlands; D Jaeck, Hôpital Universitaire Hautepierre, Strasbourg, France; J P Arnaud, Centre Hospitalier Universitaire d'Angers, France; D Smith, Clatterbridge Centre for Oncology NHS Trust, Wirral Merseyside, UK; D Sherlock, North Manchester General Hospital, Manchester, UK; B Ammori, Manchester Royal Infirmary, Manchester, UK; A Gillams, UCL Hospitals, London, UK; M El-Serafi, National Cancer Institute, Cairo, Egypt; B Glimelius, Karolinska University Hospital Solna, Stockholm, Sweden; P Hellman, Akademiska Sjukhuset, Uppsala, Sweden 863. Uppsala Akademiska (SE). We thank all patients who consented to enter the study. This study was undertaken by the EORTC GITCG (European Organisation for Research and Treatment of Cancer Gastro-Intestinal Tract Cancer Group) in collaboration with NCRI CCSG (National Cancer Research Institution Colorectal Study Group) and was partly managed by the Cancer Research UK and UCL Cancer Trial Centre in collaboration with EORTC. TR was responsible for the design of the trial. CP, FVC, JP, IBR, JAL, WB contributed patients to the study and reviewed the report. MAL, MPL, BN, EVC and GP contributed to the trial management and reviewed the report. MM analyzed the trial data, contributed to the writing of the report and approved the final version. All authors approved the final version of the report.
references
- 1.House MG, Ito H, Gonen M, et al. Survival after hepatic resection for metastatic colorectal cancer: trends in outcomes for 1,600 patients during two decades at a single institution. J Am Coll Surg. 2010;210(5):744–745. doi: 10.1016/j.jamcollsurg.2009.12.040. [DOI] [PubMed] [Google Scholar]
- 2.Kopetz S, Chang GJ, Overman MJ, et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol. 2009;27(22):3677–3683. doi: 10.1200/JCO.2008.20.5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mitry E, Fields AL, Bleiberg H, et al. Adjuvant chemotherapy after potentially curative resection of metastases from colorectal cancer: a pooled analysis of two randomized trials. J Clin Oncol. 2008;26(30):4906–4911. doi: 10.1200/JCO.2008.17.3781. [DOI] [PubMed] [Google Scholar]
- 4.Saltz LB, Clarke S, Diaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26(12):2013–2019. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]
- 5.Tol J, Koopman M, Cats A, et al. Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancer. N Engl J Med. 2009;360(6):563–572. doi: 10.1056/NEJMoa0808268. [DOI] [PubMed] [Google Scholar]
- 6.Van CE, Kohne CH, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360(14):1408–1417. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 7.Sanoff HK, Sargent DJ, Campbell ME, et al. Five-year data and prognostic factor analysis of oxaliplatin and irinotecan combinations for advanced colorectal cancer: N9741. J Clin Oncol. 2008;26(35):5721–5727. doi: 10.1200/JCO.2008.17.7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Baere T, Elias D, Dromain C, et al. Radiofrequency ablation of 100 hepatic metastases with a mean follow-up of more than 1 year. AJR Am J Roentgenol. 2000;175(6):1619–1625. doi: 10.2214/ajr.175.6.1751619. [DOI] [PubMed] [Google Scholar]
- 9.Martin RC, Scoggins CR, McMasters KM. Safety and efficacy of microwave ablation of hepatic tumors: a prospective review of a 5-year experience. Ann Surg Oncol. 2010;17(1):171–178. doi: 10.1245/s10434-009-0686-z. [DOI] [PubMed] [Google Scholar]
- 10.Vogl TJ, Straub R, Eichler K, et al. Colorectal carcinoma metastases in liver: laser-induced interstitial thermotherapy—local tumor control rate and survival data. Radiology. 2004;230(2):450–458. doi: 10.1148/radiol.2302020646. [DOI] [PubMed] [Google Scholar]
- 11.Abdalla EK, Vauthey JN, Ellis LM, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg. 2004;239(6):818–825. doi: 10.1097/01.sla.0000128305.90650.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gillams AR, Lees WR. Five-year survival in 309 patients with colorectal liver metastases treated with radiofrequency ablation. Eur Radiol. 2009;19(5):1206–1213. doi: 10.1007/s00330-008-1258-5. [DOI] [PubMed] [Google Scholar]
- 13.Mulier S, Ni Y, Jamart J, et al. Local recurrence after hepatic radiofrequency coagulation: multivariate meta-analysis and review of contributing factors. Ann Surg. 2005;242(2):158–171. doi: 10.1097/01.sla.0000171032.99149.fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruers TJ, Joosten JJ, Wiering B, et al. Comparison between local ablative therapy and chemotherapy for non-resectable colorectal liver metastases: a prospective study. Ann Surg Oncol. 2007;14(3):1161–1169. doi: 10.1245/s10434-006-9312-5. [DOI] [PubMed] [Google Scholar]
- 15.Siperstein AE, Berber E, Ballem N, Parikh RT. Survival after radiofrequency ablation of colorectal liver metastases: 10-year experience. Ann Surg. 2007;246(4):559–565. doi: 10.1097/SLA.0b013e318155a7b6. [DOI] [PubMed] [Google Scholar]
- 16.Crocetti L, de BT, Lencioni R. Quality improvement guidelines for radiofrequency ablation of liver tumours. Cardiovasc Intervent Radiol. 2010;33(1):11–17. doi: 10.1007/s00270-009-9736-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gervais DA, Goldberg SN, Brown DB, et al. Society of Interventional Radiology position statement on percutaneous radiofrequency ablation for the treatment of liver tumors. J Vasc Interv Radiol. 2009;20(7 Suppl):S342–S347. doi: 10.1016/j.jvir.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 18.Wong SL, Mangu PB, Choti MA, et al. American Society of Clinical Oncology 2009 clinical evidence review on radiofrequency ablation of hepatic metastases from colorectal cancer. J Clin Oncol. 2010;28(3):493–508. doi: 10.1200/JCO.2009.23.4450. [DOI] [PubMed] [Google Scholar]
- 19.de Gramont A, Figer A, Seymour M, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18(16):2938–2947. doi: 10.1200/JCO.2000.18.16.2938. [DOI] [PubMed] [Google Scholar]
- 20.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 21.Tournigand C, Andre T, Achille E, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004;22(2):229–237. doi: 10.1200/JCO.2004.05.113. [DOI] [PubMed] [Google Scholar]
- 22.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 23.Barone C, Nuzzo G, Cassano A, et al. Final analysis of colorectal cancer patients treated with irinotecan and 5-fluorouracil plus folinic acid neoadjuvant chemotherapy for unresectable liver metastases. Br J Cancer. 2007;97(8):1035–1039. doi: 10.1038/sj.bjc.6603988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alberts SR, Horvath WL, Sternfeld WC, et al. Oxaliplatin, fluorouracil, and leucovorin for patients with unresectable liver-only metastases from colorectal cancer: a North Central Cancer Treatment Group phase II study. J Clin Oncol. 2005;23(36):9243–9249. doi: 10.1200/JCO.2005.07.740. [DOI] [PubMed] [Google Scholar]
- 25.Ychou M, Viret F, Kramar A, et al. Tritherapy with fluorouracil/leucovorin, irinotecan and oxaliplatin (FOLFIRINOX): a phase II study in colorectal cancer patients with non-resectable liver metastases. Cancer Chemother Pharmacol. 2008;62(2):195–201. doi: 10.1007/s00280-007-0588-3. [DOI] [PubMed] [Google Scholar]
- 26.Nordlinger B, Sorbye H, Glimelius B, et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet. 2008;371(9617):1007–1016. doi: 10.1016/S0140-6736(08)60455-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.